Abstract
A major area of cancer research focuses on improving the specificity of therapeutic agents by engineering drug-delivery vehicles that target overexpressed receptors on tumor cells. One of the most commonly used approaches involves targeting of folate receptors using folic acid conjugated to a drug-containing macromolecular cargo. Once internalized via endocytosis, the drugs must be released from these constructs in order to avoid being trapped in the endosomes. Here, we describe the synthesis of a small-molecule conjugate that couples folic acid to doxorubicin via a photocleavable linker. Using HPLC we show that the doxorubicin can be released with light rapidly and with high efficiency. This approach has advantages over macromolecular systems due to its simplicity and efficiency.
Keywords: Folic Acid, Photocage, Endosomal entrapment, Drug Release, HPLC
Graphical abstract

Targeted therapeutics promise to greatly reduce the adverse side effects associated with current cancer chemotherapeutics.1 Targeted therapies typically act either by interfering with upregulated oncogenic pathways or through enabling cancer cell selective delivery by binding to an overexpressed receptor on the cell membrane of cancer cells.2–5 In the latter approach, a receptor targeting ligand is covalently coupled to a cytotoxic agent, which is then selectively delivered to the tumor cells.
Folic acid (FA-vitamin B9) is a precursor to tetrahydrofolate, 1-carbon donor which is essential for the synthesis of nucleotide bases. Commonly prescribed as nutritional supplement, FA has high affinity towards folate receptors (FRs), which facilitates its internalization. Two independent and mechanistically different systems mediate cellular uptake of FA in mammalian cells. The Reduced Folate Carrier (RFC) is a low affinity (Kd ~ 10−6 M), high-capacity membrane-spanning anion transport protein that delivers reduced FAs across the plasma membrane in a bidirectional fashion. Folate Receptor (FRα) is a high affinity (Kd ~ 10−10 M), single chain FA-binding protein which internalizes FA through active-receptor mediated endocytosis.6,7 FRα is frequently overexpressed in epithelial tumors, particularly in ovarian cancer where 90% of patients have elevated FRα levels.8,9 For this reason, FR targeting is being pursued for imaging and therapeutic purposes by conjugating FA with imaging or drug molecules.10–13 Folate-containing conjugates bind to FRα and are internalized via endocytosis. Once internalized the cargo needs to be released in order to prevent endosomal entrapment.14
Endosomal escape can be facilitated through cleavage of the bond between folate and a cell permeable drug, enabling it to pass through the endosomal membrane. A common approach to achieve this involves the use of disulfide bonds, which are cleaved only under reducing conditions inside the endosomes.15
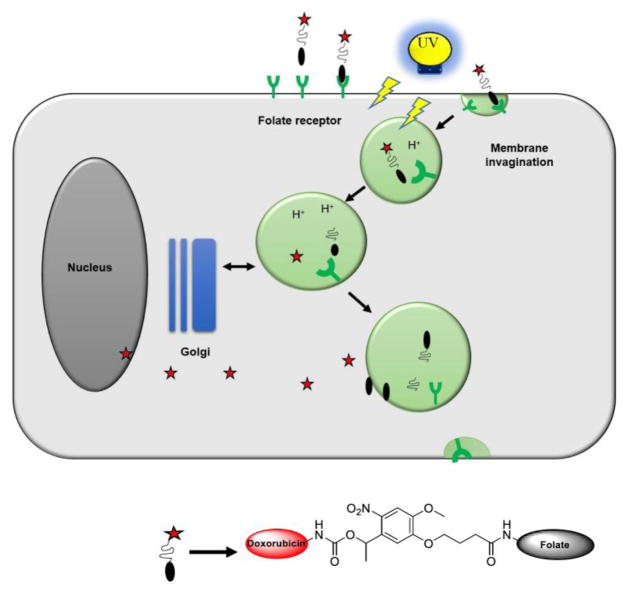
Another potential modality for drug release is light.16–28 Light has the advantages of being non-invasive, and spatially and temporally controllable for added selectivity. A folate targeted drug release system based on light would also offer an oxygen-independent alternative to photodynamic therapy,29,30 and other light-activated therapeutic strategies.31–34 Here, we describe such a targeted drug release system prepared by attaching doxorubicin to folic acid via a light-cleavable bond. Such a conjugate should selectively bind to and concentrate in tumor cells expressing the folate receptor (Fig. 1). When irradiated with light, the drug molecule will be released from the endosomes, eventually causing cell death with minimal collateral damage.
