Abstract
Purpose
The ability of human melanotransferrin (hMTf) to carry a therapeutic concentration of trastuzumab (BTA) in the brain after conjugation (in the form of trastuzumab-melanotransferrin conjugate, BT2111 conjugate) was investigated by measuring the reduction of the number and size of metastatic human HER2+ breast cancer tumors in a preclinical model of brain metastases of breast cancer.
Methods
Human metastatic brain seeking breast cancer cells were injected in NuNu mice (n=6–12 per group) which then developed experimental brain metastases. Drug uptake was analyzed in relation to metastasis size and blood-tumor barrier permeability. To investigate in-vivo activity against brain metastases, equimolar doses of the conjugate, and relevant controls (hMTF and BTA) in separate groups were administered biweekly after intracardiac injection of the metastatic cancer cells.
Results
The trastuzumab-melanotransferrin conjugate (BT2111) reduced the number of preclinical human HER2+ breast cancer metastases in the brain by 68% compared to control groups. Tumors which remained after treatment were 46% smaller than the control groups. In contrast, BTA alone had no effect on reducing number of metastases, and was associated with only a minimal reduction in metastasis size.
Conclusions
The results suggest the novel trastuzumab-melanotransferrin conjugate (BT2111) may have utility in treating brain metastasis and validate hMTf as a potential vector for antibody transport across the Blood Brain Barrier (BBB).
Keywords: Antibody trastuzumab-melanotransferrin conjugate (BT2111) metastatic HER2+ breast cancer tumors, Brain Metastases, Trastuzumab (BTA), Human melanotransferrin (hMTf), Blood Brain Barrier (BBB)
4. Introduction
Brain metastasis of breast cancer is of concern due to increasing mortality rates and poor quality of life after diagnosis. The incidence of brain metastasis in breast cancer patients range from 10–16% in patients with disseminated breast cancer (1). For women with symptomatic CNS metastases, the median survival is approximately 4 months (2), with less than 2% of women surviving two years post diagnosis (3). Head computed tomography (CT) scan and brain magnetic resonance imaging (MRI) (4) suggest that that an additional 15% of asymptomatic metastatic breast cancer patients may have occult brain metastases and further, it is suggested that approximately 30% of disseminated breast cancer patients have lesions discovered upon autopsy (5, 6).
Hormone receptor status is a correlated risk factor in developing brain metastases of breast cancer (7). Breast cancer is subcategorized based on the receptor status to endocrine receptor (ER, estrogen or progesterone receptor), HER2 (human epidermal growth factor receptor 2), or triple negative (negative for estrogen, progesterone, or HER2 receptors) (7). Both triple negative and Her2 positive primary tumors have been reported to be correlated with the development of (8–10). Importantly, HER2-positive breast cancers tend to grow and metastasize faster compared to HER2-negative breast cancers (11, 12).
Trastuzumab, BTA, (Herceptin, Genentech/ Roche), a humanized monoclonal antibody, is widely used to target HER2+ breast cancer since it binds to the extracellular juxtamembrane domain of HER2 and inhibits proliferation and survival of HER2-dependent tumors (13). Trastuzumab is approved by the Food and Drug Administration (FDA) for patients with HER2 positive breast cancers (14). The first clinical study of trastuzumab (15) showed therapy decreased mortality at 1 year (22% vs. 33%, P=0.008) and produced a longer median survival (25.1 vs. 20.3 months, P=0.046) with a 20% reduction of death risk. Unfortunately, when breast cancer progresses to brain, Trastuzumab has limited efficacy because it poorly crosses the blood-brain barrier (BBB) and blood-tumor barrier (BTB) (16).
An approach to translocate pharmaceuticals across the BBB is to design drugs or carriers to use BBB transporters to allow penetration into the CNS. One such mechanism that can be used to facilitate uptake of active pharmaceutical ingredients across the BBB is melanotransferrin (hMTf) or melanoma tumor antigen (p97). Melanotransferrin (hMTf) has been identified as a potential vector to transport drugs across the BBB for the treatment of brain diseases. Human melanotransferrin (hMTf) is a glycosylated, bi-lobed protein belonging to the transferrin family of iron binding proteins, which include transferrin, lactoferrin, and ova transferrin among others (17). They are characterized by conserved sequences of amino acids organized into an N lobe and a C lobe with each lobe having the capacity to bind one molecule of iron. The exception is melanotransferrin, which can bind only one molecule of iron due to an amino acid substitution in the iron-binding site of lobe C (17). hMTf is a chemical and structural homolog of transferrin (Tf) (18). Contrary to Tf, hMTf does not bind to the transferrin receptor but involves directly or indirectly a receptor of the family of Low Density Lipoprotein Receptor related Protein (LRP) such as LRP-1 (19, 20). These data have been demonstrated in an in-vitro BBB transcytosis assay as Tf never competed with hMTf for transcytosis suggesting two separate receptors (20).
Previous studies have shown that hMTf can cross the BBB to deliver iron (21) and can also shuttle doxorubicin to brain tumors (22). hMTf has been demonstrated to cross very efficiently the BBB as measured by in-vitro BBB model using bovine brain capillary endothelial cells (BBCEC) and using the in-situ brain perfusion methods (23). Finally, hMTf has been demonstrated to direct adenovirus (Ad5) across the BBB by transcytosis across an in-vitro model of the BBB (24).
In the current work, we investigated brain and brain metastases uptake of a melanotransferrin (hMTf, P97)-trastuzumab (BTA) conjugate (BT2111), and determined if the conjugate had efficacy in reducing the number and size of metastatic lesions in a preclinical model of brain metastases of breast cancer. BT2111 is a conjugate comprised of a melanotransferrin, a unique blood-brain barrier transporter, and herceptin (Trastuzumab, BTA). BT2111 is a novel recombinant conjugated protein developed by Bioasis Technologies Inc.(25), and currently in the non-clinical development as an intravenous product for the treatment of brain metastasis of breast cancer.
5. Materials and methods
5.1. Chemicals
Texas Red (Sulforhodamine 101) was purchased from Invitrogen (Carlsbad, CA). Cold conjugate (Trastuzumab-hMTf Conjugate; p97-BTA conjugate; BT2111), trastuzumab (Herceptin; BTA, MW ~ 145,532 Da) and human melanotransferrin (p97; hMTf, MW ~ 80,000 Da) were supplied by BiOasis, Inc. as stock solutions of 5, 5 and 10 mg/mL respectively (Purity of >99% (26)). The estimated molecular weight (MW) of BT2111 was ~ 270,000 Da based on conjugation ratios. BT2111 was formulated in 50 mM potassium phosphate buffer and 150 mM sodium chloride adjusted at pH of 6.7. Trastuzumab (Roche) was buffer-exchanged into 50 mM potassium phosphate buffer and 150 mM sodium chloride adjusted at pH of 6.7. Test articles (BT2111 conjugate, BTA and hMTf) were radiolabeled with 125I. 125l-labeled p97, trastuzumab, and p97- trastuzumab proteins purity is >99%, as measured by HPLC(26). Several batches of radioactive test articles were prepared and the specific radioactivity of test articles used in this study differed from one batch to another. The specific radioactivity of the predominant batch used in this study for BT2111 conjugate, BTA and p97 was 0.2899, 0.5686 and 0.31 µCi/µg respectively. The purity of the radioactive materials was not confirmed by HPLC analysis. Information on synthesis methods, stability, purity, composition, or other characteristics that define the test article(s) components is on file with BiOasis, Inc. All other chemicals are of analytical grade and were purchased from Sigma-Aldrich (St. Louis, MO).
5.2. Animals
Homozygous Female NuNu(27) (Crl:NU-Foxn1nu, Strain code 088) mice (25–30 g) were obtained from Charles River Laboratories (Kingston, NY) and used for all experiments in this study. This outbred, isolator maintained, hairless, albino mouse lacks a thymus and is unable to produce T cells. The Nu Nu mouse strain was selected because these animals are immunodeficient and will not mount an immune response against injected human cells. This immunodeficient strain is utilized as an animal model of choice in oncology research (27). All studies were approved by the Animal Care and Use Committee at Texas Tech University Health Sciences Center, and conducted in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals. Animals were acclimated to laboratory conditions before study initiation at least for a week.
