Abstract
Background:
Panitumumab, a fully human monoclonal antibody targeting epidermal growth factor receptor, is used in combination with chemotherapy for patients with metastatic colorectal cancer (mCRC). However, the effects of panitumumab in combination with irrinotecan-based chemotherapy remain uncertain. Therefore, we conducted this meta-analysis to assess the efficacy and safety of combination treatment of panitumumab plus chemotherapy in the treatment of mCRC.
Methods:
By searching electronic databases (PubMed, Embase, and Web of Science), all clinical trials which assessed the effects of panitumumab plus irrinotecan-based chemotherapy in mCRC would be included. Main outcome measures included progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and adverse events. Pooled estimates were calculated by a fixed-effects model or random-effects model, according to the heterogeneity among the included studies.
Results:
Eleven trials with a total number of 1338 patients met the inclusion criteria and were included in this meta-analysis. The combination treatment of panitumumab and irrinotecan-based chemotherapy was associated with a median PFS of 5.83 months, OS of 11.15 months, and ORR of 33%. Subgroup analysis showed that, in the first-line and second-line treatment, the combination therapy for PFS was 9.27 and 5.01 months, for OS was 8.87 and 11.68 months, and for ORR was 61% and 26%, respectively. In the wild-type and mutant KRAS populations, the combination therapy for PFS was 5.76 and 5.27 months, for OS was 11.15 and 10.64 months, and for ORR was 37% and 18%, respectively. Moreover, combination therapy also induced an incidence of 56% treatment-related adverse events.
Conclusion:
Panitumumab plus irrinotecan-based chemotherapy is effective and well-tolerated in the treatment of patients with mCRC, especially in those with wild-type KRAS tumors.
Keywords: chemotherapy, irinotecan, meta-analysis, metastatic colorectal cancer, panitumumab
1. Introduction
Worldwide, one million patients are diagnosed annually with colorectal cancer (CRC), and 50% of them will develop metastatic disease.[1] Over the past decades, owning to the introduction of conventional chemotherapeutic agents (irinotecan and oxaliplatin), and biologic agents (bevacizumab, cetuximab, and panitumumab), the median survival of patients with metastatic CRC (mCRC) have been prolonged.[2–6]
Several clinical trials have demonstrated the beneficial effects of adding bevacizumab or cetuximab to chemotherapy in the treatment of patients with mCRC.[4,6,7] However, approximately 70% of patients would have disease progression after the first-line therapy, and will receive at least 1 subsequent line of systemic treatment.[5] Oxaliplatin-based therapy is usually recommended as the initial treatment for patients with mCRC. And for those patients who have disease progression, irinotecan-based regiments with biologic therapies would be used as the second-line therapy options.[8,9] Irinotecan could be administered either alone or in combination with leucovorin and 5-fluorouracil.
Panitumumab is a fully human monoclonal antibody that targets epidermal growth factor receptor (EGFR), and it has been used as monotherapy for patients with wild-type (WT) KRAS tumors who have disease progression after the standard chemotherapy.[10,11]KRAS gene status is a predictive marker for the treatment effects of anti-EGFR therapies in mCRC[12]; patients with WT KRAS tumors have beneficial effects, whereas those with mutant (MT) KRAS tumors do not derive clinical benefit.[6,9,13–15] We conducted this meta-analysis to evaluate the efficacy and safety of panitumumab in combination with irrinotecan-based chemotherapy regimens for mCRC.
2. Materials and methods
The ethical approval is not necessary for the meta-analysis.
2.1. Search strategy
We conducted a comprehensive literature search in PubMed, Embase, and Web of Science database from inception through December 12, 2015. The literature search was updated on September 12, 2016. The following search terms were used: ((“secondary”[Subheading] OR “secondary”[All Fields] OR “metastatic”[All Fields]) AND (“colorectal neoplasms”[MeSH Terms] OR (“colorectal”[All Fields] AND “neoplasms”[All Fields]) OR “colorectal neoplasms”[All Fields] OR (“colorectal”[All Fields] AND “cancer”[All Fields]) OR “colorectal cancer”[All Fields])) AND (“panitumumab”[Supplementary Concept] OR “panitumumab”[All Fields]) AND (“irinotecan”[Supplementary Concept] OR “irinotecan”[All Fields]). In addition, we also manually checked the reference lists of identified studies to include other potentially eligible trials.
2.2. Review strategy
We used the Endnote bibliographic software to build up an electronic library of citations identified in the literature searches. The literature searches of PubMed, Embase, and Web of Science database were conducted using Endnote, and duplicate records were deleted. Two independent reviewers (SZ and QC) were trained to perform the title/abstract review, and full-text review. Disagreements between the reviewers were resolved by consensus and discussion.
