Abstract
Purpose
We examined the relation between malnutrition, lifestyle factors, and bone health in AN via dual-energy X-ray absorptiometry (DXA) and peripheral quantitative computed tomography (pQCT).
Methods
Seventy adolescent girls with AN and 132 normal-weighted controls underwent pQCT tibial measures including trabecular volumetric bone mineral density (vBMD), cortical vBMD, and cortical thickness. Participants with AN underwent DXA measures of the axial skeleton. We assessed the association of DXA and pQCT measures with clinical and lifestyle variables.
Results
BMI Z-score and ideal body weight percentage were positively correlated with trabecular vBMD, cortical CSA, and section modulus (p<0.04). Exercise was associated with all pQCT measures, but only with hip BMD by DXA. In AN, use of antidepressants was associated with lower pQCT measures (p<0.03).
Conclusions
Antidepressants may negatively, and exercise positively, influence BMD in adolescents with eating disorders. These findings offer a provocative look at two longstanding questions.
Keywords: Anorexia nervosa, malnutrition, peripheral quantitative computed tomography, DXA, exercise, antidepressant
Introduction
Bone loss frequently complicates anorexia nervosa (AN)1,2; patients have a seven-fold increased fracture incidence3. Studies using dual energy X-ray absorptiometry (DXA) demonstrate significant reductions in bone mineral density (BMD)1. Several factors correlate with BMD in AN: body mass index (BMI), age at menarche, lean body mass, and illness duration1. Less is known about the impact of modifiable factors, such as physical activity or medication use, on the peripheral skeleton or bone geometry of girls with AN. We sought to determine the association between these factors and skeletal health as measured by DXA and peripheral quantitative computed tomography (pQCT). We hypothesized that physical activity would be protective for skeletal health even in the setting of AN, while use of anti-depressant medications would not significantly impact bone measurements in these patients.
Materials and Methods
Participant Selection/Measurements
Details have been published previously.4 We recruited female adolescents (ages 11–20 years) from two Eating Disorders Programs for participation in a clinical trial (Clinicaltrials.gov, NCT01343771). Eligible patients were post-menarchal or had a bone age ≥ 13 years, and met DSM-V diagnostic criteria for AN. Subjects were excluded if they had other medical conditions known to affect bone health (such as celiac disease or cystic fibrosis), or if they were receiving medications known to affect the skeleton (such as oral contraceptives). Control subjects were recruited from general adolescent medicine clinics at the same sites. Control subjects were normal-weighted, free of concomitant chronic diseases, and did not regularly use medications over the year prior to study enrollment. We report baseline data from the first research visit for 70 females with AN and 132 healthy control subjects (ages 11–18 years). The study was IRB approved; informed consent was obtained.
Bone measurements were completed at the baseline visit. DXA (Discovery A, Hologic, Inc.) measurements of bone mineral content (BMC, g) and BMD (g/cm2) were obtained for the total body less head (TBLH), spine, and total hip. Body composition was measured by DXA. pQCT left tibial cross-sectional measurements were obtained (Stratec XCT 3000, Orthometrix) at tibial length percentages proximal to the growth plate (3%, 38%, 66%) for trabecular and cortical volumetric BMD (vBMD, mg/cm3), cortical BMC (mg) and cross-sectional area (CSA, mm2), cortical thickness (mm), and muscle CSA (mm2). Results were converted to Z-scores.
Anthropometrics were obtained in all participants. The Hamwi method for women was used to calculated percentage ideal body weight (% IBW) = 100 lbs for 60 inches tall, and 5 lbs/inch for each inch over 5 feet5,6. . Participants provided health information, and completed the Youth/Adolescent Activity Questionnaire to quantify exercise.4
We used Pearson correlation and Student t-test to assess associations between clinical markers and measures, and analysis of covariance to compare groups for a given level of weekly exercise.
