Abstract
Epothilones are microtubule inhibitors that are promising alternatives to paclitaxel due to enhanced anticancer efficacy. While epothilones are slightly more water soluble than paclitaxel and more active against paclitaxel-resistant cells, they still require formulation with Cremophor EL and/or cosolvents and drug resistance still limits therapeutic efficacy. In this report, we showed that the combinational treatment of epothilone B (EpoB), 17-N-allylamino-17-demethoxygeldanamycin (17-AAG, Hsp90 inhibitor), and rapamycin (mTOR inhibitor) displays strong anticancer activity in vitro and in vivo. To address the poor water solubility of this 3 drug-combination, they were co-loaded into poly(ethylene glycol)-block-poly(D,L-lactic acid) (PEG-b-PLA) micelles, and the 3-in-1 loaded PEG-b-PLA micelle (m-EAR) was characterized in terms of drug loading efficiency, particle size, release kinetics. The m-EAR achieved high levels of all three drugs in water; formed micelles with hydrodynamic diameters at ca. 30 nm and released the drugs in a sustained manner in vitro at rates slower than individually loaded PEG-b-PLA micelles. In A549-derived xenograft mice, m-EAR (2.0, 15.0, and 7.5 mg/kg) caused tumor regression after four weekly injections, whereas EpoB alone (2.0 mg/kg) was the same as control. No severe changes in body weight relative to PBS control were observed, attesting to the safety of m-EAR. Collectively, these results suggest that m-EAR provides a simple, but effective and safe EpoB-based combination nanomedicine for cancer therapy.
Keywords: Epothilone B, polymeric micelle, non-small cell lung cancer, drug combination, multiple drug solubilization
Graphical abstract
Epothilones have gained increasing attention due to much greater anticancer efficacy compared to paclitaxel (PTX). Epothilones induce cell cycle arrest at the G2-M transition and microtubule polymerization at nanomolar concentration, leading to cancer cell death (Goodin et al., 2004). Compared to PTX, epothilones possess several advantages: Low susceptibility to drug efflux transporters, increased solubility and more manageable toxic profile in human patients (Bollag et al., 1995; Bystricky and Chau, 2011). Bollag et al. has demonstrated that epothilone B (EpoB) has higher cytotoxicity and mitotic arrest at the G2-M transition against P-glycoprotein-expressing multi-drug resistant KBV-1 cells than PTX (Bollag et al., 1995). Others have found that EpoB has strong in vivo anticancer activity at tolerated doses in several human cancer cell-derived xenograft models (Lin et al., 2005; O'Reilly et al., 2005; Pietras et al., 2003; Rothermel et al., 2003). However, researchers have concluded that single chemotherapy such as PTX and epothilones has reached a plateau in efficacy as a primary treatment modality (Bagnyukova et al., 2010; LoPiccolo et al., 2008). This is due to the growing recognition that cancer cells possess a capacity to activate compensatory signaling pathways, leading to drug resistance toward microtubule inhibitors (Hasenstein et al., 2012).
To overcome drug resistance towards paclitaxel, the axis of mammalian target of rapamycin (mTOR) and heat shock protein 90 (Hsp90) has been widely investigated in pre-clinical and clinical studies. Rapamycin has improved the anticancer activity of PTX in MDA-MB-468 derived xenograft mice (Mondesire et al., 2004). Combination therapy of rapamycin and 17-N-allylamino-17-demethoxygeldanamycin (17-AAG) has resulted in strong anticancer efficacy against breast cancer cells, blunting Akt activation due to mTOR inhibition (Roforth and Tan, 2008). 17-AAG has improved the anticancer efficacy of PTX in ovarian, lung and breast cancer xenograft models (Nguyen et al., 2001; Sausville, 2001; Solit et al., 2003). 17-AAG binds Hsp90 and inhibits client protein maturation, e.g. HER2 expression and Akt activation of cancer cells, increasing anticancer activity of PTX (Basso et al., 2002; Schulte and Neckers, 1998). Furthermore, all three drugs have not only displayed cytotoxicity against cancer cells but also anti-angiogenesis ability, (Bocci et al., 2002; Guba et al., 2002; Kaur et al., 2004). All these data suggest that combination therapy of 17-AAG and rapamycin might enhance anticancer efficacy of EpoB, achieved by the simultaneous inhibition of mTOR and Hsp90.