2.3. Inclusion criteria
All clinical trials that assessed the efficacy and safety of panitumumab plus irinotecan-based chemotherapy for mCRC were considered eligible for analysis. The following inclusion criteria were applied: the study population was patients with histologically or cytologically confirmed mCRC; patients were treated with panitumumab and irinotecan-based chemotherapy; results reported data on progression-free survival (PFS), overall survival (OS), and overall response rate (ORR), and adverse events.
2.4. Data extraction
We created a standardized Excel file for data extraction. Two independent investigators (MC and ZW) extracted the following data from the included studies: leader author, year of publication, number of patients, characteristics, the treatment regimens, line of treatment, the status of KRAS gene, the median duration with 95% confidence interval (CI) of PFS and OS, ORR, and incidence of adverse events. When several publications from the same trial were present, we only included the most informative article to avoid duplication of information.
2.5. Statistical analysis
Before the original data were synthesized, we tested the heterogeneity across the studies using I2 statistics and Cochrane Q chi-square test.[16] An I2value of <25, 25 to 50, 50 to 75, or >75% indicated no, low, moderate or high heterogeneity existed across the studies.[17] Studies with an I2 >50% or a P value <0.1 were considered to have significant heterogeneity.[18] If significant heterogeneity was identified, a random-effects model was used to pool the estimate[19]; otherwise, the fixed-effects model was preferred to summarize the data.[20] When considerable heterogeneity was found, we also performed sensitivity analysis or subgroup analysis to explore the potential sources of heterogeneity. Because the number of included studies was less than 10, the assessment of publication bias was not performed. A P value of less than 0.05 was considered statistically significant. All analyses were performed using STATA version 12.0 (Stata Corporation, College Station, TX).
3. Results
3.1. Study identification and selection
The selection process is presented in Fig. 1. The initial database search yielded 1062 records. Of them, 657 were excluded because of duplicate records, and 392 were excluded based on title/abstracts review for various reasons (abstract, letters, case report, reviews, or unrelated with our topics). Also, the remaining 13 studies were reviewed for the full-text information evaluation, 2 of which were excluded because 1 did not provide outcome of interest and 1 assessed the monotherapy efficacy of panitumumab. Finally, 9 studies[21–31] were included in this meta-analysis.
Figure 1.
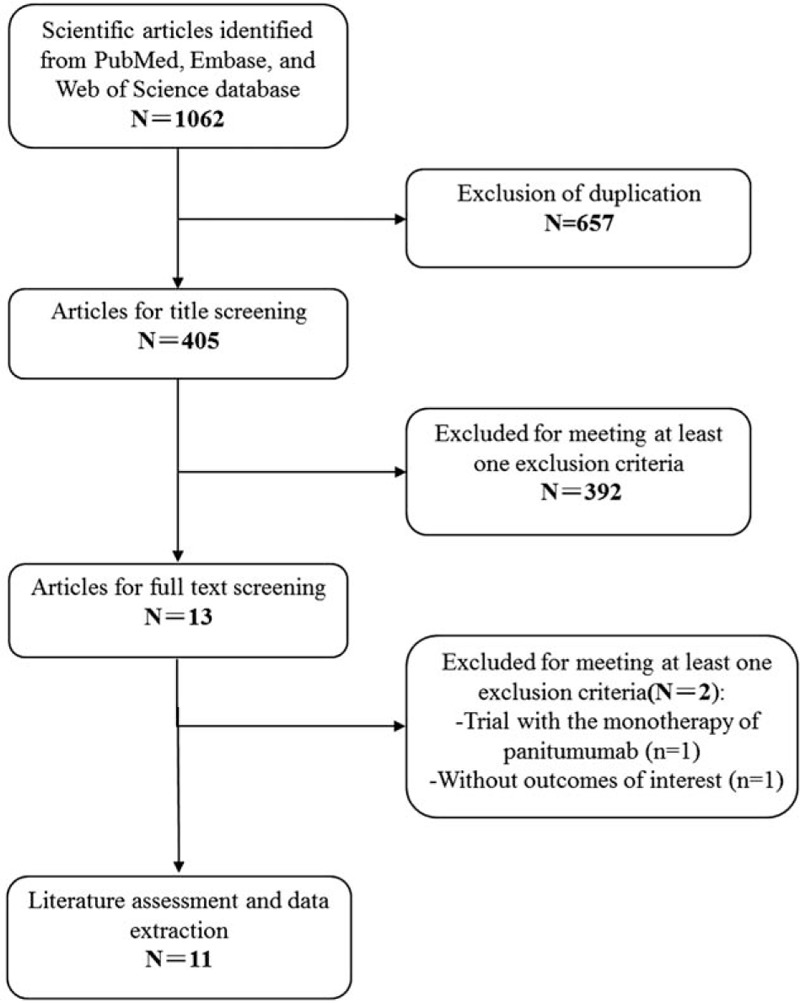
Eligibility of studies for inclusion in meta-analysis.