5.3. Cell Culture
Human metastatic triple-negative breast cancer over-expressing Her2 cells (MDA-MB-231-BRHER2/eGFP) were cultured in DMEM (10-17-CV, Cellgro Inc.) supplemented with 10% FBS and Zeocin (300 µg per 500 mL media, Invitrogen). MDA-MB-231-BRHER2/eGFP cells were selected because they over-express HER2 receptors and as such are sensitive to herceptin treatment. All cells were used in passages 1–10 and maintained at 37°C with 5% CO2. Transfection was conducted using retroviral vector pLEGFP-CD from BD Biosciences. EGFP expression (95% to 99%) was confirmed by fluorescent microscopy after being selected in the presence of 0.8 mg/mL G418. Cells are passaged in DMEM, 10% FBS with 2 mL L-Glutamine. The cells were subsequently transfected with pCMV4.ErbB2 full-length human cDNA and pSVzeo to create the MDA-MB-231-BRHER2/eGFP line. The sequence of the Her-2 insert in pCMV4.ErbB2 was confirmed by sequencing. Stable colonies were selected in the presence of 0.75mg/mL zeocin (Invitrogen). For preparation of MDA-MB-231-BRHER2/eGFP cells for intracardiac injection, cells were grown to 70% confluency, trypsinized, and rinsed twice in 4°C PBS to remove all traces of serum. Cells were resuspended in serum free 4°C DMEM media and placed on ice.
5.4. Development of Metastases and Administration of Drugs
Mice were anesthetized with isoflurane and inoculated with the breast cancer cell line (MDA-MB-231-BR-Her2: 1.75 × 105/100 µL DMEM/mouse) in the left cardiac ventricle. MDA-MB-231-BRHER2/eGFP cells are brain seeking metastatic cells that upon injection into the left cardiac ventricle, circulate in the peripheral vasculature, arrest in brain capillaries, extravasate across the blood brain barrier (BBB), and develop metastatic lesions predominantly in brain (28).
5.4.1. Test articles uptake into brain and brain metastases of breast cancer after intravenous administration in mice: preclinical pharmacokinetics and comparison with systemic tissues
To examine the BT2111 conjugate uptake into brain and brain metastases of breast cancer, animals were administered radiolabeled BT2111 conjugate (17 mg/kg), trastuzumab, BTA (10 mg/kg) and melanotransferrin, hMTf (7 mg/kg) intravenously and sacrificed at three time points for each group (30 min, 2 and 8 hours). IV administration was used to ensure 100% test article delivery into circulation. A dose level of BTA (10 mg/kg), route of administration and dosing schedule were selected based on literature data for this drug(29). hMTf and BT2111 conjugate were injected at corresponding molar equivalent doses. At least 3 animals per time point were used in this study except for hMTf/0.5 hour group (4 mice), BT2111/8 hour (4 mice) and BT2111/2 hours (6 mice). Injections of 125I-labeled Test Articles (14 µCi/mouse) were performed week 5–6 after cell injections when animals start exhibiting neurological symptoms. The levels of radioactivity in the brain and brain metastasis were determined using radiotracer imaging of brain sections. Before euthanasia, the animals were injected with Texas Red-Dextran. The uptake of BT2111 versus hMTf and BTA was analyzed in relation to metastasis size, blood-tumor barrier permeability, and time of circulation. In addition to the brains, blood was collected immediately after the animals were euthanized (before the removal of the brain). The radioactivity of whole blood was measured and expressed as cpm/mL and then converted to nCi/mL and nCi/g.
In addition to blood, samples were collected from other tissues and organs for comparative analysis (brain stem, heart, lung, liver, spleen, one kidney, muscle, fat, and intestine). Parts of organs or tissues were collected, washed and weighted. Weights were recorded for each organ or tissue collected. Radioactivity was measured immediately after collection and expressed as cpm/mg and then converted to nCi/g. The tissue/blood concentration ratios were determined based on ratio of radioactivity in tissue to blood normalized by weight.
5.4.2. Test articles effect on the number and size of brain metastases of breast cancer after intraperitoneal administration in mice
Furthermore, we compared the BT2111 conjugate and hMTf versus BTA activity in-vivo against brain metastases of breast cancer. Following cell administration, the animals were randomized into treatment groups. 8–9 mice/group were used in the vehicle control and BT2111 groups; 13 mice/group in the hMTf and BTA groups. The test facility assigned the increased number of animals to the hMTf and BTA groups because of anticipated higher mortality rates as compared to the BT2111 group. In this study, animals were administered BT2111 conjugate (17 mg/kg), BTA (10 mg/kg) and hMTf (7 mg/kg) intravenously biweekly. Treatment was initiated on day 21 after the initial intracardiac injections of MDA-MB-231-BRHER2/eGFP. All animals within each group were sacrificed on day 35 after the intracardiac injection of MDA-MB-231-BRHER2/eGFP cells. The different drugs and controls efficacy was assessed by counting the number and size of metastases in brain at the termination of the experiment. All experiments were conducted in accordance with approved animal use protocols. Animal observations were performed and body weights were measured periodically.
5.5. Harvesting of The Brain
Animals were euthanized, and brain tissue was removed, and placed in isopentane (-65°C). Brain was removed rapidly (less than 60 seconds) since slower removal may cause changes in the spatial distribution of small molecular weight tracers (30, 31). Brains were sliced (20 µm) using a cryostat (Leica Microsystems, Wetzler, Germany) and sections were mounted on glass slides, air dried, and stored at −80 °C.
5.6. Quantitative autoradiography (QAR)
Autoradiograms were generated by co-exposing the sections on Fuji film (FLA 7000, Stamford, CT) with tissue-calibrated 1H-standards for 2–3 weeks. Quantitative analysis of the regional radioactivity was performed using a micro-computer image device (MCID) (Linton, Cambridge, England). Test article concentrations in tumor and normal brain areas were expressed in nCi/g based on the calibration curve per mm2.
5.7. Immunofluorescence
Slides adjacent to the slides used for QAR analysis were used for immunofluorescent staining. All chemicals were of analytical grade and were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. Tissues were rehydrated briefly in phosphate buffered saline (PBS) and fixed in cold 4% paraformaldehyde (4°C) for 30 minutes. After three washings with PBS, the slides were then covered in 1% sodium dodecyl sulfate for 5 minutes and rinsed again with PBS. Primary antibodies [Anti-Cytokeratin mouse monoclonal IgG antibody (MNF 116, Abcam) that reacts with human tissue and Anti-LRP1 (low density lipoprotein receptor-related protein 1) rabbit monoclonal IgG antibody (AB 92544, Abcam) that reacts with human tissue] were diluted 1:500 in 5% goat serum and were incubated with slides overnight at 4°C following by washing.
Sections were covered in 0.03% hydrogen peroxide + 0.1% sodium azide for 10 minutes and subsequently washed. After a second blocking with 10% goat serum for 30 minutes, slides were incubated with secondary antibodies (Molecular Probes) diluted in 5% goat serum at 1:1000 [Alexa Fluor® 488 Goat Anti-Mouse IgG (labeled with bright, photostable, green-fluorescent Alexa Fluor 488 dye) and Alexa Fluor® 594 Donkey Anti-Rabbit IgG1 (labeled with bright, photostable, red-fluorescent Alexa Fluor 594 dye, spectrally similar to Texas Red® dye) and is prepared from affinity-purified antibodies that react with the Fc portion of the heavy chain of mouse IgG1]. DAPI (Invitrogen, 4’,6-diamidino-2-phenylindole, dilactate,) was added to this solution at 1 mg/mL 1:500 and slides were incubated for 1 hour at room temperature. Slides were again washed, mountant (DAKO) was added, and coverslips were applied. Fluorescence analyses on whole brain sections were performed using an Olympus MVX10 microscope with a 2× objective (NA=0.5) and an optical zoom of (0.63–6.3)×. Excitation and emission filters were 470±40 and 525±50 nm for eGFP, 560±55 and 645±75 nm for Texas Red dextran and 740±35nm and 780 longpass filter for near-infrared indocyanine green. Fluorescent image analysis was performed using Slidebook 5.0 program (Olympus).
On selected slides processed for immunochistochemistry staining, excitation and emission filters were 470±40 and 525±50 nm for green (Alexa Fluor AF488), 560±55 and 645±75 nm for red (Alexa Fluor 594), and 320±50 and 430±60 nm for DAPI blue. Immunohisto-fluorescent images were taken with an Olympus IX81 microscope (Center Valley, PA).
5.8. Unidirectional uptake transfer constants (Kin)
Kin values were then calculated from brain distribution volume versus time as indicated in the literature (28).
5.9. Survival study
Animals were injected with MDA-MD-231Br-Luc into the left cardiac ventricle and imaged using Bioluminescent imaging (BLI, IVIS Lumineer XV (PerkinElmer)) to confirm a successful injection. All animals included in the study displayed BLI signal in brain indicating a successful intracardiac injection. Metastases were allowed to develop for 21 days. On the 21st day, treatment of vehicle, BT2111 conjugate (17 mg/kg), BTA (10 mg/kg) and hMTf (7 mg/kg) was started via intraperitoneal (IP) injection as usually administered in trastuzumab treatment and repeated biweekly. Animals were also imaged for BLI once weekly. Animals were sacrificed when neurological symptoms became noticeable (as previously mentioned). Animals were sacrificed under anesthesia (ketamine/xylazine; 100mg/kg and 8mg/kg respectively).
