Abstract
This study aimed to evaluate the safety and curative effect of percutaneous endoscopic transforaminal lumbar spinal canal decompression in the treatment of lumbar spinal stenosis.
This retrospective study recruited 64 patients with lumbar spinal stenosis who underwent percutaneous endoscopic lumbar spinal canal decompression via surgical approach of posterolateral intervertebral foramen. The postoperation neurological function and pain status were evaluated by the visual analog scale (VAS) score of pain and the Oswestry disability index (ODI), and the patient satisfaction was evaluated according to the MacNab outcome criteria. The data, including preoperative comorbidities, operation time, the quantity of bleeding, bed rest time, and intraoperative and postoperative complications, were recorded.
The mean operation time was 78 min, the mean quantity of bleeding was 20 mL and bed rest time was 6 h to 3 days. All patients were followed-up for 4 months to 5 years. The mean preoperative VAS score was 7.7 ± 1.2, while postoperative 3 months, 6 months, and final follow-up VAS scores were 2.8 ± 0.7, 2.1 ± 0.6, and 0.8 ± 0.6, respectively (P < 0.001). The mean preoperative ODI score was 72.4 ± 1.2, while postoperative 3 months, 6 months, and final follow-up ODI scores were 29.7 ± 4.9, 23.9 ± 4.0, and 12.5 ± 3.9, respectively (P < 0.001). The excellent and good rate reached 73.4% at the final follow-up.
The percutaneous endoscopic transforaminal lumbar spinal canal decompression is an easy, safe, and effective minimally invasive surgery for patients with lumbar spinal stenosis.
Keywords: follow-up, lumbar spinal canal decompression, lumbar spinal stenosis, minimally invasive surgery, posterolateral intervertebral foramen
Highlights
Postoperative mean VAS was significantly reduced compared with preoperation.
Postoperative mean ODI was significantly reduced compared with preoperation.
The excellent and good rate reached 73.4% at the final follow-up.
The percutaneous endoscopic transforaminal spinal decompression is effective.
1. Introduction
Lumbar spinal stenosis is a common lumbar vertebrae disease in the elderly population, can lead to leg pain and low back pain especially when walking.[1] It is usually caused by the herniation of intervertebral disc, the gradual narrowing of the spinal canal, and the hypertrophy of vertebral plate.[1] This degenerative condition severely affects walking ability of patients, thereby resulting in the poor quality of life.[2] The spinal surgery used for the decompression of the spinal canal is considered as an effective treatment to improve walking ability.[3] The classic operation process is that the paraspinal muscles and spinous process are dissected sequentially, then the interspinous ligaments are removed.[4] Due to the scarring of the epidural space and segmental instability caused by the open surgery, it is necessary to develop the effective minimally invasive surgery for lumbar spinal stenosis.[5]
Percutaneous endoscopic transforaminal lumbar discectomy (PETLD) is considered as a safe and effective minimally invasive surgery.[6] The surgical approach of PETLD is posterolateral intervertebral foramen or interlaminae space.[7] PETLD can be used for decompression of nerve tissue, but not destroy the stable structure of spinal posterior.[8] Currently, PETLD has been widely applied in central spinal canal stenosis caused by hypertrophy of ligamentum flavum and protrusion of intervertebral disc, nerve root canal stenosis caused by hyperostosis, and lumbar foraminal stenosis caused by hyperplasia of articular process.[9–11] However, few studies investigated the curative effect of percutaneous endoscopic transforaminal lumbar spinal canal decompression for the treatment of lumbar spinal stenosis.
The current study investigated the outcomes of 64 patients with lumbar spinal stenosis who underwent percutaneous endoscopic decompression via surgical approach of posterolateral intervertebral foramen, aimed to evaluate the safety and curative effect of percutaneous endoscopic transforaminal lumbar spinal canal decompression in the treatment of lumbar spinal stenosis.
2. Materials and methods
2.1. Patients
From February 2010 to February 2015, a total of 64 patients with lumbar spinal stenosis (40 males and 24 females; age 46–86 years) were enrolled in this retrospective study. Among these 64 patients, there were 6 patients with lateral crural stolidity, 3 patients with positive straight leg raising test, 45 patients with positive waist backward stretch test, and 8 patients with muscle weakness. All the patients performed dynamic X-ray scattering, magnetic resonance imaging, or computed tomography (CT) scan before the operation to define the pathological type and diseased region. The inclusion criteria were as follows: the main symptom was radicular pain or intermittent claudication; patients were diagnosed with single segmental lumbar spinal stenosis or with the mild second segmental lumbar spinal stenosis while lumbar disc herniation as the main symptom; and patients had mild and moderate articular process hyperplasia and/or ligamentum flavum hypertrophy. The patients with flank pain as the main symptom, unstable lumbar vertebrae and severe reduced vertebral canal volume, and/or degenerative scoliosis were excluded. This study was approved by the Ethics Committee of our hospital.