Figure 1.
Schematic representation of targeted delivery of photocaged drug (Adapted from Hilgenbrink et al.13)
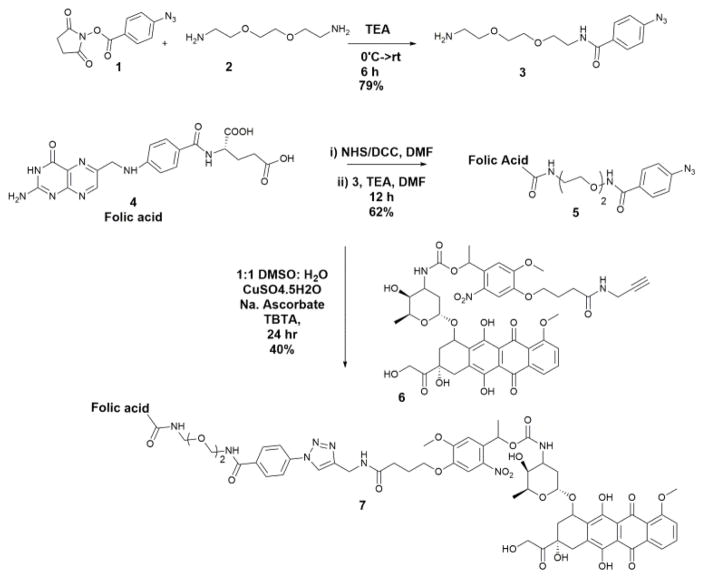
We envisioned linking the Folic acid to a photocaged doxorubicin using click chemistry. Previously, we established a protocol for the synthesis of a photocaged-doxorubicin with an alkyne functional group (6) Scheme 1. 21 To prepare the required folate-azide we coupled 4-Azidobenzoic succinimidyl ester (1) to bis-amino PEG linker (2) via an amide bond to give compound (3). We then prepared the N-hydroxysuccinimide ester of Folic acid (4) using reaction conditions that are known to be selective for the γ-COOH. 35 This intermediate was coupled with compound (3) to give azido folate conjugate (5) in moderate yield. This azide-derivatized folic acid was coupled to photocaged doxorubicin (6) using standard click chemistry to give PhotoDox-Fol (7). The drug conjugate was purified using HPLC and characterized using mass spectrometry (SI-7).
Scheme 1.
Synthesis of PhotoDox-Fol
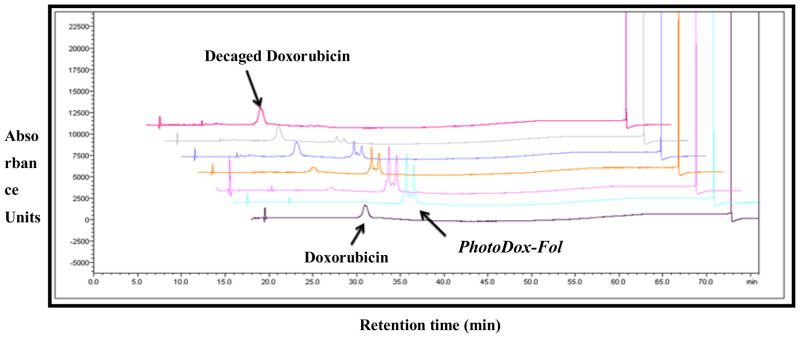
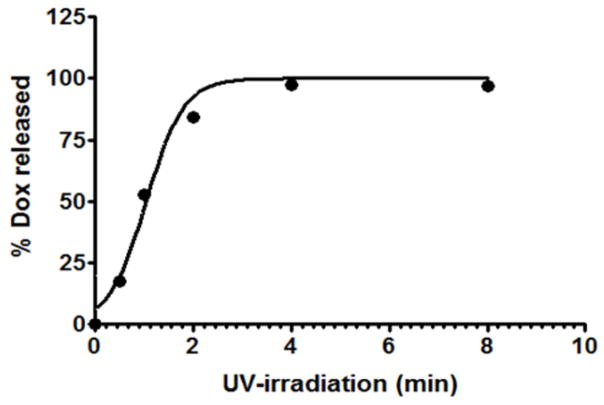
To demonstrate the efficiency of our drug conjugate to release drug in the presence of UV-light, we performed a photolysis assay, wherein the release of doxorubicin from the PhotoDox-Fol (7) was analyzed using HPLC.36 During the course of the reaction, the peaks corresponding to the PhotoDox-Fol (7) disappeared with concomitant increase in the intensity of a peak with the same retention time as Dox (Fig. 2), confirming time-dependent Dox release in the presence of light. The majority of doxorubicin was released rapidly within first 4 minutes of irradiating with the UV light (Fig. 3).