5.10. Bioluminescent imaging
Mice were injected with D-luciferin potassium salt (150mg/kg; PerkinElmer, Waltham, MA) dissolved in sterile 1× PBS via intraperitoneal injection and then anesthetized under 2% isoflurane. To determine the appropriate time to image after injection, five control mice were imaged sequentially every five minutes for 100 minutes immediately after IP injection of D-luciferin potassium salt (150mg/kg) and BLI signal in brain was measured over time to identify the peak signal and time course. It was determined that 15 minutes after IP injection of D-luciferin the BLI provided a consistent peak intensity which reflects the substrates kinetic profile. Fifteen minutes after IP injection of D-luciferin, darkfield images of mice were acquired with an IVIS Lumineer XV (PerkinElmer) to detect bioluminescence. Animals were imaged 1, 3, 6, 9, 12, 24, 48, 72, 96, 120, 144, and 168 hours post intracardiac injection. Regions of interest (ROIs) were drawn according to the circumference of the cranium and all data were reported as radiance (photons/sec/cm2/seradian).
5.11. Data analysis
Statistical significance by one-way ANOVA followed by Bonferroni’s multiple comparison’s test. All differences were considered statistically significant at p< 0.05. Data is reported as Mean ± Standard Error of Mean (SEM) (GraphPad Prism 5.0, San Diego, CA). Results associated with drug levels determined in tumor and brain distant to tumor (BDT) areas represent Mean values of combined readings from all tumor and BDT areas in the study group without separation by individual animal data. In case of Kin analysis, values obtained at different time points were also pooled together.
6. Results
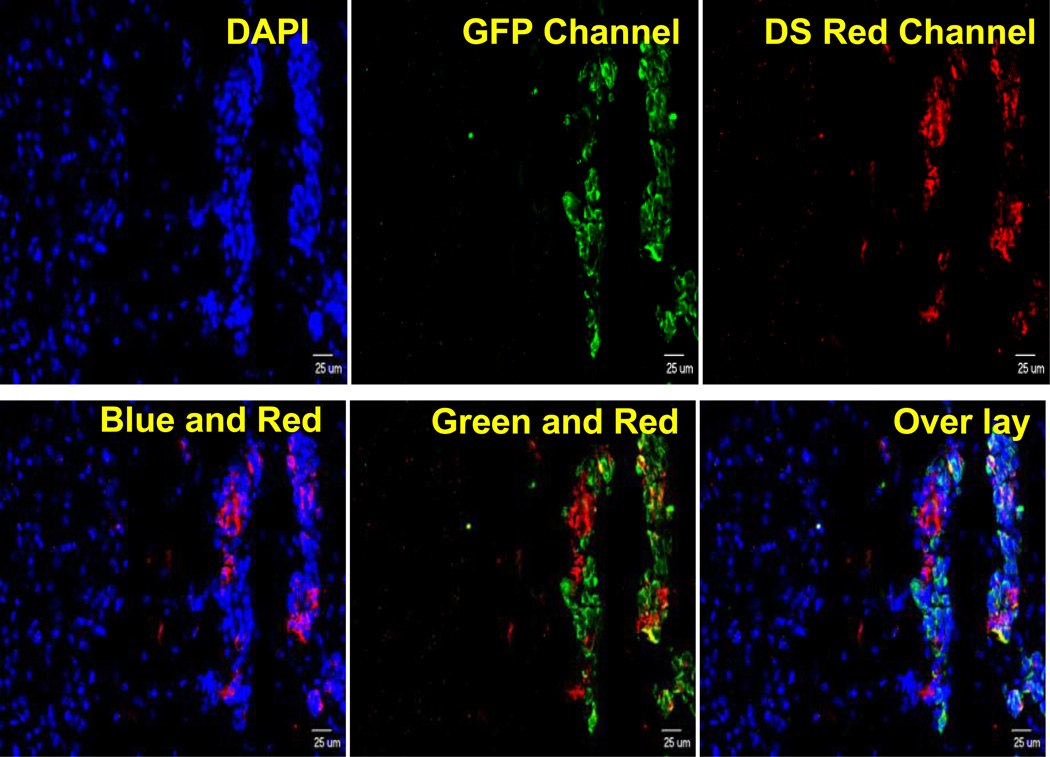
Brain metastasis and primary tumors have been associated with the presence of different receptor target sites drug delivery (32). One receptor site is the low density lipoprotein receptor-related protein (LRP-1), which has a rapid rate of endocytosis (t½< 30 s)(32). LRP-1 is highly expressed at the BBB and is upregulated in brain tumors (33). LRP-1 has been involved with transcytosis of several proteins and peptides such as tissue plasminogen activator (tPA), receptor associated peptide (RAP), and melanotransferrin (hMTf) across the BBB (20, 32). Fluorescence images showing LRP Expression in MDA-MB-231-BRHER2/eGFP brain metastases are shown in Fig 1. DAPI (Blue), anti-cytokeratin mouse monoclonal IgG antibody (green) and anti-LRP-1 rabbit monoclonal IgG antibody (red, AB 92544, Abcam) were used to localize cell nucleus, to stain epithelial cells and illustrate LRP 1 expression respectively. The LRP-1 fluorescence (red) was predominantly co-localized with cytokeratin staining of epithelial cells (green fluorescence).
Figure 1.
Fluorescence images showing LRP Expression in MDA-MB-231Br brain metastases (Blue: DAPI to localize cell nucleus, Green: Cytokeratin to stain epithelial cells, Red: LRP 1). Primary antibodies used are Anti-Cytokeratin mouse monoclonal IgG antibody (MNF 116, Abcam) and Anti-LRP1 (low density lipoprotein receptor-related protein 1) rabbit monoclonal IgG antibody (AB 92544, Abcam). Excitation and emission filters were 470±40 and 525±50 nm for green (Alexa Fluor AF488), 560±55 and 645±75 nm for red (Alexa Fluor 594), and 320±50 and 430±60 nm for DAPI blue.
Test drugs (hMTf, BTA or BT2111) uptake into brain and brain metastases of breast cancer after intravenous administration in mice was investigated. MDA-MB-231-BRHER2/eGFP cells were cultured, harvested and injected into the left cardiac ventricle of NU/NU mice. Animals start exhibiting neurological symptoms, 5 to 6 weeks after cell injections on average. As soon as neurological symptoms start, animals were injected with 125I-labeled hMTf, BTA or BT2111 (14 µCi/mouse). The animals were sacrificed at various time points (30 min, 2 and 8 hours) and levels of radioactivity in the brain and brain metastasis were determined using autoradiography imaging of brain sections. The uptake of BT2111 vs. hMTf and BTA was analyzed in relation to metastasis size, blood-tumor barrier permeability, and time of circulation.
Test article concentrations in blood and selected organs and tissues (brain stem, heart, lung, liver, spleen, muscle, fat, and intestine) were determined by radioactive counting. The majority of BTA remained in blood circulation with low tissue penetration. At 2 hrs, the BTA tissue/blood concentration ratio was the highest in well perfused organs such as kidneys (0.34), spleen (0.28), liver, lung and heart (0.13–0.15) while lower levels were observed in muscles, fat and intestine (0.04–0.06). The lowest ratios of BTA were recorded in the brainstem (0.0054). hMTf showed significantly higher tissue/blood concentration ratios as compared to BTA, with test article concentrations in selected tissues being higher than in blood. Specifically, at 2 hours the following tissue/blood concentration ratios were observed: liver (4.0), spleen (2.47), kidney (1.22), fat (1.03), lung, intestine, heart, and muscle (0.17–0.46). hMTf tissue/blood concentration ratio for the brainstem was 0.071 13-times higher than BTA). Conjugation of BTA to hMTf resulted in higher tissue/blood concentration ratios as compared to free BTA. The highest ratios were observed at 2 hrs as compared to 30 min and 8 hrs. The highest tissue/blood ratio was observed in lungs (1.86), followed by liver (1.23), spleen (0.89), muscle, fat, intestine (0.28–0.43). Similar to hMTf, a relatively low level of BT2111 was noted in the heart (0.12).