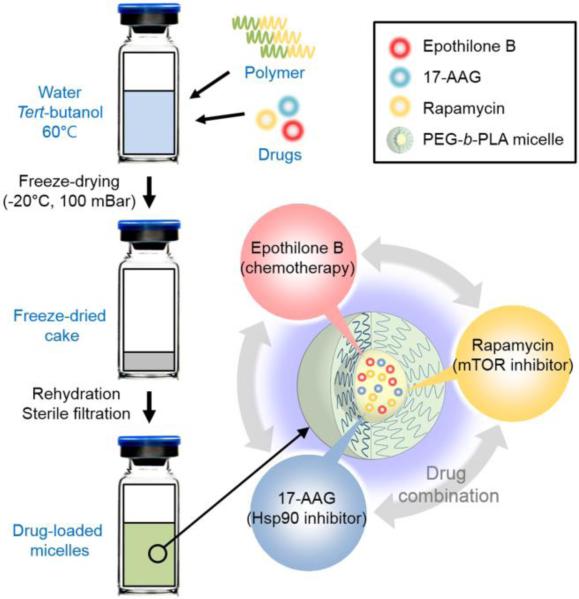
In this study, a 3-in-1 poly(ethylene glycol)-block-poly(D,L-lactic acid) (PEG-b-PLA) micelle containing EpoB, 17-AAG and rapamycin (m-EAR) has been prepared based on freeze-drying method (Fig. 1). Epothilones have required drug solubilization by cosolvents and/or Cremophor EL for intravenous injection or infusion for pre-clinical evaluation in human xenograft models and clinical trials. However, cosolvents and/or Cremophor EL are toxic; in the latter case, Cremophor EL causes life-threatening hypersensitivity reactions in ca. 3% of breast cancer patients despite pre-treatment with corticosteroid and antihistamine. Alternatively, PEG-b-PLA micelles act as a nanocarrier for poorly water-soluble anticancer agents, e.g. permitting 2-fold dose escalation of PTX relative to Taxol® (Kim et al., 2007). PEG-b-PLA micelles have been shown to solubilize sagophilone, an epothilone analog, and physicochemical properties of sagophilone-loaded PEG-b-PLA micelles have been characterized in vitro (Richter et al., 2010).
Fig. 1.
Schematic representation of 3-in-1 PEG-b-PLA micelle preparation.
Table 1 summarizes drug loading efficiency of PEG-b-PLA micelles: Single drug-loaded micelles (EpoB micelle: m-E, 17-AAG micelle: m-A and rapamycin micelle: m-R); two drug (EpoB/17-AAG micelle: m-EA, 17-AAG/rapamycin micelle: m-AR, 17-AAG/rapamycin micelle: m-ER) or three drug-loaded micelles (m-EAR). PEG-b-PLA micelles increased the individual aqueous solubility of EpoB, 17-AAG and rapamycin (solubility in water: 25 μg/mL, 20 μg/mL, and 2.6 μg/mL, respectively) by 11-, 137- and 503-fold, resulting in drug level at 0.28, 2.74 and 1.31 mg/mL, respectively (Table 1) (Kaur et al., 2004; Simamora et al., 2001). Notably, % drug loading efficiency for each drug in 2-in-1 and m-EAR was similar with that of single 1-in-1 drug-loaded micelles (70~90%), showing that PEG-b-PLA micelles have capacity for not only one drug but also two or three drugs. The results on m-EAR are consistent with our recent work on PEG-b-PLA micelles that has proven that they can solubilize not only one drug but also two or three drugs as a multi-drug nanocarrier, including PTX, 17-AAG and rapamycin (Shin et al., 2009; Tomoda et al., 2016). The average hydrodynamic diameters of PEG-b-PLA micelles was 25~30 nm, regardless of drug loading efficiency (Fig. S1 and Table S1), and PDI values were < 0.1, indicating low polydispersity. Based on particle size analysis by DLS, all drug-loaded PEG-b-PLA micelles were stable for five days at ambient temperature (Fig. S2). However, purple precipitate was observed only in m-A after five days, indicating the loss of physical stability of 17-AAG (Fig. S3). In contrast, m-A prepared by a polymer film hydration method had higher 17-AAG loading and was more stable (Shin et al., 2011), indicating that polymer film hydration method is better for m-A between two methods. The molecular rationale for differences for 17-AAG requires further study. No precipitation was observed in m-EA, m-AR, and m-EAR.