Figure 2.
Time dependent release of doxorubicin from the folic acid-photocaged drug conjugate under UV light. A solution of compound (7) was irradiated with UV light and samples were removed at various time points (0.5, 1, 2, 4 and 8 min) and were analyzed by RP-HPLC at λ = 480 nm. The non-irradiated compound (t = 0) and free doxorubicin are included as standards. PhotoDox-Fol exists as two diastereomers due to the racemic benzylic carbon on the nitroveratryl linker. We are able to resolve these diastereomers via HPLC in accord with previous work (ref 21).
Figure 3.
Graph representing increase in the % doxorubicin released from drug conjugate over period of 8 minutes. The values for % doxorubicin released were determined by quantifying the intensity of peak area of doxorubicin relative to Photodox-Fol intensity.
Existing reports that combine folate targeting using light sensitive vehicles commonly employ macromolecules such as dendrimers, micelles, etc. 37–39 For instance, Baker Jr. and colleagues reported synthesis of G5-PAMAM dendrimer coupled to folic acid and methotrexate (MTX), a cytotoxic analog of folic acid and, successfully tested its efficiency to target folate receptors on HeLa cells under light irradiation. 39 In a different approach, Yeh and group reported synthesis of biodegradable PLGA-lipid hybrid nanoconjugate (PLGA= poly (D, L-lactide-co-glycolide)), encapsulating Taxol (anti-microtubule agent), while the surface was coupled to photocaged folic acid to inhibit efficient permeability in cells and hence cytotoxicity. Irradiation with UV light led to uncaging of the drug, eventually leading to enhanced cell death in KB cells. 37 Although these strategies have potential advantages of avidity and the ability to release multiple drug molecules per receptor binding event, there are also some disadvantages such as slower drug release, limited endocytosis and issues with the carriers that are left behind.
PhotoDox-Fol has several advantages compared to these approaches. First, because PhotoDox-Fol is a low molecular weight compound, it is expected to be more easily endocytosed. Second, upon release, our conjugate does not leave behind any potentially toxic or hard-to-clear nanoparticles or polymers. Third, drug release from the conjugate under UV irradiation is very rapid and highly efficient even at low doses (1.1 J/cm2) (Fig. 2 and 3). Under identical conditions, the release kinetics are significantly faster than a non-folate targeted version of the molecule which required 20 minutes for full release. 21 UV-light at these low doses is also not likely to be cytotoxic on its own. 37,40,41 All these attributes make our approach amenable for rapid release of cytotoxins within folate receptor positive cancer cells.
One ongoing challenge with the use of light and therapy is the limited tissue penetration of UV light in tissue. Several recent advances in the use of longer, single-photon release such as BODIPY 42–44 and Cyanine molecules, 45 or two photon activation of photocages,46 should ultimately enable dual targeted constructs like PhotoDox-Fol to be applicable in vivo. For ultimate use, more cytotoxic cargoes than Dox will enable enhanced potency in deeper regions of tissue, since less efficient release will be required for potent activity.