3.2. Characteristics of eligible studies
The study characteristics are shown in Table 1. These studies were published between 2007 and 2016. The sample size ranged from 35 to 541 (total of 1338 patients). Of the 9 included studies, 2 were in Japan,[30,31] 1 was conducted in USA,[21] 1 in France,[22] 1 in Germany,[24] 1 in China,[25] 1 in Spain,[26] and the remaining 5 were conducted in multicenter cities.[23,27–29] Among the 7 trials that tested the KRAS status, 1122 patients had known KRAS status, including 706 (62.9%) patients with WT KRAS and 416 (39.8%) patients with MT KRAS. The dosage of panitumumab was 6 mg/kg in most of the included trials, except for 2 trials, in which the dosages of panitumumab were 9 mg/kg[26] and 2.5 mg/kg,[28] respectively. In 3 of the 9 trials, the combination treatment of panitumumab plus irinotecan-based chemotherapy was used as a first-line therapy, and in the remaining 6 trials, it was used as a second-line therapy.
Table 1.
Baseline characteristics of patients in the trials included in the meta-analysis.
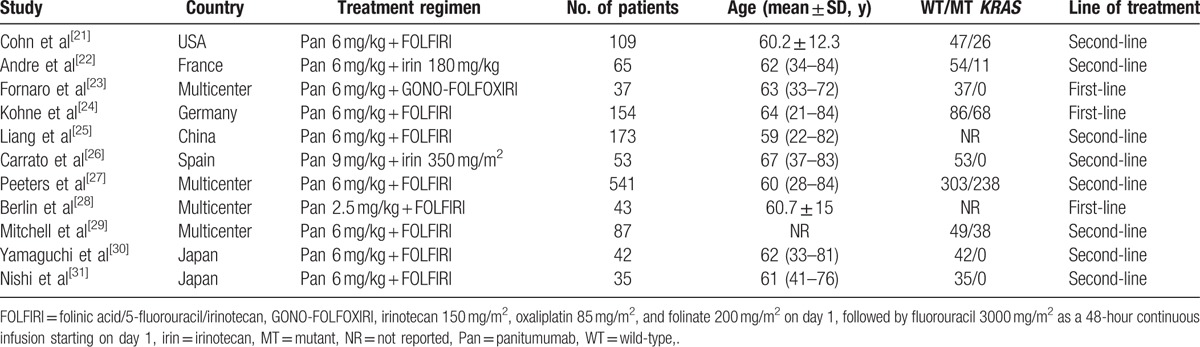
In the trial conducted by Mitchell et al,[29] patients with mCRC were divided into 2 groups: panitumumab 6 mg/kg + FOLFIRI every 2 weeks, and panitumumab 9 mg/kg + irinotecan every 3 weeks. Since the dosage of panitumumab in most of the trials was 6 mg/kg, we only extracted the interest data from patients in the prior group.
3.3. Progression-free survival
All the studies reported the data of PFS.[21–31] The median PFS among these studies ranged from 2.70 to 11.3 months. Pooled estimates indicated that combination treatment of panitumumab and irinotecan-based chemotherapy had a median PFS of 5.83 months (95% CI 4.78, 7.11 months) (Fig. 2). There was significant heterogeneity among the included studies (I2 = 91.9%, P < 0.001). Therefore, we performed sensitivity analysis to explore potential sources of heterogeneity. When we excluded the trial conducted by Fornaro et al,[23] which observed the longest median PFS of 11.3 months, the heterogeneity disappeared (I2 = 45.7%, P = 0.21). However, the pooled estimates did not change substantially (ES = 5.53, 95% CI 4.69, 6.52 months).
Figure 2.
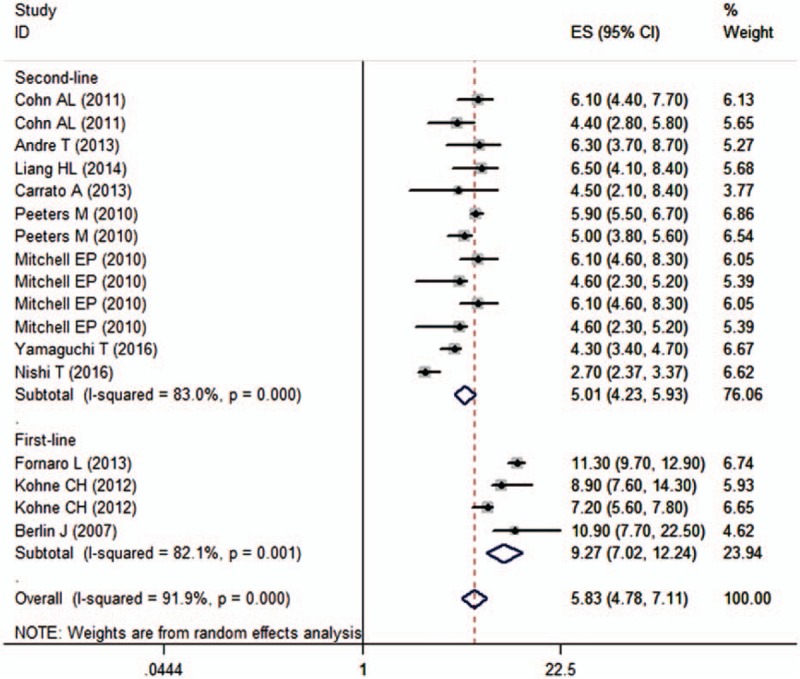
Progression-free survival (PFS) according to line of treatment.