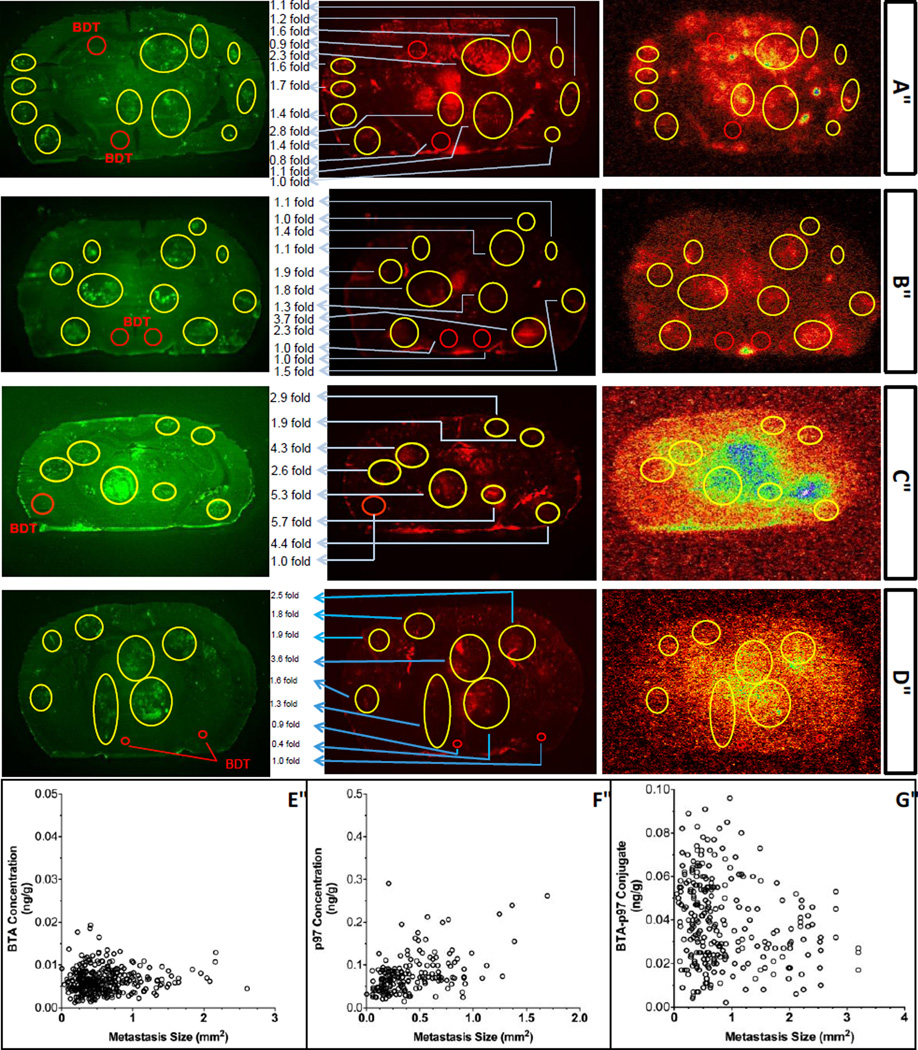
Fig 2 shows representative images of brain tumors and distribution of Trastuzumab (BTA), hMTf (p97) and BT2111 (BTA-p97) at either 0.5, 2 or 8 hours post injection. The choice of the time points in Fig 2A–2D was dependent on the highest brain tissue/blood concentration ratio for each moiety, either P97, BTA or BT2111. For each moiety, the time point corresponding to the highest brain tissue/blood concentration ratio was used. The test article concentrations at 0.5, 2 and 8 hours post injection were determined in areas containing brain tumors and compared to the concentrations detected in areas distant to tumors. All test articles demonstrated higher brain/blood concentration ratio in the tumor area as compared to the normal brain area. Specifically, the ratios in the tumors at 0.5 and 2 hours were increased 7.9 and 5.1 times (BTA), 3.2 and 1.8 times (hMTf), 4.0 and 1.4 times (BT2111), respectively, as compared with the tumor-free brain areas. With respect to the time course, all test articles exhibited higher brain/blood concentration ratio at later time points (2 or 8 hours) as compared to 30 min. Similarly to other tissues, BTA demonstrated the lowest tissue penetration with the brain/blood concentration ratios of approximately 1 × 10−7 and 4 × 10−7 in tumor-free areas and 8 × 10-7 and 19 × 10−7 in tumor lesions at 0.5 and 2 hours, respectively. Corresponding hMTf ratios were higher by 2 orders of magnitude: approximately 1 × 10−5 and 6 × 10−5 in normal areas and 3 × 10−5 and 10 × 10−5 in tumors, respectively. The ratios of BT2111 were significantly higher than the corresponding ratios of the free drug. Namely, at 0.5, 2 and 8 hours the brain area/blood concentration ratios were approximately 0.2 × 10−5, 9 × 10−5, and 29 × 10−5 in the tumor-free areas and 0.8 × 10−5, 13 × 10−5, and 51 × 10−5 in tumors, respectively. In general, the BT2111 values were comparable to the hMTf values but were 10 to 225 times higher than the corresponding ratio values of BTA. In addition to relative values, the concentrations (pg/g) of the test articles in tumor areas were estimated. BTA concentrations were 6.4 pg/g at both 0.5 and 2 hrs post injection. BT2111 demonstrated significantly higher levels of 33 and 48 pg/g at 2 and 8 hours. BT2111 concentrations were lower as compared with hMTf (77 and 79 pg/g at 0.5 and 2 hrs).
Figure 2.
Representative images of brain tumors and test articles distribution (Trastuzumab (BTA) at 2 hours post injection (A), hMTf (p97) at 30 minutes post injection (B), BT2111 (BTA-p97) at 2 hours post injection (C), BT2111 (BTA-p97) at 8 hours post injection (D)) and size of metastatic lesions in the isolated brain of test NuNu mice after administration of BTA (E), hMTf (F), and BT2111 (G) at 0.5, 2 and 8 hours time points. The choice of the time points was dependent on the highest brain tissue/blood concentration ratio for each moiety either P97, BTA or BT2111. For each moiety, the time point corresponding to the highest brain tissue/blood concentration ratio was used. Left column (A–D): Brain metastasis induced by administration of MDA-MB-231-BRHER2/eGFP cells (green fluorescence due to presence of eGFP); red circles indicate brain distant to tumor areas (BDT) and yellow circles indicate tumor areas. Central column (A–D): Distribution of Texas Red-Dextran describing the areas in the brain or brain metastases which are leaky or which have a disrupted BBB Right column (A–D): Autoradiogram showing the amount of radioactivity distributed in the brain slices.
Size of metastatic lesions in the isolated brain of test NuNu mice after administration of BTA, hMTf, and BT2111 at 0.5, 2 and 8 hours’ time points is shown in Fig 2. There was no correlation between any of the test articles and the tumor size in which their concentration was determined. These results suggest that all test articles exhibit similar penetration into the tumors of different sizes, from ~0.1 mm2 and up to ~4 mm2. Under the conditions of the study, the pharmacological effect on the tumor size is not anticipated since drug concentrations were determined within 0.5–8 hours post single dose administration.
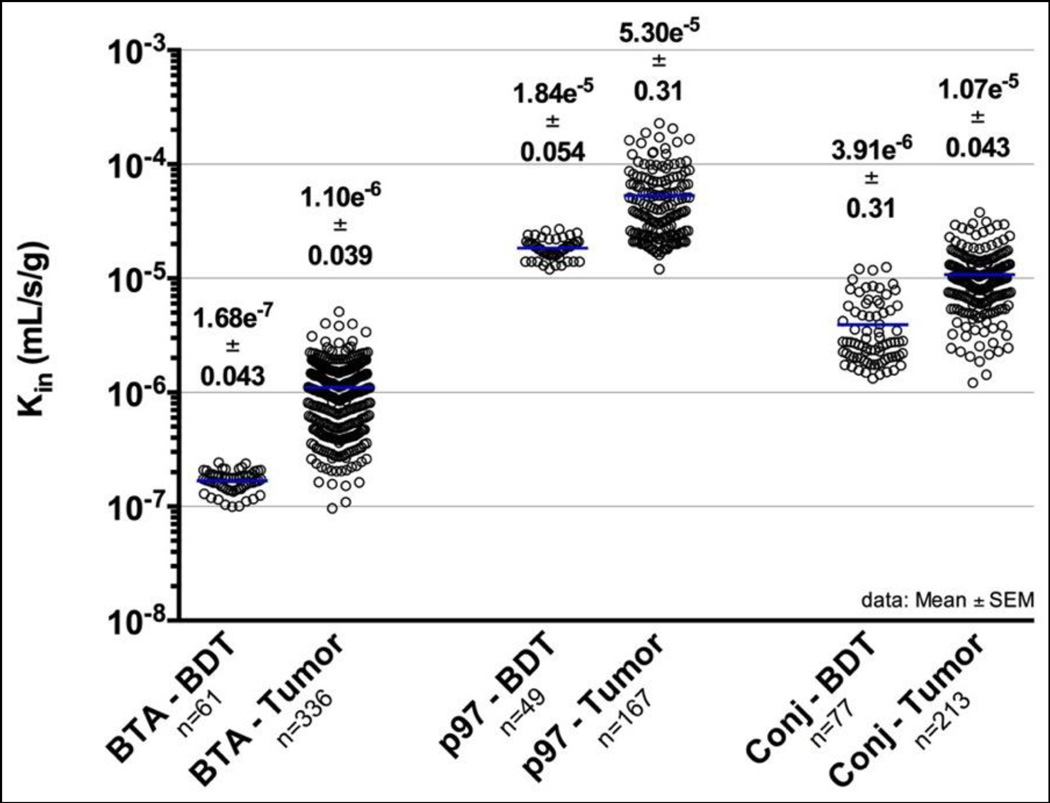
Kin values were determined separately for normal and tumor areas of the brain (Figs. 3 & 4 and Table 1). Uptake of BTA in “normal” brain (brain distant to tumor; BDT) was 0.17 ± 0.004 × 10−6 mL/s/g and nearly 10 fold higher on average in metastatic lesions (1.1 ± 0.04 × 10−6 mL/s/g). hMTf uptake into BDT (18.4 ± 0.5 × 10−6 mL/s/g) and brain metastases (53.0 ± 3.1 × 10−6 mL/s/g) was significantly higher (p<0.01) than that of the BTA alone. BT2111 was nearly 30 fold higher (p<0.01) than that of the BTA alone in BDT (3.91 ± 0.3 × 10−6 mL/s/g) and 10 fold higher (p<0.01) in metastases (10.7 ± 0.43 × 10−6 mL/s/g).