Results
Adolescents with AN differed from control subjects in BMI and %IBW (Table). Most subjects exercised regularly; all reported participation in weight-bearing activities such as running, walking, and use of equipment such as an elliptical machine. Controls who exercised reported less activity (mean ± SD, 5.5 ± 4.4 hr/wk) than AN participants (8.5 ± 4.9 hr/wk, p<0.001). Twenty-nine participants (41%) with AN used antidepressants regularly for a minimum of 3 months prior to study enrollment; no control subjects did. All 29 took a selective serotonin reuptake inhibitor (SSRI); of those, n=7 also took an antipsychotic and n=2 also took a tricyclic antidepressant. No subjects consumed alcohol or smoked.
Table 1.
Characteristics of 70 adolescent girls with anorexia nervosa and 132 female control subjects of comparable age.
| Characteristic | AN* | Control | |||
|---|---|---|---|---|---|
|
| |||||
| Mean ± SD | Min, Max | Mean ± SD | Min, Max | p-value | |
| Age, yr | 15.5 ± 1.9 | 11.5, 18.9 | 16.9 ± 2.3 | 11.0, 20.8 | <0.0001 |
| Height, cm | 160.5 ± 7.9 | 142.0, 178.0 | 161.9 ± 7.3 | 134.7, 178.4 | 0.28 |
| Weight, kg | 48.4 ± 6.5 | 25.9, 59.4 | 59.4 ± 9.9 | 30.8, 81.4 | <0.0001 |
| BMI, kg/m2 | 18.7 ± 1.7 | 12.8, 22.4 | 22.6 ± 3.1 | 15.6, 29.7 | <0.0001 |
| BMI Z-score | −0.60 ± 0.79 | −3.13, 0.82 | 0.41 ± 0.74 | −1.52, 2.01 | <0.0001 |
| % IBW | 91.0 ± 9.4 | 56.9, 111.0 | 109.6 ± 15.4 | 67.7, 146.2 | <0.0001 |
| Lean mass, kg | 34.5 ± 4.9 | 18.9, 50.0 | |||
| Fat mass, kg | 11.9 ± 3.3 | 3.6, 18.6 | |||
| Body fat, % | 24.4 ± 5.2 | 11.0, 33.5 | |||
|
|
|||||
| Median | Min, Max | ||||
|
|
|||||
| Duration of AN, mo | 4.5 | 1, 60 | |||
| Duration of amenorrhea, mo* | 4 | 1, 18 | |||
|
| |||||
| N (%) | N (%) | p-value | |||
|
| |||||
| Race | <0.0001 | ||||
| White | 61 (87) | 48 (36) | |||
| Black | — | 31 (23) | |||
| Asian | 2 (3) | 22 (17) | |||
| Other | 7 (10) | 28 (21) | |||
| Native American | — | 3 (2) | |||
| Hispanic ethnicity | 4 (6) | 32 (24) | 0.008 | ||
| Regular exercise | 52 (74) | 111 (84) | 0.13 | ||
| Antidepressant use | 29 (41) | 0 (0) | P<0.0001 | ||
| Vitamin D insufficient (25OHD <30 ng/mL) | 34 (49) | — | |||
| Vitamin D deficient (25OHD <20 ng/mL) | 7 (10) | — | |||
Rounded down; premenarchal participants excluded.
BMI, body mass index; AN, anorexia nervosa; 25OHD, 25-hydroxy-vitamin D
BMI Z-score was positively associated with every BMD and BMC measure by DXA in AN (r range 0.44 to 0.57, p<0.001). Illness duration inversely correlated with spinal BMD Z-scores only (Pearson r= −0.32, p=0.006). Amenorrhea duration was negatively associated with TBLH and spinal Z-scores (r= −0.26 to −0.27, p=0.04). Peripherally, BMI Z-score correlated positively with trabecular vBMD, cortical BMC, cortical CSA, and muscle CSA Z-scores (r 0.35 to 0.50; p<0.005). Correlations between these pQCT measures and %IBW were similar (r 0.22 to 0.42, p<0.07). Neither the duration of illness nor duration of amenorrhea was associated with pQCT outcomes.
