Abstract
Parkinson disease (PD) is usually accompanied by numerous nonmotor symptoms (NMS), such as neuropsychiatric symptoms, sleep disorders, autonomic dysfunctions, and sensory disturbances. However, it is not clear that the factors influencing the occurrence of NMS and its sequence with motor symptoms (MS).
We conducted comprehensive assessments of NMS by using 13 scales in 1119 PD patients.
A total of 70.8% PD patients present NMS. Olfactory dysfunction tends to occur in PD patients with older age, more severe depression, sleep problems, and autonomic dysfunctions. Older patients are more likely to have olfactory dysfunction before MS than younger patients. Rapid eye movement behavior disorder is more prone to happen in patients with older age, older onset age, more severe depression, sleep problems, and autonomic dysfunctions. Patients with rapid eye movement behavior disorder before MS are older in onset age than after group.
Olfactory dysfunction, constipation, rapid eye movement behavior disorder, and depression, as early warning NMSs of PD, connected to each other. There is a clinical heterogeneity that older patients are more likely to have NMS before MS, while younger patients are opposite.
Keywords: clinical heterogeneity, olfactory dysfunction, rapid eye movement sleep behavior disorder
1. Introduction
Parkinson disease (PD) is a progressive, neurodegenerative disorder, greatly affecting the quality of life of patients, and bringing heavy burden for caregivers. Besides the major 4 motor symptoms (MS) including resting tremor, bradykinesia, rigidity, and postural and gait instability, PD is usually accompanied by numerous nonmotor symptoms (NMS), such as neuropsychiatric symptoms, sleep disorders, autonomic dysfunctions, and sensory disturbances. At present, there is a lack of clinical epidemiological study on sporadic PD in large population over a thousand evaluating MS and NMS by comprehensive-related scales. Therefore, we conducted assessments of MS and NMS by using 13 scales in over a thousand PD patients, in order to provide an important evidence for understanding the features of clinical symptoms, particularly NMS of PD, and find out what affects their occurrence and sequence with MS.
2. Methods
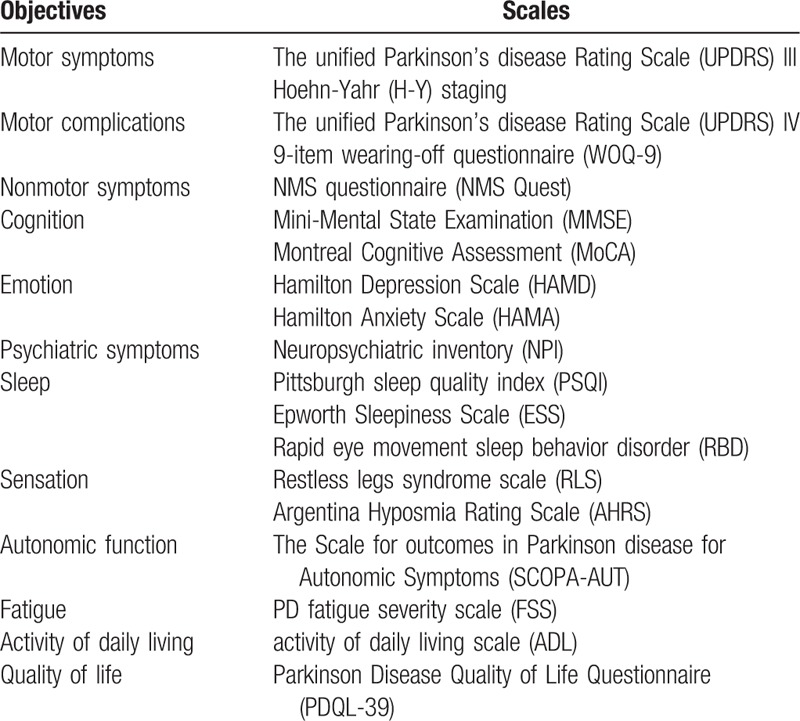
This study was approved by Beijing Tiantan Hospital review board. Written informed consents were obtained from all participants in this study. We recruited 1119 PD patients consecutively from the Department of Geriatrics and Neurology, Beijing Tiantan Hospital, Capital Medical University, from January 2011 to August 2015. Patients were diagnosed with PD according to UK Parkinson's Disease Society Brain Bank criteria.[1] Scales for evaluating of MS, motor complications, NMS, activity of daily living, and quality of life see Table 1. In order to keep the results of scales accurate and correct, all staff participating in the evaluation have been specially trained and passed the consistency check.