Figure 3.
Unidirectional drug uptake into brain transfer constant (Kin) values of Trastuzumab (BTA), P97 (hMTf), and BT2111 conjugate (Conj) at 0.5, 2 and 8 hours time points in tumor and Brain Distant to Tumor (normal brain, BDT) areas. Results are presented as Mean±STDEV. Combined data are plotted for all time points.
Figure 4.
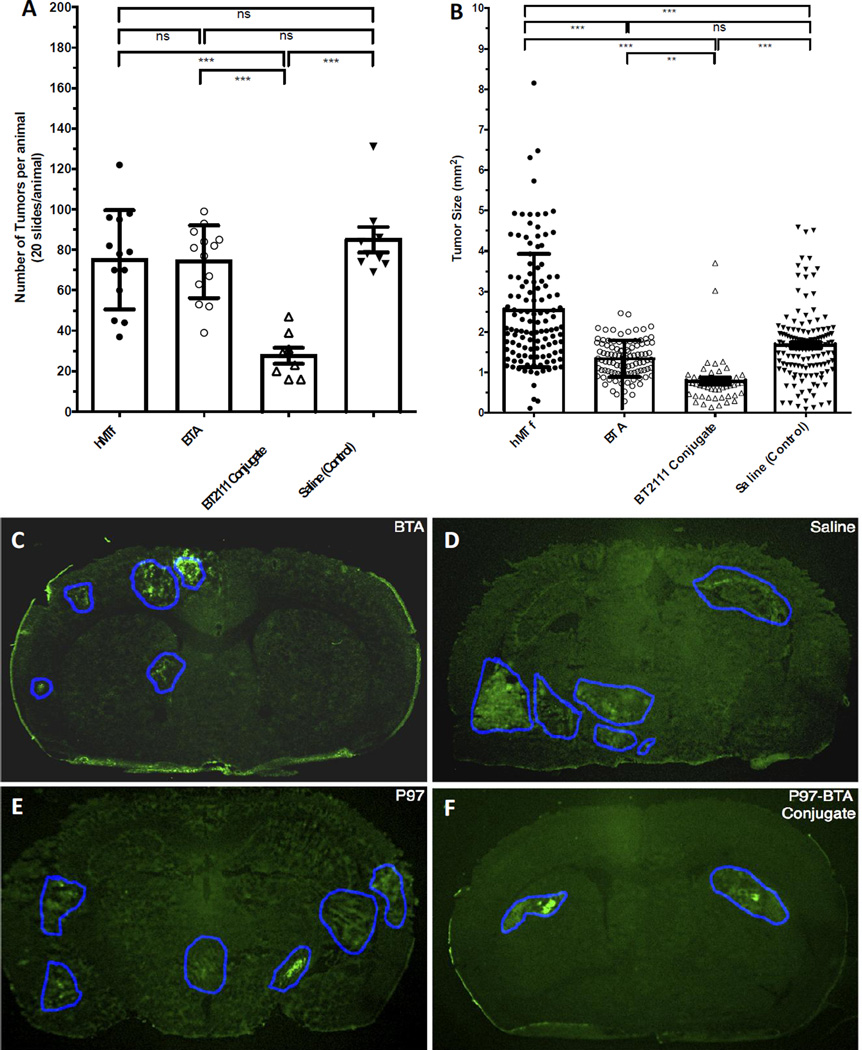
Number (Figure 4A) and size (Figure 4B) of metastatic lesions in the isolated brain of test NuNu mice after administration of saline, P97 (hMTf), BTA and P97-Trastuzumab conjugate (BT2111) biweekly for two weeks. The P Value for the number of metastatic lesions for BT2111 vs. control, BTA or hMTf groups was found to be < 0.0001. Tumor size was based on pooled data from all individual values in each group. The P Value for the size of metastatic lesions for BT2111 vs. control or hMTf groups was found to be < 0.0001 and for BT2111 vs. BTA group was found to be < 0.001. Figures (4C–4F) show representative fluorescent images from each group highlighting the metastatic lesions size in the isolated brain of test NuNu mice after administration of saline, hMTf, BTA and BT2111 conjugate biweekly for two weeks.
Table 1.
Unidirectional drug uptake into brain transfer constant (Kin) values. Results are presented as Mean ± STDEV
| Test article | Kin (mL/s/g) |
|||
|---|---|---|---|---|
| BDTa area | Number | Tumor area | Number | |
| BTA | (1.68±0.33)×10−7 | 61 | (11.01±7.21)×10−7 | 336 |
| hMTf (p97) | (1.84±0.38)×10−5 | 49 | (5.30±3.97)×10−5 | 167 |
| BT2111 | (2.31±0.60)×10−6 | 52 | (11.70±8.29)×10−6 | 101 |
| (7.32±2.41)×10−6 | 24 | (9.88±3.44)×10−6 | 112 | |
| (3.89±2.71)×10−6 | 76 | (10.74±6.28)×10−6 | 213 | |
Brain Distant to Tumor (normal brain, BDT) area
To quantify the effect of BT2111 on tumor cell colonization of the brain, the number and size of metastatic lesions were determined in brain sections. The results are summarized in tables 2 and 3 and presented in Fig 4. MDA-MB-231-BRHER2/eGFP cells were cultured, harvested and injected into the left cardiac ventricle of mice. BT2111 (17 mg/kg), BTA (10 mg/kg), hMTf (7 mg/kg) and vehicle control were injected twice per week intraperitoneally starting from day 21 post cell injections. All animals were sacrificed on day 35 post intracardiac injection of MDA-MB-231-BRHER2/eGFP cells. On the day of euthanasia, animals in the control group (vehicle), hMTf and BTA-treated groups exhibited clinical signs with almost all animals in the control group showing severe signs. The appearance of animals in the BT2111 group was significantly better as compared with the vehicle control group. The brains were collected; brain sections prepared and tumor number and size were determined based on fluorescent EGFP detection of the transfected tumor cells. Fig 4 shows representative fluorescent images from each group highlighting the metastatic lesions size in the isolated brain of test NuNu mice after administration of saline, hMTf, BTA and BT2111 biweekly for two weeks.
Table 2.
Number of metastatic lesions in the isolated brain of test NuNu mice after administration of saline, hMTf, BTA and BT2111 biweekly for two weeks.
| Group | Dose, IP, biweekly for 2 weeks (mg/kg) |
Number of mice/group |
Number of metastatic lesions per animal (20 slides per animal) | |
|---|---|---|---|---|
| Mean ± STDEV | Range | |||
| Saline (control) |
NA | 9 | 85.0±18.9 | 69 – 131 |
| BT2111 | 17 | 8 | 27.6±11.1a | 16 – 47 |
| BTA | 10 | 13 | 74.2±18.0 | 39 – 99 |
| hMTf | 7 | 13 | 75.1±24.6 | 37 – 122 |
The P Value for BT2111 vs. control, BTA or hMTf groups was found to be < 0.0001.
Table 3.