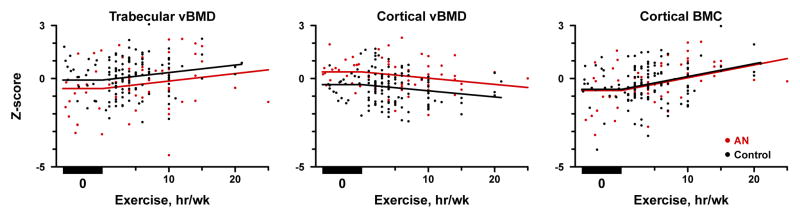
By DXA, only hip BMD Z-score was related to exercise in AN (+0.06 ± 0.03 per hour of exercise/week, estimate ± SE, p=0.02). pQCT measures were associated not only with presence or absence of exercise, but amount of exercise performed. In AN, reported exercise positively correlated with trabecular vBMD (p=0.01) and cortical BMC Z-scores (Figure; p<0.001). Bone size was similarly impacted; more exercise was associated with higher cortical CSA and cortical thickness Z-score (p<0.001). In contrast, cortical vBMD Z-scores were negatively associated with exercise in AN (Figure, p=0.007). Weekly exercise impacted cortical BMC, CSA, and thickness Z-scores similarly between girls with AN and controls (p>0.40 for group x exercise interaction). Girls with AN showed consistently lower trabecular vBMD-Z scores and higher cortical vBMD Z-scores over the range of exercise (0–25 hr/wk).
Figure.
Association of trabecular volumetric bone mineral density (vBMD) Z-scores (Panel A), cortical vBMD Z-scores (Panel B), and cortical bone mineral content (BMC) Z-scores (Panel C) with reported hours of exercise per week among adolescent girls with anorexia nervosa (AN, red lines) and healthy female control subjects (Control, black lines). In all 3 panels, a significant association between the number of hours of weekly exercise and the pQCT measure is observed (p<0.01 for all), following a parallel course for AN and Control (p>0.40 for group × exercise interaction). Panels A and B: Significant differences between AN and Controls remain across the range of exercise for trabecular vBMD Z-score (AN-Control −0.48 ± 0.18, estimate ± standard error, p=0.008) and cortical vBMD Z-score (0.71 ± 0.14, p<0.001). Panel C: No difference between AN and Controls for cortical BMC Z-score (p=0.66).
Hip BMD Z-scores were lower with antidepressant use versus non-use in AN (−0.69 ± 1.3 vs. − 0.05 ± 1.1, p=0.03). Hip BMC Z-scores were also negatively impacted (BMC Z-score −0.56 lower with antidepressants, p=0.02). No differences were seen at TBLH or hip. With pQCT, cortical BMC (−0.57 ± 1.18), cortical CSA (−0.66 ± 1.20), and muscle CSA (−0.78 ± 1.04) Z-scores were lower with antidepressants compared to non-use, by differences of −0.60 to −0.61 units (p<0.03). Trabecular vBMD, cortical thickness, and section modulus Z-score differences were similar (−0.64, −0.55, −0.47) although marginally non-significant (0.06<p<0.08). Antidepressant use did not impact cortical vBMD Z-scores (p=0.84).
We adjusted the comparisons regarding antidepressant use in AN for BMI and exercise. BMI Z-scores were not different between anti-depressant users (BMI Z-score mean −0.64 ± 0.81) and non-users (BMI Z-score −0.57 ± 0.78, p=0.71). Accordingly, BMI did not impact differences in skeletal measurements between the two groups. In contrast, adolescents with AN who used antidepressants reported significantly less exercise than those who did not (median 0 hr/wk versus 7 hr/wk, p=0.002). Adjusting for exercise did tend to attenuate the antidepressant differences for pQCT measures, particularly those at the 38% site.
Discussion
While DXA cannot fully characterize skeletal wellness, pQCT provides additional insight into the density and structure of the peripheral skeleton. Herein, we revealed skeletal deficits by pQCT in adolescents with AN. The high levels of weight-bearing activity in this cohort may have led to some compensation despite low muscle mass. Bone size and BMD by pQCT at several sites were positively affected by exercise, supporting our hypothesis. The effect size of exercise on cortical BMC, CSA, and thickness Z-scores was similar between girls with AN and healthy adolescents. However, trabecular and cortical vBMD Z-scores differed between groups at a given exercise level. Our findings suggest that exercise strengthens muscles around bone to confer skeletal gains, particularly at cortical sites, even in the face of an eating disorder. Clinicians need to remain cautious in the endorsement of exercise recommendations. Skeletal benefits need to be weighed against medical risk, and considering caloric expenditure, fracture risk, and purging through hyper-exercise. Additionally, exercise does not appear to solve all skeletal aberrations that occur in AN; trabecular and cortical vBMD Z-score deficits persisted in AN compared to controls despite the observed associations.