Table 1.
Evaluating scales for 1119 Parkinson disease patients.
Statistical analyses were performed with SPSS Statistics 20.0 (IBM Corporation, NY). The cases with incomplete information will be deleted before statistical analyses. Epidemiology, medication, and the incidence of NMS, MS of PD patients were statistically analyzed; and 2 major NMS in PD patients, that olfactory dysfunction and RBD were further analyzed. According to accompanied with or without above 2 NMS, PD patients were divided into 2 groups and compared; for patients with those above 2 NMS, were further subdivided into 2 groups according the order of NMS and MS and then compared. Continuous variables were presented as means ± standard deviations and compared by 2-tailed t test if they were normally distributed, and presented as median (quartile) and compared by nonparametric test if they were not normally distributed. Discrete variables were compared by Chi square test. P value was significant when it was <0.05.
3. Results
-
(1)
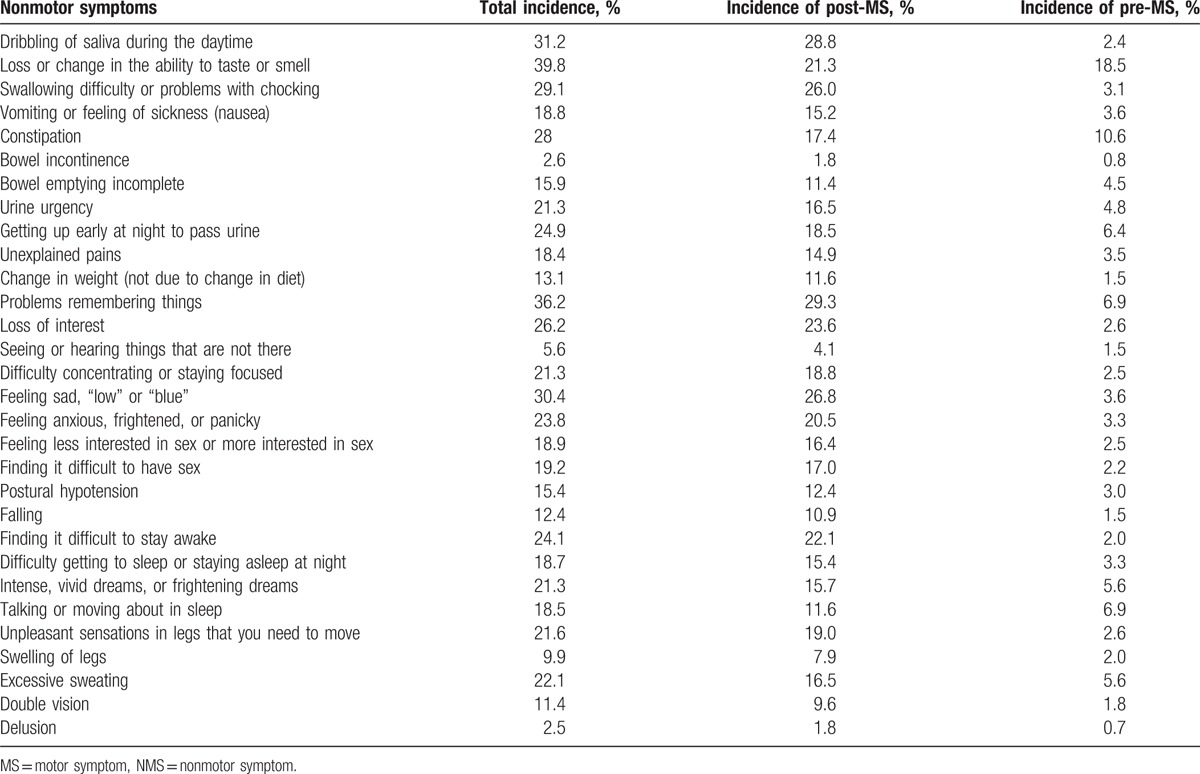
Demographic and clinical characteristics of PD patients are shown in Table 2. According to NMS Quest, the incidences of NMS see Table 3.
-
(2)
Clinical features of 2 pre-MS NMS
Table 2.
Demographic characteristics of 1119 Parkinson disease patients.