Size of metastatic lesions in the isolated brain of test NuNu mice after administration of saline, hMTf, BTA and BT2111 biweekly for two weeks. Tumor size was based on pooled data from all individual values in each group.
| Group | Tumor size based on pooled data from all individual values in group | |
|---|---|---|
| N | Tumor size (mm2) Mean ± STDEV |
|
| Saline control | N = 765 | 1.654 ± 1.673 |
| BT2111 | N=223 | 0.710 ± 0.727 a,b,c |
| BTA | N = 962 | 1.402 ± 1.217 |
| hMTf | N = 976 | 2.259 ± 2.743 a,d |
- BT2111 or hMTf vs. saline control group P value < 0.0001
- BT2111 vs. BTA group P value < 0.001
- BT2111 vs. hMTf group P value < 0.0001
- hMTf vs. BTA group P value < 0.0001
BT2111 reduced the number of preclinical human HER2+ breast cancer metastases in the brain of control animals (85 ± 6.3) by 68% (27.6 ± 3.9; P<0.001) (Fig 4A). Further, lesions in control animals were 54% larger (1.7 ± 0.7 µm2) than those in the BT2111 treated animals (0.78 ± 0.6 µm2; P<0.001) (Fig 4B). In contrast, BTA alone had no effect on reducing the number of metastases (74 ± 5.0, P>0.05) and was associated with only a minimal (21%) reduction in metastasis size (1.34 ± 0.04 µm2).
7. Discussion
A limitation with the use of Trastuzumab is the continued progression of brain metastases despite therapy (22). A large meta-analysis (16), demonstrated the incidence of brain metastases increased as a first site of relapse with prolonged exposure to trastuzumab antibody. The specific trials included, four phase three randomized controlled trials (NSABP B31, NCCTG N9831, HERA, and PACS), which had 125 out of 4921 breast cancer patients develop brain metastasis as the site of first recurrence for an overall incidence of 2.56%. In contrast, 78 out of 4099 breast cancer patients who did not receive trastuzumab developed brain metastasis with an incidence of 1.94%. Further work has shown that the ratio of brain metastases to total recurrence events was 16.94% (95% CI, 10.85% – 24.07%) and 8.33% (95% CI, 6.49%–10.86%) for patients treated with trastuzumab and those not treated with trastuzumab respectively (34). It has been suggested that the increased incidence of brain metastasis with trastuzumab therapy maybe a result of increased survival time (35).
A reason for the continued progression may be that trastuzumab as an antibody, has poor penetration across the intact BBB (36). For example, patients who have cranial lesions, Trastuzumab levels in their cerebrospinal fluid can be ~300-fold lower than concentrations in plasma (37) suggesting poor CNS penetration. In a retrospective study (38), healthcare administrative data demonstrated 40 patients developed secondary brain metastases of 681 patients on trastuzumab therapy. Given the poor CNS distribution in both healthy and lesioned brains, it is reasonable to suggest trastuzumab treatment does not increase risk for brain metastases but rather controls peripheral systemic disorders (39).
Melanotransferrin (hMTf, p97) is a naturally occurring transport protein found at low concentrations in the blood (under 7.5 ng/ml = 0.08 nM in healthy adults) (40, 41) and is predicted to be one of the oldest members of the Tf family (40, 41). Recombinant MTf is actively transported across the BBB in an in vitro model of BBB transcytosis (42, 43), with transport rate of 10–15 times higher than that of Tf (42, 43). Biodistribution studies have shown preferential distribution of MTf in brain tissue (20, 43, 44), likely facilitated through a receptor-mediated transcytosis involving a member of the low-density lipoprotein receptor-related protein family (LRP) (20, 21, 45, 46).
This vector has a unique capacity to carry drugs into brain since the concentration of MTf is not high enough to significantly competitively inhibit binding of exogenously injected MTf conjugates to the receptor (40). Further, MTf-drug conjugates have been shown to traverse the brain capillary endothelium within minutes, with end brain concentrations 10 fold higher than unconjugated drug (22). Furthermore, as an autologous human protein, immune hypersensitivity or elimination via neutralizing antibodies are less likely to be an issue in clinical setting. Lastly, since MTf traverse the BBB as part of its normal function, MTf does not appear to pose toxicity related issues for the delivery of MTf-drug conjugate into the brain. Subsequently, MTf poses less risk than cytokines TNF, which could disrubt the BBB by generating a focal inflammatory response(47). Melanotransferrin (hMTf, P97) is branded Transcend™ vector platform by BiOasis Inc. (48).
With regard to using this strategy for the treatment of CNS tumors, it has been shown that MTf-adriamycin conjugates inhibit growth of subcutaneous gliomas, prolong survival of animals with mammary tumors or intracranial gliomas (22). The conjugate MTf has also been used to transport monoclonal antibodies across the BBB for the treatment of brain cancers. This study is the first to test whether hMTf can be used in the treatment of a human Her2+ brain metastasis of breast cancer model.
In order to overcome the trastuzumab inability to cross the BBB and treat brain metastases of HER2+ breast cancer, BT2111, a novel bioconjugate of trastuzumab (BTA) was developed by biOasis Inc., which is based on the melanotransferrin (hMTf, p97) Transcend™vector platform. Administration of BT2111 to NuNu nude mice with brain metastasis induced by administration of MDA-MB-231-BRHER2/eGFP cells was characterized by significantly improved penetration through the BBB as compared to BTA. This effect was associated with the following increased parameters: 1) The brain /blood concentration ratio of BT2111 in normal and tumor areas of the brain was 10 to 225 times higher than the corresponding ratio for BTA and 2) BT2111 has a Kin value that was 40 times higher in tumor-free areas of the brain and 10 times higher in tumor areas of the brain as compared to BTA. In addition, administration of BT2111 was characterized by significantly higher tissue/blood concentration ratios in other organs and tissues of NuNu mice as compared to BTA.
Administration of BT2111 (17 mg/kg, IP, biweekly, starting from day 21 post tumor injection) resulted in statistically significant reduction of the number (68% reduction) of brain tumors induced by administration of human metastatic breast cancer cells (MDA-MB-231-BRHER2/eGFP) to female NuNu mice. The remaining tumors were significantly smaller in size (57–60% reduction) than the tumors observed in the control animals. In contrast, administration of BTA (10 mg/kg) and hMTf (7 mg/kg) was not accompanied by reduction of the brain tumor numbers or by decrease of tumor sizes.
To our knowledge, trastuzumab has not been conjugated to a delivery peptide or vehicle as a chemotherapeutic. Currently, trastuzumab is either used alone or in combination therapy for breast cancer and its brain metastases. In a case study reported by Şendur et al (49), lapatinib and capecitabine combination was used followed by trastuzumab in HER2-positive brain metastatic breast cancer. No progression of cranial metastases was found post-treatment. In another case report (50), one case out of three HER-2 positive breast cancer patients with brain metastasis maintained brain metastases post-treatment with a combination of weekly trastuzumab plus vinorelbine after brain metastasis. In an animal study, it was observed that the combination of a HER2 inhibitor with an anti–VEGF receptor-2 (VEGFR2) antibody along trastuzumab and lapatinib treatment significantly slows tumor growth in the brain, (51). According to the authors, trastuzumab may transiently act as an antiangiogenic in a leptomeningeal metastasis model of HER2-overexpressing breast cancer through, resulting in trastuzumab and lapatinib controlled tumor progression in breast but failing to contain tumor growth in brain (51).
In spite of lack of reports on the use of trastuzumab bioconjugates for its antitumor activity, previous studies have used trastuzumab as a bioconjugate to specifically deliver antitumor or diagnostic agents to HER2-positive cells. For example, bioconjugated 64Cu-DOTA PET imaging agent with trastuzumab for the detection of breast cancer and its primary brain metastasis on 6 patients. The pilot tumor images of 64Cu-DOTA-trastuzumab PET demonstrated successful tumor uptake and visualization of HER2-positive primary breast carcinoma in the 6 patients but only its metastatic lesions in the brain of 2 patients (52) In a similar study by Kurihara et al. (53) among five patients, metastatic brain lesions could be visualized by 64Cu-DOTA-trastuzumab PET in all of cases. In both studies, the number of cases were too small to suggest that trastuzumab can pass the BBB and penetrate into CNS lesions.
A major trastuzumab bioconjugate, is Ado-trastuzumab emtansine (T-DM1) (54), that links trastuzumab, with maytansinoid, DM1, a potent microtubule-disrupting agent. Initial phase III clinical trials demonstrated that the conjugate prolonged progression-free and overall survival with less toxicity than lapatinib plus capecitabinen in patients with advanced HER2-positive breast cancer (55). In 2013, T-DM1 received FDA approval for the treatment of patients with HER2-positive metastatic breast cancer who had previously received trastuzumab and a taxane, separately or in combination, the first antibody bioconjugate to receive full approval based on a randomized study (56). Another example, XMT-1522, which conjugates a modified trastuzumab antibody to ~15 auristatin molecules using Fleximer, a biodegradable hydrophilic polymer (57).