Antidepressant use was inversely associated with hip BMD by DXA, but did not impact the spine. Adult investigations7–9 and studies of AN10,11 have shown similar skeletal effects of antidepressants. We found that trabecular vBMD, cortical CSA, cortical thickness, section modulus, and maximal muscle CSA by pQCT were negatively correlated with antidepressant use. The adverse effects of these medications on cortical bone, which comprises 80% of the skeleton, are particularly concerning. However, these differences in pQCT measures were attenuated at the 38% site by the association between antidepressant use and lower levels of exercise in AN. Our findings suggest that exercise may ameliorate some of the negative impact of antidepressants on the cortical skeleton.
Study limitations merit acknowledgement. The sample size was relatively small, and study design, cross-sectional. Our recruitment strategy aimed to examine bone health across a broader spectrum of disease than captured previously. Our results may not be generalizable to patients with severe disease.
In conclusion, we found that the impact of malnutrition and accompanying systemic alterations on the peripheral skeleton of adolescents with AN may be similar to that of the axial skeleton. Our findings around exercise and antidepressant use offer a provocative new look at two longstanding management issues for these patients that should drive future trials.
Table 2.
Correlation between tibial pQCT Z-scores or DXA measures with markers of disease severity in 70 adolescent girls with anorexia nervosa
| Site | DXA Z-score | Correlation with BMI Z-score | Correlation with duration of illness |
|---|---|---|---|
| TBLH | BMD Z-score | 0.53* | −0.21 |
| BMC Z-score | 0.57* | −0.19 | |
|
| |||
| Spine | BMD Z-score | 0.53* | −0.32† |
| BMC Z-score | 0.49* | −0.26† | |
|
| |||
| Hip | BMD Z-score | 0.47* | −0.17 |
| BMC Z-score | 0.44* | −0.03 | |
|
| |||
| Site | pQCT Z-score | Correlation with BMI Z-score | Correlation with duration of illness |
|
| |||
| 3% | Trabecular vBMD | 0.42* | −0.16 |
|
| |||
| 38% | Cortical vBMD | −0.05 | −0.13 |
| Cortical BMC | 0.46* | −0.15 | |
| Cortical CSA | 0.47* | −0.13 | |
| Cortical thickness | 0.35† | −0.07 | |
| Polar section modulus | 0.45* | −0.15 | |
|
| |||
| 66% | Maximum muscle CSA | 0.50* | 0.03 |
DXA, dual energy X-ray absorptiometry; pQCT, peripheral quantitative computed tomography; BMD, bone mineral density; BMC, bone mineral content; CSA, cross sectional area.
p<0.0001.
p<0.05.
Table 3.