We also conducted subgroup analysis based on line of treatment and KRAS status. The pooled results of studies showed that combination treatment of panitumumab and irinotecan-based chemotherapy was associated with a median PFS of 9.27 months (95% CI 7.02, 12.24 months) and 5.01 months (95% CI 4.23, 5.93 months) when it was used as the first-line and second-line therapy, respectively (Fig. 2). Moreover, the median PFS for patients with WT KRAS tumors and MT KRAS tumors was 5.76 months (95% CI 4.41, 7.51 months) and 5.27 months (95% CI 3.89, 7.13 months), respectively.
3.4. Overall survival
Nine studies reported the data of OS.[21,22,24–27,29–31] The median OS among these studies ranged from 6.3 to 15.4 months. The aggregated results indicated that mCRC patients treated with panitumumab in combination with irinotecan-based chemotherapy had a median OS of 11.15 months (95% CI 9.47, 13.13 months) (Fig. 3). There was significant heterogeneity among the included studies (I2 = 82.3%, P < 0.001). Thus, we conducted sensitivity analysis to explore potential source of heterogeneity. Exclusion of any single trial did not change the pooled estimates substantially, which ranged from 10.86 months (95% CI 9.15, 12.90 months) to 11.77 months (95% CI 10.18, 13.59 months). However, significant heterogeneity was still present.
Figure 3.
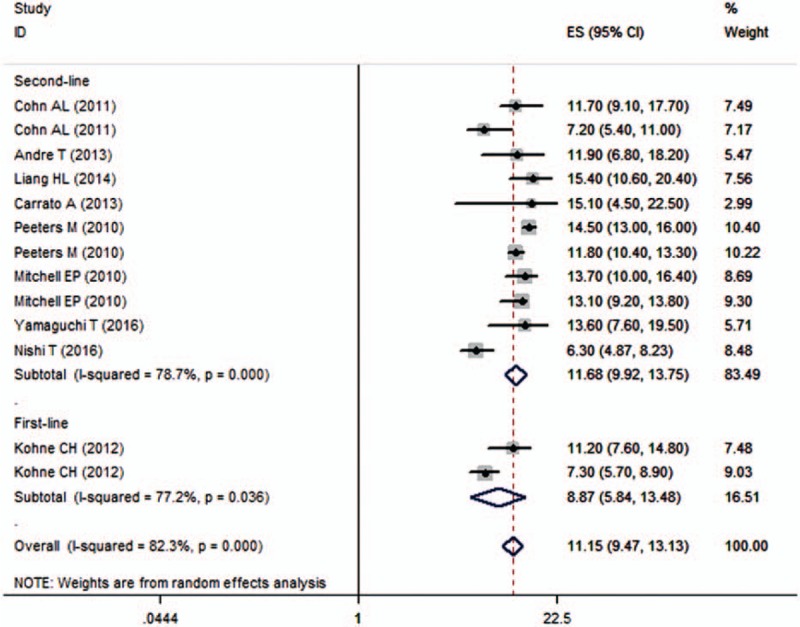
Overall survival (OS) according to line of treatment.
Subgroup analysis based on line of treatment showed that panitumumab in combination with irinotecan-based chemotherapy was associated with a median OS of 8.87 months (95% CI 5.84, 13.48 months) and 111.68 months (95% CI 9.92, 13.75 months) when it was used as the first-line and second-line therapy, respectively (Fig. 3). Subgroup analysis based on KRAS status showed that the OS for patients with WT and MTKRAS tumors were 11.15 months (95% CI 8.55, 14.54 months) and 10.64 months (95% CI 8.35, 13.56 months), respectively.
3.5. Overall response rate
Ten studies reported the data of ORR.[21–27,29–31] The ORR among these studies ranged from 8% to 89%. The pooled results indicated that the ORR for patients with mCRC was 33% (95% CI 22%, 45%) (Fig. 4). There was significant heterogeneity among the included studies (I2 = 95.8%, P < 0.001). Thus, we conducted sensitivity analysis to explore potential source of heterogeneity. Exclusion of any single trial did not change the pooled estimates substantially, which ranged from 29% to 36%. However, the heterogeneity was still present.