The results presented in this study provide evidence that BT2111 has efficacy in treating brain metastasis in preclinical models and validates the role hMTf has as a vector (Transcend™ vector platform) for the transport of antibodies across the BBB. This study has shown that Transcend can deliver Herceptin®, a chemotherapeutic used to treat HER2-positive breast cancer, in sufficient quantity to the brain to significantly reduce the number of HER2-positive metastatic brain tumors. The ability to more effectively permeate both the blood-brain and the blood-tumor barrier opens the door for the creation of new drugs designed to treat a wide variety of neurological diseases and disorders. Additionally, Transcend™ vector platform offers the potential to take existing clinically approved drugs that are near their end of patent life and extend them.
Abbreviations
- BT2111, BTA-MTf
Antibody trastuzumab-melanotransferrin conjugate
- MDA-MB-231-Her2
Metastatic brain seeking breast cancer cells
- BTA
Trastuzumab
- hMTf
Melanotransferrin
- BBB
Blood Brain Barrier
- BTB
Blood Tumor Barrier
- Kin
Drug uptake
- CT
Computed Tomography
- MRI
Magnetic Resonance Imaging
- ER
Estrogen Receptor
- hMTf
Melanotransferrin
- p97
Melanoma tumor antigen
- Tf
Transferrin
- LRP
Low Density Lipoprotein Receptor related Protein
- FDA
Food and Drug Administration
- HER2, HER-2/neu or c-erbB-2
Human epidermal growth factor receptor 2
- PBS
Phosphate Buffered Saline
- MNF
Anti-Cytokeratin mouse monoclonal IgG antibody
- BDT
Brain Distant to Tumor
References
- 1.Leone JP, Lee AV, Brufsky AM. Prognostic factors and survival of patients with brain metastasis from breast cancer who underwent craniotomy. Cancer Medicine. 2015;4(7):989–994. doi: 10.1002/cam4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colzani E, Liljegren A, Johansson AL, Adolfsson J, Hellborg H, Hall PF, Czene K. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. Journal of Clinical Oncology. 2011;29(30):4014–4021. doi: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 3.Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48(2):384–394. doi: 10.1002/1097-0142(19810715)48:2<384::aid-cncr2820480227>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Miller KD, Weathers T, Haney LG, Timmerman R, Dickler M, Shen J, Sledge GW., Jr Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Annals of Oncology. 2003;14(7):1072–1077. doi: 10.1093/annonc/mdg300. [DOI] [PubMed] [Google Scholar]
- 5.Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52(12):2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Riihimaki M, Thomsen H, Brandt A, Sundquist J, Hemminki K. Death causes in breast cancer patients. Annals of Oncology. 2012;23(3):604–610. doi: 10.1093/annonc/mdr160. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Munoz A, Plata-Fernandez Y, Fernandez M, Jaen-Morago A, Fernandez-Navarro M, de la Torre-Cabrera C, Ramirez-Tortosa C, Pascual J, Alba E, Sanchez-Rovira P. Tumor histological subtyping determined by hormone receptors and HER2 status defines different pathological complete response and outcome to dose-dense neoadjuvant chemotherapy in breast cancer patients. Clinical and Translational Oncology. 2013 doi: 10.1007/s12094-013-1116-z. [DOI] [PubMed] [Google Scholar]
- 8.de la Monte SM, Hutchins GM, Moore GW. Estrogen and progesterone receptors in prediction of metastatic behavior of breast carcinoma. American Journal of Medicine. 1984;76(1):11–17. doi: 10.1016/0002-9343(84)90738-1. [DOI] [PubMed] [Google Scholar]
- 9.Knight WA, Livingston RB, Gregory EJ, McGuire WL. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer research. 1977;37(12):4669–4671. [PubMed] [Google Scholar]
- 10.Sugimoto H, Nakagawa T, Sato T, Nagahara M, Ishiba T, Kasahara M, Kawachi H, Kubota K, Sugihara K. A long-surviving case of HER2-positive breast cancer with brain metastasis treated by multidisciplinary therapy. Gan to kagaku ryoho Cancer & chemotherapy. 2012;39(12):2071–2073. [PubMed] [Google Scholar]
- 11.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 13.Park YH, Park MJ, Ji SH, Yi SY, Lim DH, Nam DH, Lee JI, Park W, Choi DH, Huh SJ, Ahn JS, Kang WK, Park K, Im YH. Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. British journal of cancer. 2009;100(6):894–900. doi: 10.1038/sj.bjc.6604941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blumenthal GM, Scher NS, Cortazar P, Chattopadhyay S, Tang S, Song P, Liu Q, Ringgold K, Pilaro AM, Tilley A, King KE, Graham L, Rellahan BL, Weinberg WC, Chi B, Thomas C, Hughes P, Ibrahim A, Justice R, Pazdur R. First FDA approval of dual anti-HER2 regimen: pertuzumab in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer. Clinical Cancer Research. 2013;19(18):4911–4916. doi: 10.1158/1078-0432.CCR-13-1212. [DOI] [PubMed] [Google Scholar]
- 15.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. New England Journal of Medicine. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 16.Olson EM, Abdel-Rasoul M, Maly J, Wu CS, Lin NU, Shapiro CL. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Annals of Oncology. 2013;24(6):1526–1533. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothenberger S, Food MR, Gabathuler R, Kennard ML, Yamada T, Yasuhara O, McGeer PL, Jefferies WA. Coincident expression and distribution of melanotransferrin and transferrin receptor in human brain capillary endothelium. Brain Research. 1996;712(1):117–121. doi: 10.1016/0006-8993(96)88505-2. [DOI] [PubMed] [Google Scholar]
- 18.Suryo Rahmanto Y, Dunn LL, Richardson DR. The melanoma tumor antigen, melanotransferrin (p97): a 25-year hallmark--from iron metabolism to tumorigenesis. Oncogene. 2007;26(42):6113–6124. doi: 10.1038/sj.onc.1210442. [DOI] [PubMed] [Google Scholar]
- 19.Yi X, Manickam DS, Brynskikh A, Kabanov AV. Agile delivery of protein therapeutics to CNS. Journal of Controlled Release. 2014;190:637–663. doi: 10.1016/j.jconrel.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demeule M, Poirier J, Jodoin J, Bertrand Y, Desrosiers RR, Dagenais C, Nguyen T, Lanthier J, Gabathuler R, Kennard M, Jefferies WA, Karkan D, Tsai S, Fenart L, Cecchelli R, Beliveau R. High transcytosis of melanotransferrin (P97) across the blood-brain barrier. Journal of neurochemistry. 2002;83(4):924–933. doi: 10.1046/j.1471-4159.2002.01201.x. [DOI] [PubMed] [Google Scholar]
- 21.Moroo I, Ujiie M, Walker BL, Tiong JW, Vitalis TZ, Karkan D, Gabathuler R, Moise AR, Jefferies WA. Identification of a novel route of iron transcytosis across the mammalian blood-brain barrier. Microcirculation. 2003;10(6):457–462. doi: 10.1038/sj.mn.7800213. [DOI] [PubMed] [Google Scholar]
- 22.Karkan D, Pfeifer C, Vitalis TZ, Arthur G, Ujiie M, Chen Q, Tsai S, Koliatis G, Gabathuler R, Jefferies WA. A unique carrier for delivery of therapeutic compounds beyond the blood-brain barrier. PloS one. 2008;3(6):e2469. doi: 10.1371/journal.pone.0002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demeule M, Regina A, Jodoin J, Laplante A, Dagenais C, Berthelet F, Moghrabi A, Beliveau R. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascular pharmacology. 2002;38(6):339–348. doi: 10.1016/s1537-1891(02)00201-x. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Han T, Everts M, Zhu ZB, Gillespie GY, Curiel DT, Wu H. Directing adenovirus across the blood-brain barrier via melanotransferrin (P97) transcytosis pathway in an in vitro model. Gene therapy. 2007;14(6):523–532. doi: 10.1038/sj.gt.3302888. [DOI] [PubMed] [Google Scholar]
- 25.biOasis. biOasis's Herceptin®-BT2111 Stops Human Breast Cancer Tumor Growth in Animals. biOasis Technologies Inc.; Available from: http://www.bioasis.ca/news/2012/120905.htm. [Google Scholar]
- 26.HUTCHISON R, VITALIS TZ, Gabathuler R. P97-antibody conjugates and methods of use. WO2013006706 A1, CA2840221A1, CN103747807A, EP2717917A1, EP2717917B1, US9150846, US20130183368, US20160053237. USA: Bioasis Technologies Inc.; US Patent. 2013