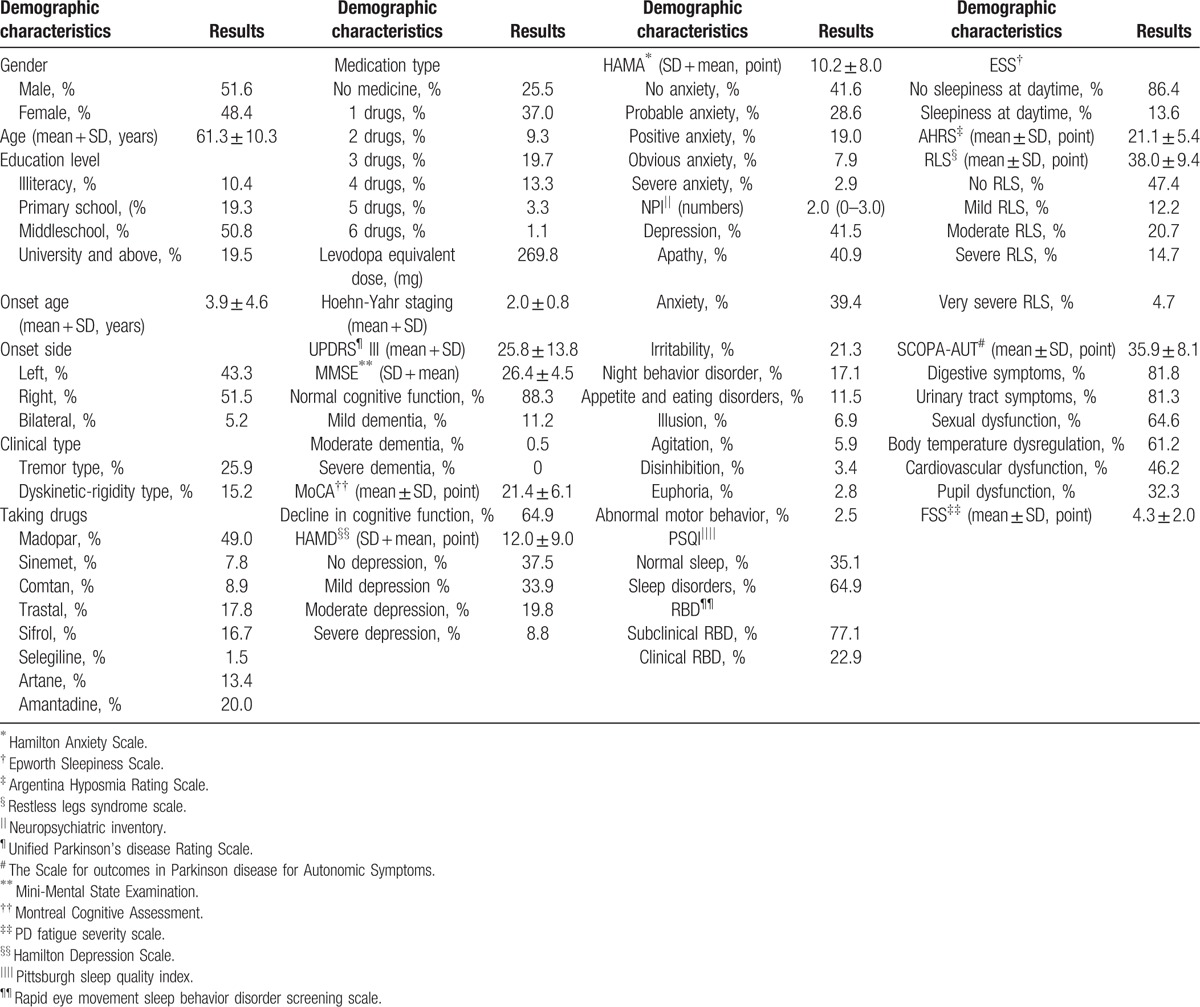
Table 3.
The incidences of NMS in Parkinson disease patients.