Table 1.
In vitro characterization of PEG-b-PLA micelles (n=3).
| Type | Micelles | Polymer (mg/mL) | Initial level of drug(s) (mg/mL) | Drug loading efficiency (%) | Individual drug level (mg/mL) | Total drug level (mg/mL) |
|---|---|---|---|---|---|---|
| 1-in-1 | m-E | 100 | 0.4 | 70.6±3.2 | 0.282±0.013 | 0.282±0.013 |
| m-A | 100 | 3 | 91.5±4.7 | 2.744±0.141 | 2.744±0.141 | |
| m-R | 100 | 1.5 | 87.6±5.1 | 1.314±0.077 | 1.314±0.077 | |
| 2-in-1 | m-EA | 100 | 0.4/3 | 83.7±4.9 82.7±4.0 |
0.335±0.019 2.482±0.121 |
2.817±0.141 |
| m-AR | 100 | 3/1.5 | 82.3±3.9 83.9±3.6 |
2.470±0.118 1.258±0.054 |
3.728±0.172 | |
| m- ER | 100 | 0.4/1.5 | 73.8±2.8 77.8±4.1 |
0.295±0.011 1.167±0.062 |
1.462±0.073 | |
| 3-in-1 | m-EAR | 100 | 0.4/3/1.5 | 88.6±0.5 83.0±0.7 82.2±0.7 |
0.354±0.002 2.491±0.021 1.232±0.010 |
4.078±0.033 |
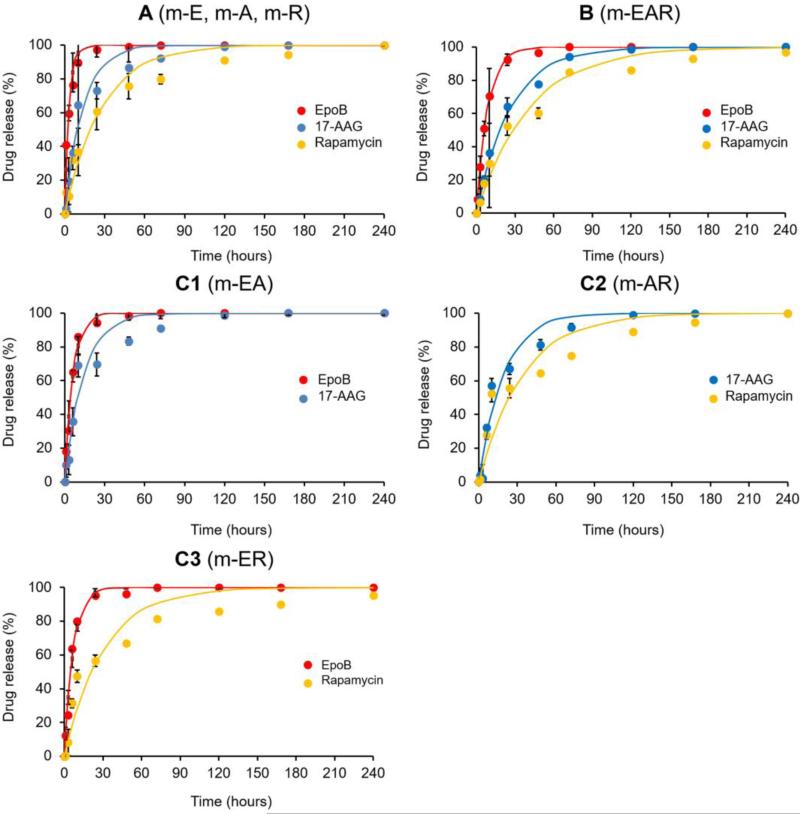
In vitro release of EpoB from PEG-b-PLA micelles was rapid with t1/2 =2.28 hours, assuming first-order kinetics (Figure 2). While the t1/2 values for 17-AAG and rapamycin were 9.42 and 19.1 hours, respectively, increasing with logPoil/water value. Notably, in vitro drug release for m-EAR was noticeably slower than 1-in-1 drug-loaded PEG-b-PLA micelles, having t1/2 values for EpoB, 17-AAG and rapamycin at 5.98, 18.0 and 28.2 hours, respectively (Table S2). This may reflect intermolecular drug interaction in the core of PEG-b-PLA micelles that allows slower drug release. Alternatively, location of co-loaded drugs in PEG-b-PLA micelles may impact drug release. 