- 27.Charles River Laboratories International I. NU/NU Nude Mouse (Crl:NU-Foxn1nu) Charles River Laboratories International, Inc.; Available from: http://www.criver.com/products-services/basic-research/find-a-model/nu-nu-nude-mouse. [Google Scholar]
- 28.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clinical Cancer Research. 2010;16(23):5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein J, Jerian S, Schechter G, Neeman T, Green MD. FDA Clinical Review of BLA 98-0369 (Herceptin®, Trastuzumab, rhuMAb HER2) In: Food and Drug Administration (FDA) CfBEaR, editor. Product Approval Information, Licensing Action. USA: CBER; 1998. p. 49. [Google Scholar]
- 30.Jay TM, Lucignani G, Crane AM, Jehle J, Sokoloff L. Measurement of local cerebral blood flow with [14C]iodoantipyrine in the mouse. Journal of Cerebral Blood Flow & Metabolism. 1988;8(1):121–129. doi: 10.1038/jcbfm.1988.16. [DOI] [PubMed] [Google Scholar]
- 31.Williams JL, Shea M, Furlan AJ, Little JR, Jones SC. Importance of freezing time when iodoantipyrine is used for measurement of cerebral blood flow. American Journal of Physiology. 1991;261(1 Pt 2):H252–H256. doi: 10.1152/ajpheart.1991.261.1.H252. [DOI] [PubMed] [Google Scholar]
- 32.de Boer AG, Gaillard PJ. Strategies to improve drug delivery across the blood-brain barrier. Clinical pharmacokinetics. 2007;46(7):553–576. doi: 10.2165/00003088-200746070-00002. [DOI] [PubMed] [Google Scholar]
- 33.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiological reviews. 2008;88(3):887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson EM, Najita JS, Sohl J, Arnaout A, Burstein HJ, Winer EP, Lin NU. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22(4):525–531. doi: 10.1016/j.breast.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loi S, de Azambuja E, Pugliano L, Sotiriou C, Piccart MJ. HER2-overexpressing breast cancer: time for the cure with less chemotherapy? Current opinion in oncology. 2011;23(6):547–558. doi: 10.1097/CCO.0b013e32834bd4c9. [DOI] [PubMed] [Google Scholar]
- 36.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiology of Disease. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 37.Pestalozzi B, Brignoli S. Traztuzumab in CSF. Journal of Clinical Oncology. 2000;18(11):2349–2351. doi: 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 38.Rossi M, Carioli G, Bonifazi M, Zambelli A, Franchi M, Moja L, Zambon A, Corrao G, La Vecchia C, Zocchetti C, Negri E. Trastuzumab for HER2+ metastatic breast cancer in clinical practice: Cardiotoxicity and overall survival. European Journal of Cancer. 2016;52:41–49. doi: 10.1016/j.ejca.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Saini KS, Azim HA, Jr, Metzger-Filho O, Loi S, Sotiriou C, de Azambuja E, Piccart M. Beyond trastuzumab: new treatment options for HER2-positive breast cancer. Breast. 2011;20(Suppl 3):S20–S27. doi: 10.1016/S0960-9776(11)70289-2. [DOI] [PubMed] [Google Scholar]
- 40.Kennard ML, Feldman H, Yamada T, Jefferies WA. Serum levels of the iron binding protein p97 are elevated in Alzheimer's disease. Nature Medicine. 1996;2(11):1230–1235. doi: 10.1038/nm1196-1230. [DOI] [PubMed] [Google Scholar]
- 41.Lambert LA, Perri H, Halbrooks PJ, Mason AB. Evolution of the transferrin family: conservation of residues associated with iron and anion binding. Comparative Biochemistry and Physiology - Part B: Biochemistry & Molecular Biology. 2005;142(2):129–141. doi: 10.1016/j.cbpb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Pardridge WM. Advances in cell biology of blood-brain barrier transport. Seminars in Cell Biology. 1991;2(6):419–426. [PubMed] [Google Scholar]
- 43.Fillebeen C, Descamps L, Dehouck MP, Fenart L, Benaissa M, Spik G, Cecchelli R, Pierce A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. Journal of Biological Chemistry. 1999;274(11):7011–7017. doi: 10.1074/jbc.274.11.7011. [DOI] [PubMed] [Google Scholar]
- 44.Friden PM, Walus LR. Transport of proteins across the blood-brain barrier via the transferrin receptor. Advances in Experimental Medicine and Biology. 1993;331:129–136. doi: 10.1007/978-1-4615-2920-0_21. [DOI] [PubMed] [Google Scholar]
- 45.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier transferrin receptor. Metabolism. 1987;36(9):892–895. doi: 10.1016/0026-0495(87)90099-0. [DOI] [PubMed] [Google Scholar]
- 46.Demeule M, Bertrand Y, Michaud-Levesque J, Jodoin J, Rolland Y, Gabathuler R, Beliveau R. Regulation of plasminogen activation: a role for melanotransferrin (p97) in cell migration. Blood. 2003;102(5):1723–1731. doi: 10.1182/blood-2003-01-0166. [DOI] [PubMed] [Google Scholar]
- 47.Villanueva MT. Drug therapy: smuggling trastuzumab into the brain. Nature Reviews Clinical Oncology. 2013;10(12):669. doi: 10.1038/nrclinonc.2013.202. [DOI] [PubMed] [Google Scholar]
- 48.biOasis. Transcend Platform. biOasis Technologies Inc.; 2016. Available from: http://www.bioasis.ca/transcend/ [Google Scholar]
- 49.Sendur MA, Uncu D, Zengin N. Longest progression-free survival with lapatinib and capecitabine combination followed by trastuzumab in HER2-positive brain metastatic breast cancer. Medical Oncology. 2014;31(4):890. doi: 10.1007/s12032-014-0890-y. [DOI] [PubMed] [Google Scholar]
- 50.Mutlu H, Buyukcelik A. The combination of weekly trastuzumab plus vinorelbine may be preferable regimen in HER-2 positive breast cancer patients with brain metastasis. Journal of Oncology Pharmacy Practice. 2015;21(4):310–312. doi: 10.1177/1078155214531514. [DOI] [PubMed] [Google Scholar]
- 51.Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, Farrar CT, Huang Y, Ager E, Kamoun W, Goel S, Snuderl M, Lussiez A, Hiddingh L, Mahmood S, Tannous BA, Eichler AF, Fukumura D, Engelman JA, Jain RK. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proceedings of the National Academy of Sciences. 2012;109(45):E3119–E3127. doi: 10.1073/pnas.1216078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, Honda N, Kodaira M, Yamamoto H, Yunokawa M, Shimizu C, Hasegawa K, Kanayama Y, Nozaki S, Kinoshita T, Wada Y, Tazawa S, Takahashi K, Watanabe Y, Fujiwara Y. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. Journal of Nuclear Medicine. 2013;54(11):1869–1875. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 53.Kurihara H, Hamada A, Yoshida M, Shimma S, Hashimoto J, Yonemori K, Tani H, Miyakita Y, Kanayama Y, Wada Y, Kodaira M, Yunokawa M, Yamamoto H, Shimizu C, Takahashi K, Watanabe Y, Fujiwara Y, Tamura K. (64)Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Research. 2015;5:8. doi: 10.1186/s13550-015-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lambert JM, Chari RV. Ado-trastuzumab Emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. Journal of Medicinal Chemistry. 2014;57(16):6949–6964. doi: 10.1021/jm500766w. [DOI] [PubMed] [Google Scholar]
- 55.Krop IE, Modi S, LoRusso PM, Pegram M, Guardino E, Althaus B, Lu D, Strasak A, Elias A. Phase 1b/2a study of trastuzumab emtansine (T-DM1), paclitaxel, and pertuzumab in HER2-positive metastatic breast cancer. Breast Cancer Research. 2016;18(1):34. doi: 10.1186/s13058-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartsch R, Berghoff AS, Vogl U, Rudas M, Bergen E, Dubsky P, Dieckmann K, Pinker K, Bago-Horvath Z, Galid A, Oehler L, Zielinski CC, Gnant M, Steger GG, Preusser M. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clinical and Experimental Metastasis. 2015;32(7):729–737. doi: 10.1007/s10585-015-9740-3. [DOI] [PubMed] [Google Scholar]
- 57.Bergstrom DA, Bodyak N, Yurkovetskiy A, Park PU, DeVit M, Yin M, Poling L, Thomas JD, Gumerov D, Xiao D, Ter-Ovanesyan E, Qin L, Uttard A, Johnson A, Lowinger TB. Abstract LB-231: A novel, highly potent HER2-targeted antibody-drug conjugate (ADC) for the treatment of low HER2-expressing tumors and combination with trastuzumab-based regimens in HER2-driven tumors. Cancer research. 2015;75(15 Supplement) LB-231. [Google Scholar]