3.1. Olfaction
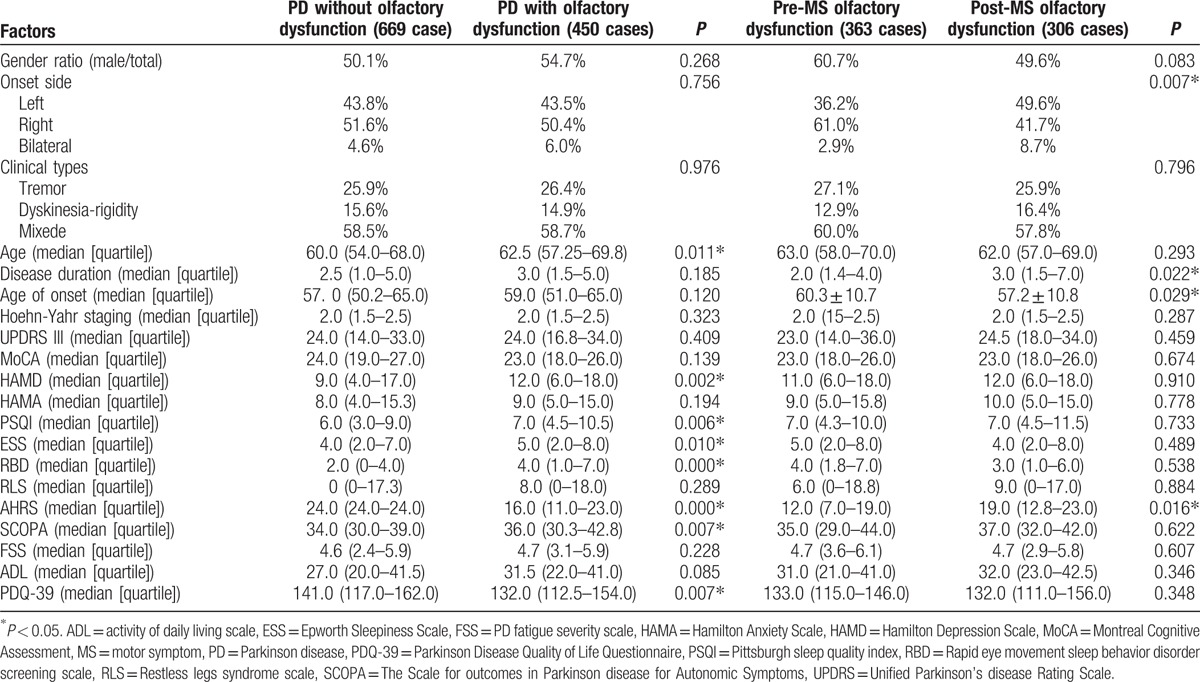
According to the 2nd item of NMS Quest, PD patients were classified as PD without and with olfactory dysfunction, and the latter were then classified as olfactory dysfunction occurring before or after MS. Results are shown in Table 4.
Table 4.
Comparison of Parkinson disease patients with or without olfactory dysfunction.
3.2. Rapid eye movement sleep behavior disorder screening scale (RBD)
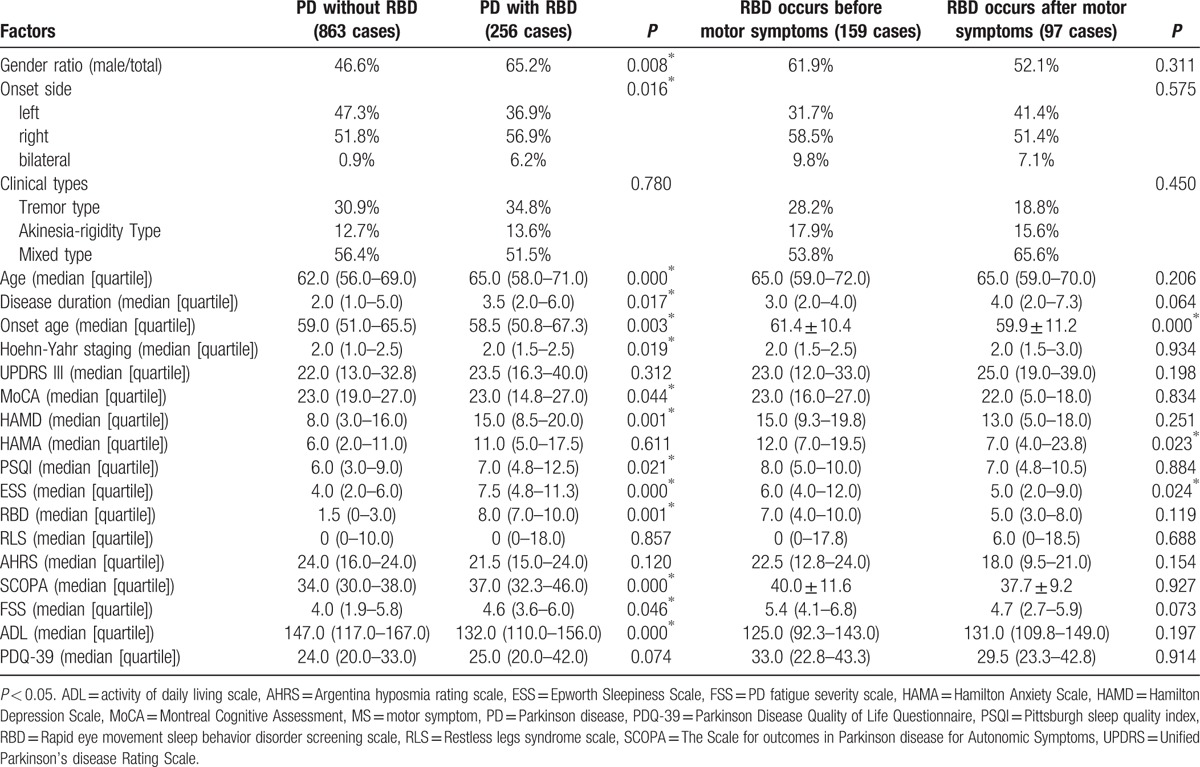
According to the 25th item of NMS screening scale, PD patients were classified as PD without RBD and PD with RBD, and the latter were further classified as RBD occurring before MS or after MS. Results were shown in Table 5.
Table 5.
comparison of Parkinson disease patients with or without rapid eye movement sleep behavior disorder.
4. Discussion
Our study showed that the incidence of PD was slightly higher in male than in female, the ratio was close to 1:1, the average age of PD patients was about 60 years, which was consistent with the domestic and international reports.[2] Education level of patients with PD was paralleled with that of age-matched (40–70 years old) citizens in our country,[3] suggesting that it was not an associated factor for PD.
By investigating into the clinical manifestations, we found that the majority PD patients were initiated from unilateral side, for which, the right side was slightly more than the left. The most common type of onset was the mixed type (which meant rigidity and resting tremor often occurred simultaneously); static tremor predominant type was less but still seen common, while the akinetic-rigidity type was rare. This implied that most PD patients presented comprehensive MS in the early stage, which was consistent with the domestic and international reports.[4]
There are still no curable anti-Parkinson medicines thus far, although our knowledge about the pathogenesis and symptom-improving drugs of PD has increased. In the past decades, several agents targeting different pathways have been explored as treatments to slow down disease progression, but promising successes are limited.[5] In this investigation, the most widely used drug was dopamine standard tablets; the 2nd was dopamine receptor agonist, which meets PD treatment guideline.[6] Because the average disease duration of our patients was approximately only 4 years, nearly half of patients could get satisfactory therapeutic effects when taking 3 kinds of anti-Parkinson drugs. However, there were still 25.5% of patients who did not receive standard treatment when first diagnosed PD, indicating insufficient understanding of diagnosis and treatment of PD in basic hospitals in our country.
For the assessment of MS, the average H-Y staging of our patients was 2.0, which meant bilateral limbs were involved, but the postural balance was not affected, and the average score of Unified Parkinson disease Rating Scale III was 25.8 ± 13.8.