1H NMR analysis of m-EAR might give insights into drug location and possibly drug-drug interactions in PEG-b-PLA micelles (Catenacci et al., 2014). One limitation of this in vitro study is noteworthy: It was done above the CMC of PEG-b-PLA micelles and reflects diffusional release, but not the contribution due to micelle disassembly, owing to dilution below the CMC after injection in blood. The rapid in vitro release of EpoB indicates that PEG-b-PLA micelles will act primarily as a non-toxic solubilizing agent but not a long-circulating nanocarrier for EpoB; however this hypothesis requires pharmacokinetic validation.
Fig. 2.
In vitro drug release profiles of various micellar formulations containing one drug only (A), three drug combination (B) and two drug combinations (C). All measurements were performed three times.
Table 2 summarizes the in vitro cytotoxicity of drug(s) against human A549 non-small cell lung cancer cells. EpoB has indeed more potent cytotoxicity against cancer cells than PTX (IC50 = 0.17 versus 3.16 nM), and this result is consistent with previous studies (Altmann et al., 2000; Bollag et al., 1995). After treatment of A549 cells with drug combinations, drug interaction (synergistic, additive or antagonistic) between EpoB, 17-AAG and rapamycin was evaluated using combination index (CI) analysis. CI values of drug combinations were calculated based on inhibitory concentration (IC35, 50, and 65) values of individual drugs and their combinations. CI values of two drug combinations were variable, showing synergy for EpoB with rapamycin, but additivity or slight antagonism for EpoB with 17-AAG. The CI value of the 3-drug combination also showed synergy, additivity or slight antagonism, depending on the fraction of A549 cell affected. In this case, the IC50 value was 0.29 nM, approaching the remarkably low value of EpoB.
Table 2.
Evaluation of drug interaction: CI analysis of 2- and 3-drug combinations (n=3).