Comparison of skeletal measures by DXA and pQCT between adolescents with anorexia nervosa who use anti-depressants and those who do not
| Anti-depressant use N=29 |
No anti-depressant use N=41 |
p-value | ||
|---|---|---|---|---|
|
| ||||
| DXA Measurements | ||||
|
| ||||
| Site | Z-score | Mean ± SD | Mean ± SD | |
|
| ||||
| Hip | BMD | −0.69 ± 1.3 | −0.05 ± 1.1 | 0.03 |
| BMC | −0.18 ± 0.98 | 0.39 ± 0.91 | 0.02 | |
|
| ||||
| TBLH | BMD | −0.49 ± 1.2 | −0.08 ± 1.2 | 0.17 |
| BMC | −0.50 ± 0.98 | −0.15 ± 0.98 | 0.14 | |
|
| ||||
| Spine | BMD | −0.79 ± 1.4 | −0.35 ± 1.3 | 0.18 |
| BMC | −0.39 ± 1.2 | 0.08 ± 1.1 | 0.09 | |
|
| ||||
| pQCT Measurements | ||||
|
| ||||
| Site | Z-score | Mean ± SD | Mean ± SD | |
|
| ||||
| 3% | Trabecular vBMD | −0.69 ± 1.51 | −0.04 ± 1.30 | 0.06 |
|
| ||||
| 38% | Cortical vBMD | 0.20 ± 0.99 | 0.16 ± 0.87 | 0.84 |
| Cortical BMC | −0.57 ± 1.18 | 0.02 ± 1.10 | 0.03 | |
| Cortical CSA | −0.66 ± 1.20 | −0.04 ± 1.09 | 0.03 | |
| Cortical thickness | −0.49 ± 1.18 | 0.05 ± 1.18 | 0.06 | |
| Polar section modulus | −0.59 ± 1.08 | −0.13 ± 1.07 | 0.08 | |
|
| ||||
| 66% | Maximum muscle CSA | −0.78 ± 1.04 | −0.17 ± 0.87 | 0.01 |
DXA, dual energy X-ray absorptiometry; pQCT, peripheral quantitative computed tomography; BMD, bone mineral density; BMC, bone mineral content; CSA, cross sectional area.
Implications and Contribution.
The impact of malnutrition and accompanying systemic alterations on the peripheral skeleton of adolescents with anorexia nervosa (AN) may be similar to that of the axial skeleton. While exercise positively influences BMD even in the setting of AN, antidepressant use appears to negatively impact the skeleton.
Acknowledgments
The authors thank Alicia McAllister, RT, CBDT, Valerie Marsocci, RT, and Nicole DaSilva, RT, CNMT for expert technical assistance; the excellent care of the Boston Children’s Hospital Clinical and Translational Study Unit (CTSU) nurses; and our patients and their families, who made this research possible. Our study was supported by NIH R01 AR060829, NICHD K23 HD060066, NIH UL1 RR-025758 (Harvard Clinical and Translational Science Center), the Boston Children’s Hospital Department of Medicine and CTSU, and the Brown Alpert Medical School Department of Orthopaedics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donaldson A, Gordon C. Skeletal complications of eating disorders. Metabolism. 2015;64(9):943–51. doi: 10.1016/j.metabol.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Curr Psychiatry Rep. 2012;14(4):406–414. doi: 10.1007/s11920-012-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA. 1991;265(9):1133–1138. [PubMed] [Google Scholar]
- 4.Divasta AD, Feldman HA, O’Donnell JM, Long J, Leonard MB, Gordon CM. Skeletal Outcomes by Peripheral Quantitative Computed Tomography and Dual-energy X-ray Absorpotiometry in Adolescent Girls with Anorexia Nervosa. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3685-5. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamwi GJ. Therapy: changing dietary concepts. In: Danowski TS, editor. Diabetes Mellitus: Diagnosis and Treatment. New York: American Diabetes Association; 1964. pp. 73–78. [Google Scholar]
- 6.Organization WH. Report of a WHO Expert Committee. Geneva: 1995. Physical Status: The Use and Interpretation of Anthropometry. [PubMed] [Google Scholar]
- 7.Moura C, Bernatsky S, Abrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS) Osteoporos Int. 2014;25(5):1473–81. doi: 10.1007/s00198-014-2649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansone RA, Sansone LA. SSRIs: bad to the bone? Innov Clin Neurosci. 2012;9(7–8):42–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzoli R, Cooper C, Reginster J-Y, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606–613. doi: 10.1016/j.bone.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Couturier J, Sy A, Johnson N, Findlay S. Bone mineral density in adolescents with eating disorders exposed to selective serotonin reuptake inhibitors. Eat Disord. 2013;21(3):238–48. doi: 10.1080/10640266.2013.779183. [DOI] [PubMed] [Google Scholar]
- 11.Feuer AJ, Demmer RT, Thai A, Vogiatzi MG. Use of selective serotonin reuptake inhibitors and bone mass in adolescents: An NHANES study. Bone. 2015;78:28–33. doi: 10.1016/j.bone.2015.04.042. [DOI] [PubMed] [Google Scholar]