Figure 4.
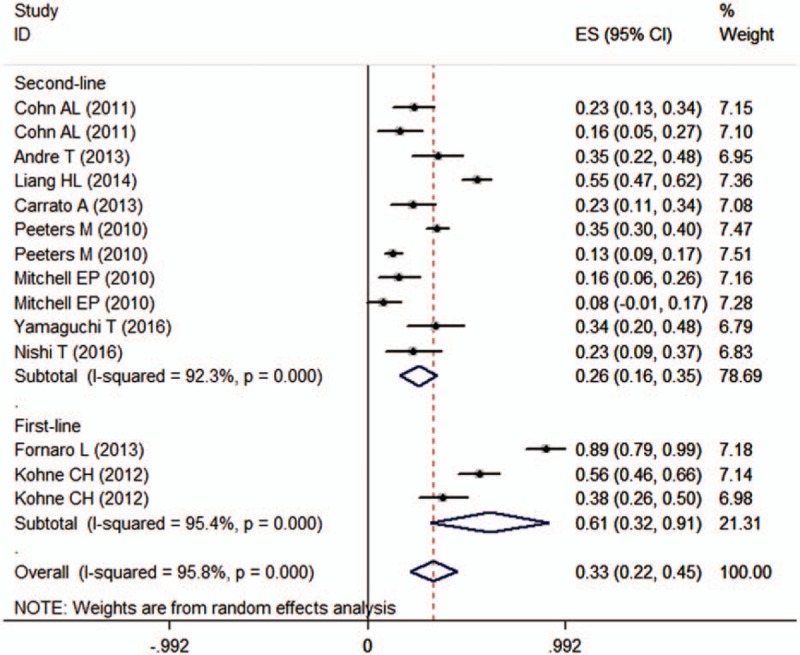
Overall response rate (ORR) according to line of treatment.
We also performed subgroup analysis based on line of treatment and KRAS status. Pooled results showed that the ORR of panitumumab plus irinotecan-based chemotherapy was 61% (95% CI 32%, 91%) and 26% (95% CI 16%, 35%), respectively, when the combination treatment was used as first-line and second-line therapy (Fig. 4). In patients with WT and MT KRAS tumors, the ORR was 37% (95% CI 23%, 52%) and 18% (95% CI 8%, 28%), respectively.
3.6. Adverse events
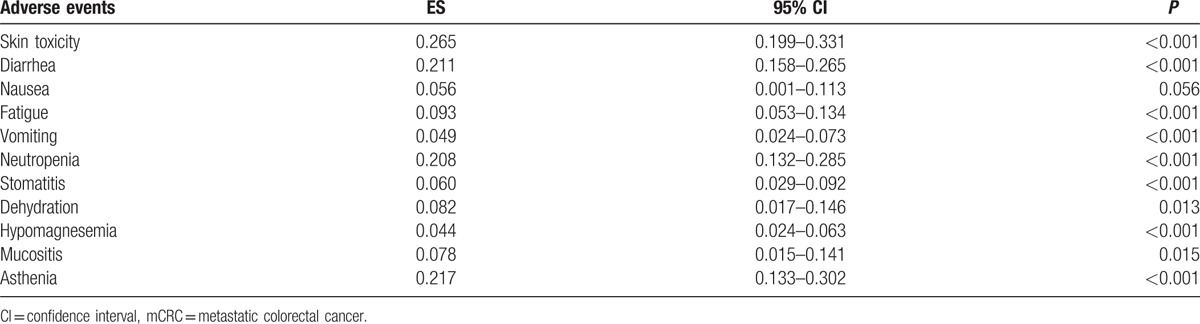
Ten studies reported the data of adverse events.[21,22,24–31] Among the studies included in this meta-analysis, the incidence of adverse events induced by the combination treatment of panitumumab plus irinotecan-based chemotherapy ranged from 20.8% to 77.4%. Pooled estimates showed that the incidence of adverse events was 56% (95% CI 49%, 64%) (Fig. 5). The most frequently observed grade 3 or 4 adverse events are listed in Table 2.
Figure 5.