Supplementary Material
Acknowledgments
We thank Prof. Richard Moran (Virginia Commonwealth University) and Prof. David Bartley (Adrian College) for advice. The authors gratefully acknowledge funding for this study from the NIH (CA167582)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Bae YH, Park K. J Controlled Release. 2011;153:198. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjornsti M, Houghton PJ. Nat Rev Cancer. 2004;4:335. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 3.Downward J. Nat Rev Cancer. 2003;3:11. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 4.Scaltriti M, Baselga J. Clin Cancer Res. 2006;12:5268. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 5.Xia W, Low PS. J Med Chem. 2010;53:6811. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Vlashi E, Low P. Subcell Biochem. 2012;56:163. doi: 10.1007/978-94-007-2199-9_9. [DOI] [PubMed] [Google Scholar]
- 7.Wibowo AS, Singh M, Reeder KM, Carter JJ, Kovach AR, Meng W, Ratnam M, Zhang F, Dann CE., 3rd Proc Natl Acad Sci U S A. 2013;110:15180. doi: 10.1073/pnas.1308827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergote IB, Marth C, Coleman RL. Cancer Metastasis Rev. 2015;34:41. doi: 10.1007/s10555-014-9539-8. [DOI] [PubMed] [Google Scholar]
- 9.Kalli KR, Oberg AL, Keeney GL, Christianson TJH, Low PS, Knutson KL, Hartmann LC. Gynecol Oncol. 108;619 doi: 10.1016/j.ygyno.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledermann JA, Canevari S, Thigpen T. Ann Oncol. 2015;26:2034. doi: 10.1093/annonc/mdv250. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasarao M, Galliford CV, Low PS. Nat Rev Drug Discov. 2015;14:203. doi: 10.1038/nrd4519. [DOI] [PubMed] [Google Scholar]
- 12.Teng L, Xie J, Teng L, Lee RJ. Expert Opin Drug Deliv. 2012;9:901. doi: 10.1517/17425247.2012.694863. [DOI] [PubMed] [Google Scholar]
- 13.Hilgenbrink AR, Low PS. J Pharm Sci. 2005;94:2135. doi: 10.1002/jps.20457. [DOI] [PubMed] [Google Scholar]
- 14.Simon SM. Drug Discov Today. 1999;4:32. doi: 10.1016/s1359-6446(98)01276-8. [DOI] [PubMed] [Google Scholar]
- 15.Vlahov IR, Leamon CP. Bioconjug Chem. 2012;23:1357. doi: 10.1021/bc2005522. [DOI] [PubMed] [Google Scholar]
- 16.Fomina N, Sankaranarayanan J, Almutairi A. Adv Drug Deliv Rev. 2012;64:1005. doi: 10.1016/j.addr.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rwei AY, Wang W, Kohane DS. Nano Today. 2015;10:451. doi: 10.1016/j.nantod.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamay Y, Adar L, Ashkenasy G, David A. Biomaterials. 2011;32:1377. doi: 10.1016/j.biomaterials.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Agasti SS, Chompoosor A, You CC, Ghosh P, Kim CK, Rotello VM. J Am Chem Soc. 2009;131:5728. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi SK, Thomas T, Li MH, Kotlyar A, Desai A, Baker JR., Jr Chem Commun (Camb) 2010;46:2632. doi: 10.1039/b927215c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dcona MM, Mitra D, Goehe RW, Gewirtz DA, Lebman DA, Hartman MC. Chem Commun (Camb) 2012;48:4755. doi: 10.1039/c2cc30819c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael Dcona M, Yu Q, Capobianco JA, Hartman MC. Chem Commun (Camb) 2015;51:8477. doi: 10.1039/c5cc01795e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonidova A, Mari C, Aebersold C, Gasser G. Organometallics. 2016;35:851. [Google Scholar]
- 24.Hu X, Tian J, Liu T, Zhang G, Liu S. Macromolecules. 2013;46:6243. [Google Scholar]