2.2. Surgical method
The posterolateral intervertebral foramen was selected as the surgical approach. The distance from skin to intervertebral foramen via the space between quadratus lumborum and musculus sacrospinalis was measured by CT images before the operation, and then served as the depth limit of first puncture. Patients were positioned prone to keep the waist backward protruding and posterolateral intervertebral space expanding enough. The intervertebral space was positioned using X-ray C-arm systems with normal perspective, and then the cross or oblique line paralleled to intervertebral space was marked away from posterior midline 12 to 16 cm using Kirschner wire, which determined the puncture position. After local or general anesthesia, a spinal needle was inserted and the localization was adjusted with a lateral view (Fig. 1). The core needle was removed and the guide wire was inserted. Endoscope was positioned through a working casing pipe that was inserted via a 0.7-cm skin incision centered on guide wire. The microvascular tissues of proximal and distal superior articular process, superior border of pedicle, and intervertebral foramen were coagulated using plasma radiofrequency at low temperature, then hyperplasia was removed using a high-speed grinding drill (Fig. 2). Next, the working casing was moved into canalis spinalis to find ligamentum flavum, and then ligamentum flavum was excised using rongeur. The localization of working casing pipe was reconfirmed with lateral view of fluoroscopy. The prominent intervertebral disc was stained using methylene blue, and then removed using pituitary rongeur under endoscope. The posterior longitudinal ligament was excised for patients with unsatisfactory decompression or lumbar spinal stenosis combined with central lumbar disc herniation. Meanwhile, intervertebral disc ablation decompression and intradiscal annuloplasty were performed. For patients with hemorrhage, hemostasis was performed as following: raising the height of transfusion bag and/or squeezing the transfusion bag; using plasma radiofrequency at low temperature; or imbedding dilating rods into working casing pipe and stopping the bleeding by compression for 5 min. After hemostasis, the incision was closed. Patients were discharged 2 to 3 days after surgery and performed strength exercises (Fig. 3).
Figure 1.
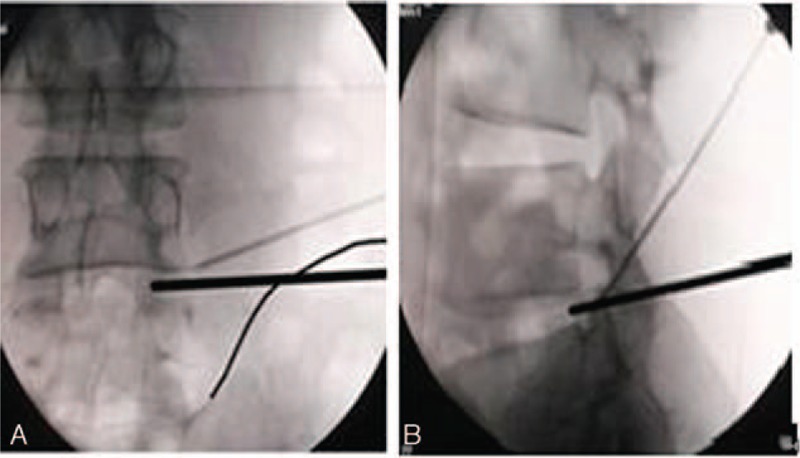
The localization of spinal needle. (A) Spinal needle entry into the tip and outer rim of superior articular process on anteroposterior fluoroscopic view. (B) Spinal needle entry into the tip of superior articular process on lateral fluoroscopic view (Note: Bold lines showing the conventional puncture path).
Figure 2.