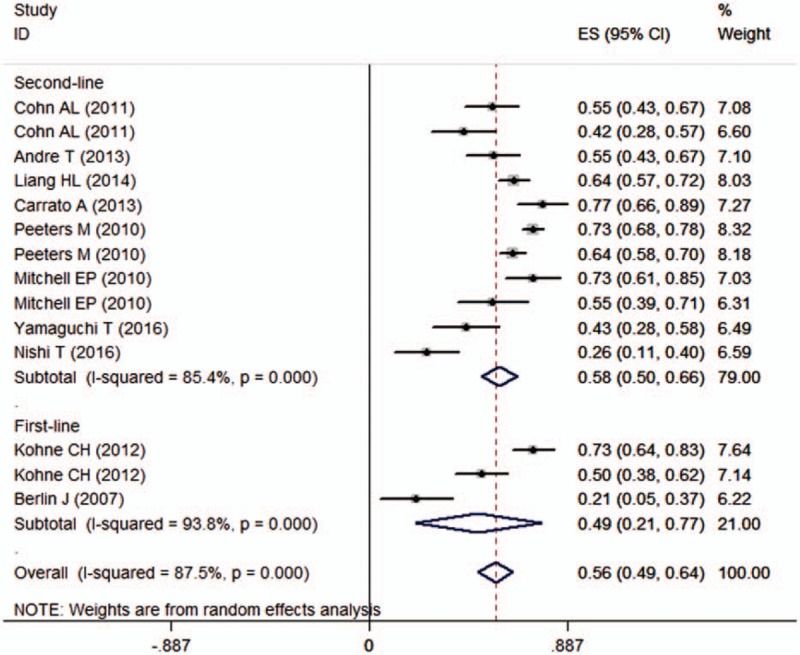
Forest plot showing the incidence of adverse events induced by panitumumab plus irinotecan-based chemotherapy.
Table 2.
Summary of the incidence of adverse events in patients with mCRC.
We also performed subgroup analysis based on line of treatment and KRAS status. Pooled results showed that the incidences of adverse events in the first-line and second-line therapy were 49% (95% CI 21%, 77%) and 58% (95% CI 50%, 66%), respectively. The incidences of adverse events in the WT and MT KRAS subpopulations were 61% (95% CI 49%, 73%) and 54% (95% CI 43%, 64%), respectively.
4. Discussion
The present study was a meta-analysis with the objective of evaluating the efficacy and safety of panitumumab in combination with irinotecan-based chemotherapy for mCRC. The current meta-analysis demonstrated that combination treatment significantly improved PFS, OS, and ORR in patients with mCRC, especially in those with WT KRAS tumors. Patients treated with combination therapy also had well-tolerated adverse events.
To the best of our knowledge, this study is the first comprehensive meta-analysis to evaluate the efficacy and safety of combination treatment of panitumumab plus irinotecan-based chemotherapy in patients with mCRC. The results of the combined treatment were comparable with either of panitumumab or irinotecan administered as single agent. Several phase 3 trials evaluated the value of irinotecan (300–350 mg/m2) in patients with mCRC who previously received the fluoropyrimidine-based chemotherapy.[3,32,33] In these clinical trials, they achieved an ORR of 7% to 16%, median time-to-progression of 2.8 to 4.4 months, and OS of 9.9 to 14.3 months.[3,32,33] Similarly, another phase 3 trial assessed the efficacy of panitumumab (6 mg/kg) in patients who previously received 2 or 3 prior chemotherapy regimens, including fluoropyrimidine, oxaliplatin, and irinotecan.[34] The ORR was 10% and median PFS was 3.2 months.[34] In another trial that included patients with WT KRAS tumors, the treatment effect was improved.[13] In that trial, patients were previously treated with oxaliplatin and irinotecan, and ORR was 17%, and median PFS and OS were 2.8 and 8.1 months, respectively.[13]
In this meta-analysis, we found that the median PFS was 5.83 months for all the mCRC population. The median PFS among the included studies varied greatly, which ranged from 2.7 to 11.3 months. The longest PFS was observed in the study conducted by Fornaro et al[23] in which panitumumab combined with FOLFOXIRI (irinotecan 150 mg/m2, oxaliplatin 85 mg/m2, folinate 200 mg/m2on day 1, followed by fluorouracil 3000 mg/m2 as 48-hour continuous infusion starting on day 1) was used as first-line treatment in mCRC patients with WT KRAS tumors. After a median follow-up of 17.7 months, the median PFS was reported to be 11.3 months (95% CI 9.7–12.9 months).[23] Another phase 2 trial also used the combined treatment of panitumumab with FOLFOXIRI; however, they only observed a median PFS of 6.1 months in the patients with WT KRAS tumors.[21] In that trial, they enrolled 109 mCRC patients who had failed to the first-line treatment with oxaliplatin-based chemotherapy plus bevacizumab. Of them, 64 patients were tested with WT KRAS tumors and 45 patients were tested with MT KRAS tumors. A median PFS of 6.1 months was observed in patients with WT KRAS tumors compared with 4.4 months in patients with MT KRAS tumors, which indicated an advantage for patients with WT KRAS tumors.[21]
Regarding the OS, we found a median OS of 11.15 months for the combination treatment of panitumumab and irinotecan-based chemotherapy. Among the included studies, the median OS for patients with WT KRAS tumors was almost similar, which ranged from 6.3 months to 15.1 months, whereas the OS for MT KRAS tumors varied substantially, which ranged from 7.2 months to 14.5 months. The longest OS for patients with MT KRAS tumors was observed in a phase 3 trial conducted by Peeters et al[27] in which mCRC patients were administered with panitumumab plus FOLFIRI every 2 weeks. Patients with 1 prior chemotherapy regimen for mCRC and Eastern Cooperative Oncology Group performance status 0 to 2 were enrolled in that trial. These patients achieved a median OS of 14.5 months.[27] Similar treatment schedule was administered in another phase 2 trial; however, the median OS was only 7.2 months.[21] In that trial, panitumumab plus FOLFIRI was administered in mCRC patients who had failure of first-line treatment with oxaliplatin-based chemotherapy plus bevacizumab.[21] However, they observed a median OS of 7.2 months and 11.7 months in the MT KRAS and WT KRAS tumors, respectively.[21] These results confirmed that WT KRAS subpopulations would achieve better survival outcomes from combination therapy of panitumumab and irinotecan-based chemotherapy than MT KRAS subpopulations.