- 25.Joshi T, Pierroz V, Mari C, Gemperle L, Ferrari S, Gasser G. Angew Chem Int Ed. 2014;53:2960. doi: 10.1002/anie.201309576. [DOI] [PubMed] [Google Scholar]
- 26.Leonidova A, Pierroz V, Rubbiani R, Lan Y, Schmitz AG, Kaech A, Sigel RKO, Ferrari S, Gasser G. Chem Sci. 2014;5:4044. [Google Scholar]
- 27.Zindler M, Pinchuk B, Renn C, Horbert R, Döbber A, Peifer C. ChemMedChem. 2015;10:1335. doi: 10.1002/cmdc.201500163. [DOI] [PubMed] [Google Scholar]
- 28.Horbert R, Pinchuk B, Davies P, Alessi D, Peifer C. ACS Chem Biol. 2015;10:2099. doi: 10.1021/acschembio.5b00174. [DOI] [PubMed] [Google Scholar]
- 29.Dolmans DE, Fukumura D, Jain RK. Nat Rev Cancer. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 30.Allison RR, Sibata CH. Photodiagnosis Photodyn Ther. 2010;7:61. doi: 10.1016/j.pdpdt.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Borowiak M, Nahaboo W, Reynders M, Nekolla K, Jalinot P, Hasserodt J, Rehberg M, Delattre M, Zahler S, Vollmar A, Trauner D, Thorn-Seshold O. Cell. 2015;162:403. doi: 10.1016/j.cell.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 32.Barhoumi A, Liu Q, Kohane DS. J Controlled Release. 2015;219:31. doi: 10.1016/j.jconrel.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Engdahl AJ, Torres EA, Lock SE, Engdahl TB, Mertz PS, Streu CN. Org Lett. 2015;17:4546. doi: 10.1021/acs.orglett.5b02262. [DOI] [PubMed] [Google Scholar]
- 34.Sheldon JE, Dcona MM, Lyons CE, Hackett JC, Hartman MCT. Org Biomol Chem. 2016;14:40. doi: 10.1039/c5ob02005k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stella B, Arpicco S, Peracchia MT, Desmaele D, Hoebeke J, Renoir M, D’Angelo J, Cattel L, Couvreur P. J Pharm Sci. 2000;89:1452. doi: 10.1002/1520-6017(200011)89:11<1452::aid-jps8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 36.Photolysis Assay.
- 37.Fan N, Cheng F, Ho JA, Yeh C. Angew Chem Int Ed. 2012;51:8806. doi: 10.1002/anie.201203339. [DOI] [PubMed] [Google Scholar]
- 38.Chien YH, Chou YL, Wang SW, Hung ST, Liau MC, Chao YJ, Su CH, Yeh CS. ACS Nano. 2013;7:8516. doi: 10.1021/nn402399m. [DOI] [PubMed] [Google Scholar]
- 39.Choi SK, Thomas TP, Li MH, Desai A, Kotlyar A, Baker JR., Jr Photochem Photobiol Sci. 2012;11:653. doi: 10.1039/c2pp05355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Govan JM, Young DD, Lusic H, Liu Q, Lively MO, Deiters A. Nucleic Acids Res. 2013;41:10518. doi: 10.1093/nar/gkt806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauers DJ, Temburni MK, Biggins JB, Ceo LM, Galileo DS, Koh JT. ACS Chem Biol. 2010;5:313. doi: 10.1021/cb9002305. [DOI] [PubMed] [Google Scholar]
- 42.Goswami PP, Syed A, Beck CL, Albright TR, Mahoney KM, Unash R, Smith EA, Winter AH. J Am Chem Soc. 2015;137:3783. doi: 10.1021/jacs.5b01297. [DOI] [PubMed] [Google Scholar]
- 43.Rubinstein N, Liu P, Miller EW, Weinstain R. Chem Commun (Camb) 2015;51:6369. doi: 10.1039/c5cc00550g. [DOI] [PubMed] [Google Scholar]
- 44.Umeda N, Takahashi H, Kamiya M, Ueno T, Komatsu T, Terai T, Hanaoka K, Nagano T, Urano Y. ACS Chem Biol. 2014;9:2242. doi: 10.1021/cb500525p. [DOI] [PubMed] [Google Scholar]
- 45.Nani RR, Gorka AP, Nagaya T, Kobayashi H, Schnermann MJ. Angew Chem Int Ed Engl. 2015;54:13635. doi: 10.1002/anie.201507391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anstaett P, Pierroz V, Ferrari S, Gasser G. Photochem Photobiol Sci. 2015;14:1821. doi: 10.1039/c5pp00245a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.