Screening of NMS suggested that the decline of smell or taste ranked top of NMS, followed by the nervous system symptoms (such as memory loss, depression, apathy, and anxiety) and autonomic symptoms (including salivation, dysphagia, constipation, nocturia, urgency to urinate, excessive sweating, and sexual dysfunction). Sleep disorders (such as insomnia, RBD, and daytime sleepiness) were also common seen, and psychiatric symptoms (such as hallucinations, delusions) ranked the last. So more attention should be paid to the decline of memory, emotion disorders, autonomic disorders, and their treatment options in PD patients.[7]
Braak classified the pathological degree of PD into 6 stages, according to the sequential presence of Lewy bodies.[8] The presence of Lewy bodies at apophysis mamillaris and anterior olfactory nucleus, nuclei originis dorsalis, reticular formation, dorsal raphe nucleus and locus coerule, nuclei pontis, hypothalamic, neocortex, and limbic system may be connected to NMSs, as olfactory sensation, sleep, autonomic nerve, mental and behavioral disorders, respectively. In addition, the effects of anti-Parkinson drugs on dopamine and non-dopamine pathways may, directly or indirectly, result in NMS.[9]
Cognitive disorder is one of the cardinal features of NMS that influences the life quality of PD patients. Detailed assessments displayed that more than half patients with PD were diagnosed mild cognitive impairment, but only 12% of patients reached the degree of dementia, which was consistent with the domestic and international reports.[10] Executive dysfunction of PD is thought to be link to DA pathway abnormalities in the frontal striatum.[11] Progressive degeneration and subsequential loss of cholinergic neurons in nucleus basalis of Meynert, and the reduction of hippocampal volume, results in dementia in PD patients.[12]
Among the emotion disturbances, the most common is depression, the 2nd is anxiety. We found that the detection rate of depression using Hamilton Depression scale was higher than NMS screening scale, this might due to depression in PD was usually accompanied by numerous somatic symptoms. Thus, the Hamilton Depression scale exhibited superiority in recognizing depression because its more complicated questionnaire design paying attention to somatic symptoms. PD patients may present depression early in the Braak stage II, when Lewy bodies deposit in dorsal raphe nucleus and locus ceruleus. The probable biochemical mechanisms are related to serotonin system, also mixed with norepinephrine and dopaminergic abnormalities in limbic lobe.[12] Anxiety is another common affective disturbance in patients with PD, the reported incidence of anxiety varies from 5% to 69%.[13] The mechanism underlying anxiety in PD remains unclear. Currently, anxiety is believed not only an emotional response to MS but also a manifestation of PD itself. A case–control study showed that anxiety features had existed for several years prior to the emergence of MS.[14]
According to NPI scale, the incidence of mental and behavioral disorders in PD patients was generally low except depression (41.5%), apathy (40.9%), and anxiety (39.4%), changes in sleep and eating condition were most common (such as nocturnal behavior disorders, appetite loss, and eating disorders), personality changes ranked the 2nd place (such as irritability, disinhibition), while illusion appeared far less. The incidence of apathy was also high. The pathogenesis of apathy is associated with the functional dysregulation of the facies orbitalis frontal lobe-ventral striatum circuit. A previous study observed that, PD related apathy was more prone to occur at “off” stage,[15] suggesting it may be correlated with the DA level of brain.
Somnipathy is a common NMS for PD patients, it could be the result of the disease itself, or secondary to other NMS, the side effect of medicine, and it may emerge over different phases of PD. It was reported that the most common somnipathy was sleep disruption,[16] referring to interfered or disrupted sleep caused by other nocturnal NMS. Previous studies showed 50% of PD patients had prolonged daytime sleepiness or unconscious drowsy. Our data showed 24.1% of PD patients experienced excessive daytime sleepiness, indicating this might be a symbolic subclinical symptom of PD.[17,18] About one third of PD patients reportedly underwent RBD.[19] The damages of nuclei pontis, locus ceruleus, and corpora rubrum may be the pathological basis of RBD.[20] Occurrence of RBD could provide a significant clue for screening in high risk population and early diagnosis of PD.
Almost 90% of the PD patients are concomitant with hyposmia to different degrees; it is considered a marked warning of PD before MS.[21] We discovered a significant smell decline in PD patients, which was due to the early involvement of olfactory related brain regions by α-synuclein according to Braak staging and has a good clinical predictive value for PD.
The causes of restless leg syndrome remain elusive currently. Clinical and functional MRI studies link the possibility to DA metabolism in central nervous system. Numerous studies held that restless leg syndrome was more common in patients with PD than healthy people, but the reported prevalence in PD patients were inconsistent, ranging from 7.90% to 20.80%.[22,23] The prevalence of restless leg syndrome in PD patients in this investigation was about 21.6%, similar to the foreign and domestic reports.
Autonomic disorders include the deregulations of sweat gland, body temperature, cardiovascular, gastrointestinal, and genitourinary systems, mainly relevant to appearance of Lewy bodies in sympathetic or parasympathetic cholinergic ganglion, and sympathetic adrenergic nerve ganglion.[9] Our data suggested that the major problems were constipation, frequent urination at night, orthostatic hypotension, polyhidrosis, and sexual dysfunction.