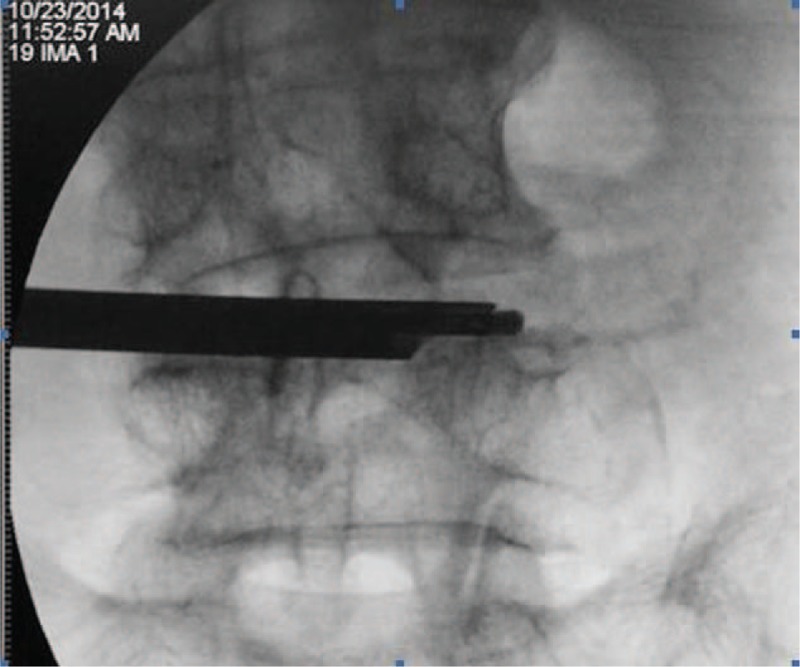
High-speed grinding drill entry into the contralateral articular process on anteroposterior fluoroscopic view.
Figure 3.
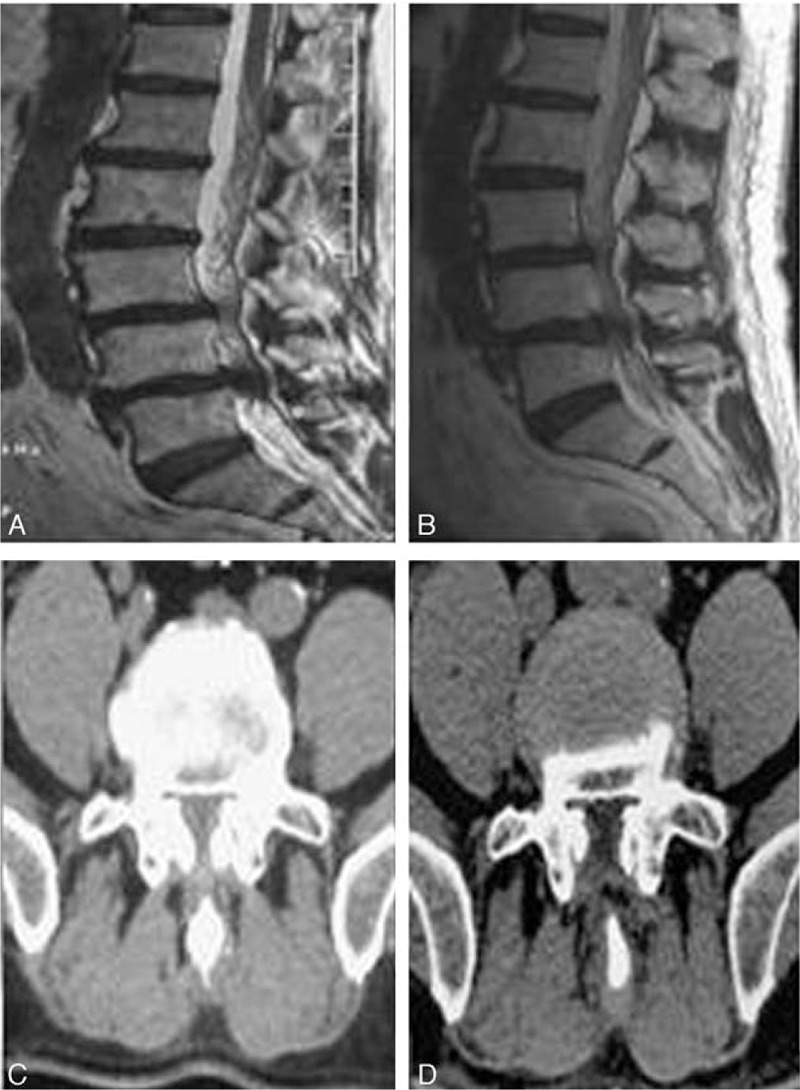
Preoperative and postoperative MRI and CT images of 1 patient (female, 77-year-old) with intermittent claudication. (A) Preoperative sagittal T2 MRI showing the pathology of L4/5 lumbar spinal stenosis; (B) postoperative 6 months sagittal T2 MRI showing the enlargement of lumbar spinal canal; (C) preoperative CT scan showing the reduction of lumbar spinal canal volume; and (D) postoperative 6 months CT scan showing the enlargement of lumbar spinal canal volume. CT = computed tomography, MRI = magnetic resonance imaging.