| Drug combination (molar ratio) |
PTX | EpoB | 17-AAG | Rapamycin | EpoB /17-AAG (1:1) |
17-AAG /Rapamycin (1:1) |
EpoB /Rapamycin (1:1) |
EpoB /17-AAG /Rapamycin (1:1:1) |
|---|---|---|---|---|---|---|---|---|
| IC35 (nM) | 1.98±0.03 | 0.10±0.00 | 8.52±0.24 | 0.07±0.01 | 0.27±0.00 | 0.12±0.00 | 0.03±0.00 | 0.09±0.00 |
| IC50 (nM) | 3.16±0.06 | 0.17±0.00 | 19.95±0.16 | 0.25±0.03 | 0.52±0.01 | 0.35±0.00 | 0.09±0.00 | 0.29±0.00 |
| IC65 (nM) | 5.59±0.24 | 0.33±0.01 | 62.97±1.71 | 2.32±0.62 | 1.28±0.04 | 1.46±0.11 | 0.30±0.01 | 1.24±0.08 |
| CI35 | - | - | - | - | 1.37 | 0.81 | 0.41 | 0.70 |
| CI50 | - | - | - | - | 1.54 | 0.70 | 0.45 | 0.97 |
| CI65 | - | - | - | - | 1.97 | 0.33 | 0.53 | 1.45 |
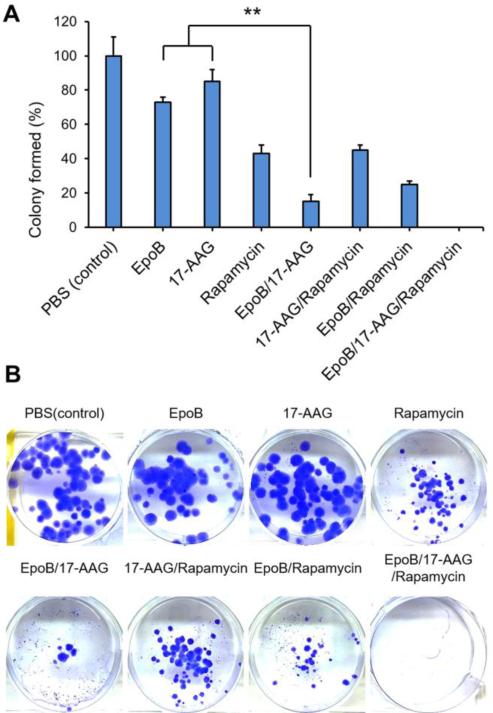
Fig. 3 shows % colony formed by A549 non-small lung cancer cells in the presence or absence of anticancer agents. EpoB (1 nM), 17-AAG (1 nM) and rapamycin (1 nM) as single agents caused a slight reduction in colonies, with rapamycin causing the highest reduction at ca. 40%. Two drug combinations were more effective, noting the low number of colonies for EpoB and 17-AAG, ca. 15%. Remarkably, no colonies were observed after treatment of EpoB/17-AAG/rapamycin (1/1/1 nM), indicating that three drug combination therapy is highly effective in cancer cell reproductive death.
Fig. 3.
(A) In vitro clonogenic assay of various formulations. Colony formation in each well after treatment of EpoB (1 nM), 17-AAG (1 nM), and rapamycin (1 nM) or their two or three combinations for two weeks was quantified. (B) Representative photographs of the colonies in each well after drug treatment (n=3).
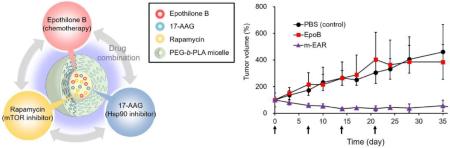
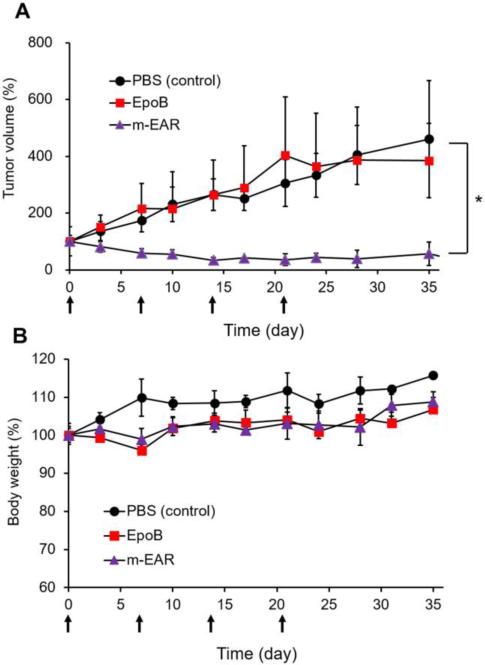
Fig. 4 shows the antitumor efficacy of m-EAR versus EpoB in an A549 xenograft model. Treatment of female athymic nude mice started after tumors were palpable, ca, 50-100 mm3. At 2.0 mg/kg, EpoB was equivalent to the control (PBS) with tumor growth increasing steadily even during weekly treatment. In contrast, four weekly injections of m-EAR at 2.0, 15.0, and 7.5 mg/kg for EpoB, 17-AAG, and rapamycin, respectively, caused tumor regression without tumor regrowth at the termination of treatment. Moreover, despite concurrent three drug injection, m-EAR caused no discernible change in body weight relative to EpoB alone, indicating an absence of overlapped toxicity. This result is due to sustained release of drugs, supporting at least partially a role of PEG-b-PLA micelles in reducing toxicity for m-EAR.