Fatigue is one of important NMS of PD; the affliction it brings along for patients is no less than MS does. According to the foreign study, 42% of patients with PD feel fatigue and one third of them deem fatigue as the most disabling factor. The significance of fatigue for PD patients has not been fully realized and only14% of the PD patients with fatigue is diagnosed. The mechanism of the fatigue is as yet undetermined and its risk factors are still disputed. Fatigue is a quite common complaint of PD patients, which exerts great influences on the activity and life quality of the patients.[24] There may be other factors that contributes to the occurrence of fatigue in PD, such as depression and daytime somnolence, which needs to be further explored.[25]
Next, we specifically focused on the cardinal NMS through further group comparison. We observed that the PD patients with olfactory dysfunction group were older, exhibited lower Argentina hyposmia rating scale scores, more severe depression, sleep and autonomic nerve disturbances, and worse quality of life. These potential connections could be explained because these mentioned NMS are all likely to occur before onset of MS, and are all pathologically involved in similar locations according to Braak staging, such as peripheral nerves, medulla oblongata, and brainstem. Besides, we discovered that the patients who already had olfactory problems before MS, displayed older age of onset, more common onset from right side, and more severe olfactory symptoms, meanwhile, the patients who developed olfactory problems after MS presented earlier age of onset, longer disease duration, and more common left-side onset, demonstrating that pathological progression is heterogeneous in individual patient. It is consistent with the typical Braak stage that the patients with right onset are more likely involved in olfactory bulb and olfactory tract at early stage have more serious smell dysfunction but develop MS much later. However, in left-onset patients, substantia nigra is involved earlier and accordingly MS appears earlier, dysosmia is milder due to later involvement of olfactory bulb and olfactory tract.
Compared to the patients without RBD, the patients accompanied by RBD showed more male, more right-side onset, older age, longer course of disease, and older age of onset, displayed higher H-Y staging, more prominent in cognitive impairment, depression, sleep disorders, autonomic nerve dysfunction, fatigue, and worse quality of life. It is suggested that right onset, older male are more susceptible to RBD. Compared to the patients who develop RBD after MS, the patients who presented RBD before MS featured older onset age, more serious anxiety, and daytime sleepiness, suggesting heterogeneity in pathological progression among individual patient. The patients who have RBD earlier often develop MS later (older age of onset), consistent with the typical Braak staging, while the patients who have RBD later often develop MS earlier (younger onset age), which means younger patients are more likely to present MS rather than NMS.
In summary, we collected the clinical information of 1119 cases of primary PD patients, conducted up to 19 scales, covering all of the MS and NMS of PD patients. The patients recruited at Beijing Tiantan hospital are from all parts of the country, therefore this sample has a good representative. It found that PD patients in our hospital present quite a number of NMS (including cognitive impairment, emotion disorder, mental and behavioral disorders, sleep disorders, autonomic dysfunctions, fatigue, and sensory abnormalities); NMS may occur before MS, PD pathology may be heterogeneous, MS always appear earlier than NMS in young patients and NMS always appear earlier than MS in older patients, and the latter is more conform to the classic Braak pathological stage.
Acknowledgments
The authors thank Run-hua Zhang for her guidance and assistance in statistics.
Footnotes
Abbreviations: MS = motor symptoms, NMS = nonmotor symptoms, PD = Parkinson disease, RBD = rapid eye movement sleep behavior disorder.
T-mZ and S-yY are the first authors.
Funding/support: This work is supported by the National Natural Science Foundation of China (81571229, 81071015, 30770745), Key Project of National Natural Science Foundation of China (81030062); Key Project of Natural Science Foundation of Beijing, China (kz201610025030, 4161004, kz200910025001), Natural Science Foundation of Beijing, China (7082032); National Key Basic Research Program of China (2011CB504100); Important National Science & Technology Specific Projects (2011ZX09102-003-01); National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2013BAI09B03); Project of Beijing Institute for Brain Disorders (BIBD-PXM2013_014226_07_000084); High Level Technical Personnel Training Project of Beijing Health System, China (2009-3-26); Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20140514); Capital Clinical Characteristic Application Research (Z12110700100000, Z121107001012161), Beijing Healthcare Research Project, China (JING-15-2, JING-15-3); Excellent Personnel Training Project of Beijing, China (20071D0300400076); Basic-Clinical Research Cooperation Funding of Capital Medical University, China (2015-JL-PT-X04, 10JL49, 14JL15); and Youth Research Funding, Beijing Tiantan Hospital, Capital Medical University, China (2014-YQN-YS-18, 2015-YQN-15, 2015-YQN-05, 2015-YQN-14, 2015-YQN-17).
The authors have no conflicts of interest to disclose.