2.3. Outcome evaluation
Postoperative follow-up was performed by regular outpatient care, phone, and Email. Patient functions were evaluated using the visual analog scale (VAS) score of pain, Oswestry disability index (ODI), and MacNab criteria before and after operation. The data, including preoperative comorbidities, operation time, the quantity of bleeding, bed rest time, and intraoperative and postoperative complications, were recorded.
2.4. Statistical analysis
Data were analyzed by SPSS 23.0 statistical analysis software (IBM Corporation, New York, NY). The continuous variables were expressed as mean ± standard deviation and analyzed using paired t test and rank-sum test. A value of P < 0.05 was considered significant.
3. Results
3.1. Clinical characteristics
Of the 64 patients, there were 36 patients with central lumbar stenosis, 26 patients with lateral recess stenosis, and 2 patients with lumbar foraminal stenosis. In total, 54 patients was diagnosed with single segmental lumbar spinal stenosis, including 2 patients with L3/4, 46 patients with L4/5, and 11 patients with L5/S1, as well as 5 patients was diagnosed with 2 segmental lumbar spinal stenosis, including 4 patients with L3/4 and L4/5, and 1 patient with L4/5 and L5/S1.
3.2. Postoperative outcomes
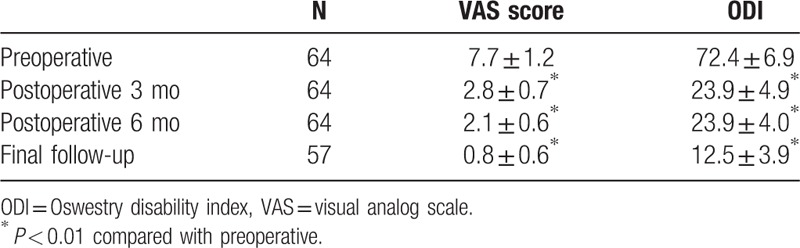
The operation time was 55 to 106 min with the average of 78 min, the average quantity of bleeding was 20 mL, and bed rest time was 6 h to 3 days. All the operations were performed during the daytime. Patients who had no complications postoperative 6 h were discharged home. All patients were followed-up for 4 months to 5 years with an average of 36 months. The mean preoperative VAS score was 7.7 ± 1.2, while postoperative 3 months, 6 months, and final follow-up VAS scores were 2.8 ± 0.7, 2.1 ± 0.6, and 0.8 ± 0.6, respectively (Table 1). The mean preoperative ODI score was 72.4 ± 1.2, while postoperative 3 months, 6 months, and final follow-up ODI scores were 29.7 ± 4.9, 23.9 ± 4.0, and 12.5 ± 3.9, respectively (Table 1). Compared with preoperation, postoperative average VAS and ODI were significantly reduced after operation for 3 months, 6 months, and the final follow-up (March 2016), respectively (P < 0.001, Table 1). Based on MacNab criteria, 47 patients (73.4%) had good results, 7 patients (10.9%) had fair results, and 3 patients (4.6%) had poor results.
Table 1.
VAS pain score and ODI score preoperatively, at 3 months, at 6 months, and at the final follow-up.
3.3. Reoperations and complications
No perioperative deaths were found in this study. Intraoperative dural laceration was found in 1 patient and healed on its own without postoperative treatment. During the follow-up period, 5 patients relapsed, among which, 2 patients underwent open spinal decompression and fusion and 3 patients received conservative treatment. In addition, 3 patients had poor therapeutic effect, then 1 patient underwent open spinal decompression and fusion and 2 patients refused the second operation due to cardiopulmonary disorders. No complications such as permanent nerve root, epidural hematoma, or superficial infection were found.