Fig. 4.
Antitumor effect of various formulations in A549 xenograft nude mice. Mice were treated weekly (indicated by arrows in the figure) and tumor growth (A) and body weight changes (B) were monitored periodically. EpoB (2.0 mg/kg). 17-AAG (15 mg/kg). Rapamycin (7.5 mg/kg) (n=5).
In summary, a 3-in-1 poly(ethylene glycol)-block-poly(D,L-lactic acid) (PEG-b-PLA) micelle containing EpoB, 17-AAG and rapamycin has been prepared, characterized and shown to be highly effective against A549 non-small cell cancer cells in vitro and in vivo. Given the high antitumor efficacy over EpoB and low acute toxicity, m-EAR deserves further research in a pre-clinical setting, aiming for clinical evaluation of m-EAR for cancer treatment.
Supplementary Material
Acknowledgements
This research was funded in part by NIH R01 AI101157.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author(s) confirm that this article content has no conflict of interest.
References
- Altmann KH, Wartmann M, O'Reilly T. Epothilones and related structures--a new class of microtubule inhibitors with potent in vivo antitumor activity. Biochim Biophys Acta. 2000;1470:M79–91. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]
- Bagnyukova TV, Serebriiskii IG, Zhou Y, Hopper-Borge EA, Golemis EA, Astsaturov I. Chemotherapy and signaling: How can targeted therapies supercharge cytotoxic agents? Cancer Biol Ther. 2010;10:839–853. doi: 10.4161/cbt.10.9.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–1166. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938–6943. [PubMed] [Google Scholar]
- Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- Bystricky B, Chau I. Patupilone in cancer treatment. Expert Opin Investig Drugs. 2011;20:107–117. doi: 10.1517/13543784.2011.542148. [DOI] [PubMed] [Google Scholar]
- Catenacci L, Mandracchia D, Sorrenti M, Colombo L, Serra M, Tripodo G. In-Solution Structural Considerations by 1H NMR and Solid-State Thermal Properties of Inulin-d-α-Tocopherol Succinate (INVITE) Micelles as Drug Delivery Systems for Hydrophobic Drugs. Macromolecular Chemistry and Physics. 2014;215:2084–2096. [Google Scholar]
- Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Hasenstein JR, Shin HC, Kasmerchak K, Buehler D, Kwon GS, Kozak KR. Antitumor activity of Triolimus: a novel multidrug-loaded micelle containing Paclitaxel, Rapamycin, and 17-AAG. Mol Cancer Ther. 2012;11:2233–2242. doi: 10.1158/1535-7163.MCT-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Belotti D, Burger AM, Fisher-Nielson K, Borsotti P, Riccardi E, Thillainathan J, Hollingshead M, Sausville EA, Giavazzi R. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin: an orally bioavailable heat shock protein 90 modulator. Clin Cancer Res. 2004;10:4813–4821. doi: 10.1158/1078-0432.CCR-03-0795. [DOI] [PubMed] [Google Scholar]
- Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, Park K, Lee MY, Heo DS. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007;18:2009–2014. doi: 10.1093/annonc/mdm374. [DOI] [PubMed] [Google Scholar]
- Lin B, Catley L, LeBlanc R, Mitsiades C, Burger R, Tai YT, Podar K, Wartmann M, Chauhan D, Griffin JD, Anderson KC. Patupilone (epothilone B) inhibits growth and survival of multiple myeloma cells in vitro and in vivo. Blood. 2005;105:350–357. doi: 10.1182/blood-2004-06-2499. [DOI] [PubMed] [Google Scholar]
- LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, Mills GB, Meric-Bernstam F. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res. 2004;10:7031–7042. doi: 10.1158/1078-0432.CCR-04-0361. [DOI] [PubMed] [Google Scholar]
- Nguyen DM, Lorang D, Chen GA, Stewart J.H.t., Tabibi E, Schrump DS. Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in vitro and in vivo analysis. Ann Thorac Surg. 2001;72:371–378. doi: 10.1016/s0003-4975(01)02787-4. discussion 378-379. [DOI] [PubMed] [Google Scholar]
- O'Reilly T, McSheehy PM, Wenger F, Hattenberger M, Muller M, Vaxelaire J, Altmann KH, Wartmann M. Patupilone (epothilone B, EPO906) inhibits growth and metastasis of experimental prostate tumors in vivo. Prostate. 2005;65:231–240. doi: 10.1002/pros.20289. [DOI] [PubMed] [Google Scholar]
- Pietras K, Stumm M, Hubert M, Buchdunger E, Rubin K, Heldin CH, McSheehy P, Wartmann M, Ostman A. STI571 enhances the therapeutic index of epothilone B by a tumor-selective increase of drug uptake. Clin Cancer Res. 2003;9:3779–3787. [PubMed] [Google Scholar]
- Richter A, Olbrich C, Krause M, Kissel T. Solubilization of sagopilone, a poorly water-soluble anticancer drug, using polymeric micelles for parenteral delivery. Int J Pharm. 2010;389:244–253. doi: 10.1016/j.ijpharm.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Roforth MM, Tan C. Combination of rapamycin and 17-allylamino-17-demethoxygeldanamycin abrogates Akt activation and potentiates mTOR blockade in breast cancer cells. Anticancer Drugs. 2008;19:681–688. doi: 10.1097/CAD.0b013e3283067681. [DOI] [PubMed] [Google Scholar]
- Rothermel J, Wartmann M, Chen T, Hohneker J. EPO906 (epothilone B): a promising novel microtubule stabilizer. Semin Oncol. 2003;30:51–55. doi: 10.1016/s0093-7754(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Sausville EA. Combining cytotoxics and 17-allylamino, 17-demethoxygeldanamycin: sequence and tumor biology matters. Commentary re: P. Munster et al., Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. Clin. Cancer Res. 2001;7:2228–2236. 2001. Clin Cancer Res 7, 2155-2158. [PubMed] [Google Scholar]
- Schulte TW, Neckers LM. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother Pharmacol. 1998;42:273–279. doi: 10.1007/s002800050817. [DOI] [PubMed] [Google Scholar]
- Shin HC, Alani AW, Cho H, Bae Y, Kolesar JM, Kwon GS. A 3-in-1 polymeric micelle nanocontainer for poorly water-soluble drugs. Mol Pharm. 2011;8:1257–1265. doi: 10.1021/mp2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HC, Alani AW, Rao DA, Rockich NC, Kwon GS. Multi-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugs. J Control Release. 2009;140:294–300. doi: 10.1016/j.jconrel.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simamora P, Alvarez JM, Yalkowsky SH. Solubilization of rapamycin. Int J Pharm. 2001;213:25–29. doi: 10.1016/s0378-5173(00)00617-7. [DOI] [PubMed] [Google Scholar]
- Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003;63:2139–2144. [PubMed] [Google Scholar]
- Tomoda K, Tam YT, Cho H, Buehler D, Kozak KR, Kwon GS. Triolimus: A Multi-Drug Loaded Polymeric Micelle Containing Paclitaxel, 17-AAG, and Rapamycin as a Novel Radiosensitizer. Macromol Biosci. 2016 doi: 10.1002/mabi.201600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.