References
- [1].Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shenoy P, Harugeri A. Elderly patients’ participation in clinical trials. Perspect Clin Res 2015;6:184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ransmayr G. Cognitive impairment in Parkinson's disease. Psychiatria Danubina 2015;27:458–61. [PubMed] [Google Scholar]
- [4].Bjornestad A, Forsaa EB, Pedersen KF, et al. Risk and course of motor complications in a population-based incident Parkinson's disease cohort. Parkinsonism Relat Disord 2016;22:48–53. [DOI] [PubMed] [Google Scholar]
- [5].Park A, Stacy M. Disease-modifying drugs in Parkinson's disease. Drugs 2015;75:2065–71. [DOI] [PubMed] [Google Scholar]
- [6].DeMaagd G, Philip A. Parkinson's disease and its management: part 4: treatment of motor complications. P&T 2015;40:747–73. [PMC free article] [PubMed] [Google Scholar]
- [7].Sauerbier A, Jenner P, Todorova A, et al. Non motor subtypes and Parkinson's disease. Parkinsonism Relat Disord 2016;22(Suppl 1):S41–6. [DOI] [PubMed] [Google Scholar]
- [8].Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- [9].Truong DD, Bhidayasiri R, Wolters E. Management of non-motor symptoms in advanced Parkinson disease. J Neurol Sci 2008;266:216–28. [DOI] [PubMed] [Google Scholar]
- [10].Huang C, Mattis P, Perrine K, et al. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology 2008;70:1470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 2010;9:1200–13. [DOI] [PubMed] [Google Scholar]
- [12].Aarsland D, Londos E, Ballard C. Parkinson's disease dementia and dementia with Lewy bodies: different aspects of one entity. Int Psychogeriatr 2009;21:216–9. [DOI] [PubMed] [Google Scholar]
- [13].Khan NL, Graham E, Critchley P, et al. Parkin disease: a phenotypic study of a large case series. Brain 2003;126:1279–92. [DOI] [PubMed] [Google Scholar]
- [14].Kulisevsky J, Pagonabarraga J, Pascual-Sedano B, et al. Prevalence and correlates of neuropsychiatric symptoms in Parkinson's disease without dementia. Mov Disord 2008;23:1889–96. [DOI] [PubMed] [Google Scholar]
- [15].Shiba M, Bower JH, Maraganore DM, et al. Anxiety disorders and depressive disorders preceding Parkinson's disease: a case-control study. Mov Disord 2000;15:669–77. [DOI] [PubMed] [Google Scholar]
- [16].Czernecki V, Pillon B, Houeto JL, et al. Motivation, reward, and Parkinson's disease: influence of dopatherapy. Neuropsychologia 2002;40:2257–67. [DOI] [PubMed] [Google Scholar]
- [17].Chaudhuri KR. Nocturnal symptom complex in PD and its management. Neurology 2003;61:S17–23. [DOI] [PubMed] [Google Scholar]
- [18].Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 2005;65:1442–6. [DOI] [PubMed] [Google Scholar]
- [19].Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 1996;46:388–93. [DOI] [PubMed] [Google Scholar]
- [20].Fantini ML, Ferini-Strambi L, Montplaisir J. Idiopathic REM sleep behavior disorder: toward a better nosologic definition. Neurology 2005;64:780–6. [DOI] [PubMed] [Google Scholar]
- [21].Ponsen MM, Stoffers D, Booij J, et al. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 2004;56:173–81. [DOI] [PubMed] [Google Scholar]
- [22].Worringham CJ, Wood JM, Kerr GK, et al. Predictors of driving assessment outcome in Parkinson's disease. Mov Disord 2006;21:230–5. [DOI] [PubMed] [Google Scholar]
- [23].Krishnan PR, Bhatia M, Behari M. Restless legs syndrome in Parkinson's disease: a case-controlled study. Mov Disord 2003;18:181–5. [DOI] [PubMed] [Google Scholar]
- [24].Havlikova E, Rosenberger J, Nagyova I, et al. Impact of fatigue on quality of life in patients with Parkinson's disease. Eur J Neurol 2008;15:475–80. [DOI] [PubMed] [Google Scholar]
- [25].Lou JS. Physical and mental fatigue in Parkinson's disease: epidemiology, pathophysiology and treatment. Drugs Aging 2009;26:195–208. [DOI] [PubMed] [Google Scholar]