4. Discussion
The increasing number of patients with lumbar spinal stenosis underwent surgical treatment due to the advances in the surgical method.[12–14] Compared with traditional discectomy, PETLD had some advantages such as clearer operative field, few trauma, and quick recovery.[15] In the present study, all the patients selected the surgical approach of posterolateral intervertebral foramen. Then, the excision of the bottom of articular process and superior border of vertebral pedicle was performed using high-speed grinding drill,[16] which enhanced the intervertebral foramen and reduced the stress of nerve root. The pituitary rongeur under endoscope was used to excise ligamentum flavum through dilated intervertebral foramen channel, which expanded the volume behind the vertebral canal. Subsequently, the prominent intervertebral disc was removed, and the posterior longitudinal ligament was excised for patients combined with central lumbar disc herniation. After operation for 3 months, 6 months, and the final follow-up, the mean VAS and ODI were significantly reduced and the excellent and good rate reached 73.4% at the final follow-up. In addition, we found 1 patient with intraoperative dural laceration, 5 patients with relapse, and 3 patients with poor curative effect. There were no complications in these patients.
Puncture technique, removing articular process using high-speed grinding drill, and effective hemostasis were closely associated with the safety of percutaneous endoscopic decompression. Compared with traditional surgical approach of intervertebral foramen during PETLD, we lengthened 1 to 2 cm puncture distance in this study, which aimed to move puncture point into ventral side and then make endoscope paralleled to intervertebral space. This method ensured the completion of the removal of anterior nucleus pulposus and posterior longitudinal ligament, as well as the excision of the hyperplastic articular process and ligamentum flavum. However, the greater risk may be caused by this puncture method including: excessive puncture depth may lead to the leakage of cerebrospinal fluid and nerve injury due to damaged dural sac; excessive puncture height may induce the damaged nerve root; and the lower puncture may injure vena cava of vertebral ventral or intestinal canal. In the present study, the depth and angle of puncture were measured by CT images before the operation to avoid these injuries. In addition, only 1 patient suffered with intraoperative dural laceration in this study, which was related to the usage of high-speed grinding drill and plasma radiofrequency at low temperature. The high-speed grinding drill and plasma radiofrequency at low temperature could ensure the safe and effective decompression under clear view. Because the terminal branch of lumbar artery crossed from superior articular process, the plasma radiofrequency at low temperature was used to coagulate the microvascular tissues of proximal and distal superior articular process, superior border of pedicle and intervertebral foramen, which avoided the bleeding caused by high-speed grinding drill. Meanwhile, the flexible high-speed grinding drill prevented the damage of dural sac and nerve root.
After final follow-up, we found 73.4% of the excellent and good rate in patients with lumbar spinal stenosis who underwent percutaneous endoscopic transforaminal lumbar spinal canal decompression. Previous studies reported that the excellent and good rate was 86.5% using percutaneous interlaminar approach in patients with central spinal canal stenosis,[17] 89% to 92% using percutaneous interlaminar approach,[18,19] and 82% using intervertebral foramen approach[20] in patients with lateral recess stenosis. Compared with these studies, the excellent and good rate using the surgical approach of posterolateral intervertebral foramen was lower in this study. This may be explained that patients with lumbar spinal stenosis were old people with long disease time and complex pathologic changes. In addition, the excellent and good rate was 69.8% in the prior 40 patients, while it was 82.5% in the later 24 patients, which was consistent with previous studies. This result suggested that the excellent and good rate was associated with the selection of surgical indications and mature surgical skills. The incidence rates of postoperative complications such as infection,[20] dural laceration,[21] and postoperative recurrence[22] were lower in PETLD than those in the traditional open surgery. In this study, 5 patients relapsed after operation for 6 months and they had a tendency of instable lumbar vertebra before operation. Two patients performed open spinal decompression and fusion, and 3 patients received conservative treatment according different aggravated degree. Thus, we suggested that percutaneous endoscopic transforaminal lumbar spinal canal decompression could be applied in patients who diagnosed with single segmental lumbar spinal stenosis, mild and moderate articular process hyperplasia and/or ligamentum flavum hypertrophy, or the mild second segmental lumbar spinal stenosis, while percutaneous endoscopic transforaminal lumbar spinal canal decompression was not unfit for patients with severe reduced vertebral canal volume and/or degenerative scoliosis, and spinal canal stenosis caused by unstable lumbar vertebrae. Unfortunately, due to the retrospective nature and small sample of this study, a prospective study with larger sample size is necessary to confirm the results.
5. Conclusions
This retrospective study suggests that percutaneous endoscopic transforaminal lumbar spinal canal decompression is an easy, safe, and effective minimally invasive surgery for patients with lumbar spinal stenosis.