Similarly, patients with different KRAS tumor status had different ORR. The ORR for all the mCRC populations was 33%, and for patients with WT KRAS and MT KRAS tumors was 37% and 18%, respectively. Among the included studies, the ORR for WT KRAS tumors ranged from 16% to 89%, and for MT KRAS tumors it ranged from 8% to 38%. The highest ORR for WT KRAS tumors was observed in the trial that used panitumumab plus FOLFOXIRI as first-line treatment,[23] whereas the lowest ORR was observed in the trial that used panitumumab plus irinotecan as second-line treatment.[22] For patients with MT KRAS tumors, a phase 2 trial that used panitumumab plus FOLFIRI (irinotecan 180 mg/m2 and leucovorin 400 mg/m2 followed by a 5-fluorouracil 400 mg/m2 bolus and a 2400–3000 mg/m2 continuous infusion) as first-line achieved the highest ORR of 38%.[24] However, in another trial that used the same treatment regimen of panitumumab plus FOLFIRI in the second-line, the ORR was only 8%,[29] which indicated that combined therapy of panitumumab and irinotecan would result in a higher ORR when it was used as first-line treatment rather than second-line treatment.
In this meta-analysis, we found that the incidence of adverse events was 56% for all the mCRC population. This result was comparable with the rate reported in the combined treatment of cetuximab plus irinotecan (62.1%).[9] The most common grades 3 to 4 adverse events associated with panitumumab and irinotecan-based chemotherapy regimens were skin toxicity, followed by asthenia, diarrhea, and neutropenia. Skin rash toxicities are regarded as a class-based effect of EGFR inhibitors. A previous trial has proved that pre-emptive management of skin toxicities is associated with a significantly lower incidence of ≥grade 2 toxicities without influencing the antitumor activity.[35]
There were several potential limitations in this meta-analysis that should be taken into account. First, this meta-analysis was conducted of 9 trials, most of which had relatively small sample size. Trials with small sample size are more likely to result in an overestimate of the treatment effects when compared with larger trials. Second, substantial heterogeneity was tested among the included studies in this meta-analysis. However, it should not be surprising when considering the variations in patients’ characteristics (age, race, primary tumor type, and site of metastatic disease), treatment schedule, and methods for KRAS status test. These factors may be responsible for the heterogeneity and have a potential impact on the pooled estimates. Third, because most of the included studies were observational trials, we could not exclude the possibility of information and selection bias, and unidentified confounders. Fourth, due to the lack of control arms, we cannot assess whether the combination treatment of panitumumab plus irinotecan-based chemotherapy was superior to panitumumab or irinotecan-based chemotherapy alone.
In conclusion, our study showed that panitumumab, in combination with irinotecan-based chemotherapy, would be a promising option for patients with mCRC, especially in those with WT KRAS tumors, since it produces pronged PFS and OS with an acceptable toxicity. However, owning to the limited data, whether combination treatment was superior to panitumumab or irinotecan-based chemotherapy alone still remains uncertain. Further well-design, larger-scale trials are needed to explore this issue.
Footnotes
Abbreviations: CI = confidence interval, CRC = colorectal cancer, EGFR = epidermal growth factor receptor, mCRC = metastatic colorectal cancer, ORR = overall response rate, OS = overall survival, PFS = progression-free survival.
QC and MC are equal contributors.
The authors report no conflicts of interest.