Footnotes
Abbreviations: CT = computed tomography, ODI = Oswestry disability index, PETLD = percutaneous endoscopic transforaminal lumbar discectomy, VAS = visual analog scale.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Feeney R. Lumbar spinal stenosis. J Pain Palliat Care Pharmacother 2016;30:150–2. [DOI] [PubMed] [Google Scholar]
- [2].Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. Jama 2010;303:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 2008;358:794–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ragab AA, Fye MA, Bohlman HH. Surgery of the lumbar spine for spinal stenosis in 118 patients 70 years of age or older. Spine 2003;28:348–53. [DOI] [PubMed] [Google Scholar]
- [5].Rahman M, Summers L, Richter B, et al. Comparison of techniques for decompressive lumbar laminectomy: the minimally invasive versus the “classic” open approach. Minim Invasive Neurosurg 2008;51:100–5. [DOI] [PubMed] [Google Scholar]
- [6].Lee S-H, Chung S-E, Ahn Y, et al. Comparative radiologic evaluation of percutaneous endoscopic lumbar discectomy and open microdiscectomy: a matched cohort analysis. Mt Sinai J Med 2006;73:795–801. [PubMed] [Google Scholar]
- [7].Lee S, Kim S-K, Lee S-H, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J 2007;16:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee DY, Lee S-H. Learning curve for percutaneous endoscopic lumbar discectomy. Neurol Med Chir 2008;48:383–9. [DOI] [PubMed] [Google Scholar]
- [9].Hoogland T, Schubert M, Miklitz B, et al. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine 2006;31:E890–7. [DOI] [PubMed] [Google Scholar]
- [10].Kafadar A, Kahraman S, Akbörü M. Percutaneous endoscopic transforaminal lumbar discectomy: a critical appraisal. Minim Invasive Neurosurg 2006;49:74–9. [DOI] [PubMed] [Google Scholar]
- [11].Wong C, Loke W. Percutaneous endoscopic transforaminal lumbar discectomy—an early experience. Malays Orthop J 2007;1:1–4. [Google Scholar]
- [12].Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am 1995;77:1036–41. [DOI] [PubMed] [Google Scholar]
- [13].Jönsson B, Annertz M, Sjöberg C, et al. A prospective and consecutive study of surgically treated lumbar spinal stenosis: part I: clinical features related to radiographic findings. Spine 1997;22:2932–7. [DOI] [PubMed] [Google Scholar]
- [14].Katz JN, Lipson SJ, Chang LC, et al. Seven- to 10-year outcome of decompressive surgery for degenerative lumbar spinal stenosis. Spine 1996;21:92–7. [DOI] [PubMed] [Google Scholar]
- [15].Tzaan W. Transforaminal percutaneous endoscopic lumbar discectomy. Chang Gung Med J 2007;30:226–34. [PubMed] [Google Scholar]
- [16].Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine 2002;27:722–31. [DOI] [PubMed] [Google Scholar]
- [17].Komp M, Hahn P, Merk H, et al. Bilateral operation of lumbar degenerative central spinal stenosis in full-endoscopic interlaminar technique with unilateral approach: prospective 2-year results of 74 patients. J Spinal Disord Tech 2011;24:281–7. [DOI] [PubMed] [Google Scholar]
- [18].Ruetten S, Komp M, Merk H, et al. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study: clinical article. J Neurosurg 2009;10:476–85. [DOI] [PubMed] [Google Scholar]
- [19].Ruetten S, Komp M, Hahn P, et al. Decompression of lumbar lateral spinal stenosis: full-endoscopic, interlaminar technique. Oper Orthop Traumatol 2013;25:31–46. [DOI] [PubMed] [Google Scholar]
- [20].Kambin P, Casey K, O’Brien E, et al. Transforaminal arthroscopic decompression of lateral recess stenosis. J Neurosurg 1996;84:462–7. [DOI] [PubMed] [Google Scholar]
- [21].Soliman HM. Irrigation endoscopic decompressive laminotomy. A new endoscopic approach for spinal stenosis decompression. Spine J 2015;15:2282–9. [DOI] [PubMed] [Google Scholar]
- [22].Telfeian AE. Transforaminal endoscopic discectomy with foraminoplasty for the treatment of spondylolisthesis. Pain Phys 2014;17:E703–8. [PubMed] [Google Scholar]