References
- [1].Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [DOI] [PubMed] [Google Scholar]
- [2].Cunningham D, Pyrhonen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet (London, England) 1998;352:1413–8. [DOI] [PubMed] [Google Scholar]
- [3].Fuchs CS, Moore MR, Harker G, et al. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol 2003;21:807–14. [DOI] [PubMed] [Google Scholar]
- [4].Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. [DOI] [PubMed] [Google Scholar]
- [5].Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229–37. [DOI] [PubMed] [Google Scholar]
- [6].Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–17. [DOI] [PubMed] [Google Scholar]
- [7].Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337–45. [DOI] [PubMed] [Google Scholar]
- [8].Abstracts of the 33rd ESMO (European Society for Medical Oncology) Congress. Stockholm, Sweden. September 12–16, 2008. Ann Oncol 2008;19(suppl 8):viii21–287. [DOI] [PubMed] [Google Scholar]
- [9].Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311–9. [DOI] [PubMed] [Google Scholar]
- [10]. Amgen. Vectibix Prescribing Information. Thousand Oaks, CA; Amgen: 2009. [Google Scholar]
- [11]. BV. AE. Vectibix Summary of Product Characteristics. Breda, the Netherlands; Amgen Europe BV: 2009. [Google Scholar]
- [12].Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757–65. [DOI] [PubMed] [Google Scholar]
- [13].Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626–34. [DOI] [PubMed] [Google Scholar]
- [14].Freeman DJ, Juan T, Reiner M, et al. Association of K-ras mutational status and clinical outcomes in patients with metastatic colorectal cancer receiving panitumumab alone. Clin Colorectal Cancer 2008;7:184–90. [DOI] [PubMed] [Google Scholar]
- [15].Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007;67:2643–8. [DOI] [PubMed] [Google Scholar]
- [16].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Armitage P, Berry G, Matthews J. Analysing means and proportions. Stat Methods Medical Res 2002;83–146. [Google Scholar]
- [19].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [20].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [21].Cohn AL, Shumaker GC, Khandelwal P, et al. An open-label, single-arm, phase 2 trial of panitumumab plus FOLFIRI as second-line therapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer 2011;10:171–7. [DOI] [PubMed] [Google Scholar]
- [22].Andre T, Blons H, Mabro M, et al. Panitumumab combined with irinotecan for patients with KRAS wild-type metastatic colorectal cancer refractory to standard chemotherapy: a GERCOR efficacy, tolerance, and translational molecular study. Ann Oncol 2013;24:412–9. [DOI] [PubMed] [Google Scholar]
- [23].Fornaro L, Lonardi S, Masi G, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol 2013;24:2062–7. [DOI] [PubMed] [Google Scholar]
- [24].Kohne CH, Hofheinz R, Mineur L, et al. First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol 2012;138:65–72. [DOI] [PubMed] [Google Scholar]
- [25].Liang HL, Hu AP, Li SL, et al. Combining bevacizumab and panitumumab with irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as second-line treatment in patients with metastatic colorectal cancer. Med Oncol 2014;31:976. [DOI] [PubMed] [Google Scholar]
- [26].Carrato A, Gomez A, Escudero P, et al. Panitumumab and irinotecan every 3 weeks is an active and convenient regimen for second-line treatment of patients with wild-type K-RAS metastatic colorectal cancer. Clin Transl Oncol 2013;15:705–11. [DOI] [PubMed] [Google Scholar]
- [27].Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706–13. [DOI] [PubMed] [Google Scholar]
- [28].Berlin J, Posey J, Tchekmedyian S, et al. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin Colorectal Cancer 2007;6:427–32. [DOI] [PubMed] [Google Scholar]
- [29].Mitchell EP, Piperdi B, Lacouture ME, et al. The efficacy and safety of panitumumab administered concomitantly with FOLFIRI or Irinotecan in second-line therapy for metastatic colorectal cancer: the secondary analysis from STEPP (Skin Toxicity Evaluation Protocol With Panitumumab) by KRAS status. Clin Colorectal Cancer 2011;10:333–9. [DOI] [PubMed] [Google Scholar]
- [30].Yamaguchi T, Iwasa S, Nagashima K, et al. Comparison of panitumumab plus irinotecan and cetuximab plus irinotecan for KRAS wild-type metastatic colorectal cancer. Anticancer Res 2016;36:3531–6. [PubMed] [Google Scholar]
- [31].Nishi T, Hamamoto Y, Nagase M, et al. Phase II trial of panitumumab with irinotecan as salvage therapy for patients with advanced or recurrent colorectal cancer (TOPIC study). Oncol Lett 2016;11:4049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Haller DG, Rothenberg ML, Wong AO, et al. Oxaliplatin plus irinotecan compared with irinotecan alone as second-line treatment after single-agent fluoropyrimidine therapy for metastatic colorectal carcinoma. J Clin Oncol 2008;26:4544–50. [DOI] [PubMed] [Google Scholar]
- [33].Kim GP, Sargent DJ, Mahoney MR, et al. Phase III noninferiority trial comparing irinotecan with oxaliplatin, fluorouracil, and leucovorin in patients with advanced colorectal carcinoma previously treated with fluorouracil: N9841. J Clin Oncol 2009;27:2848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658–64. [DOI] [PubMed] [Google Scholar]
- [35].Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:1351–7. [DOI] [PubMed] [Google Scholar]


