Abstract
Background:
Cognitive and physical performance can be negatively affected by chronic pain. This study evaluates the effect of combined physical-, cognitive-, and mindfulness training (PCMT) on cognitive and physical performance.
Methods:
From a large pharmaceutical company in Denmark we randomly allocated 112 female laboratory technicians with chronic upper limb pain to group-based PCMT at the worksite or a reference group for 10 weeks. Neurocognitive performance was measured by the computerized central nervous system vital signs neurocognitive assessment battery. Physical function was assessed in terms of shoulder external rotation strength and rate of force development in a custom-made dynamometer setup.
Results:
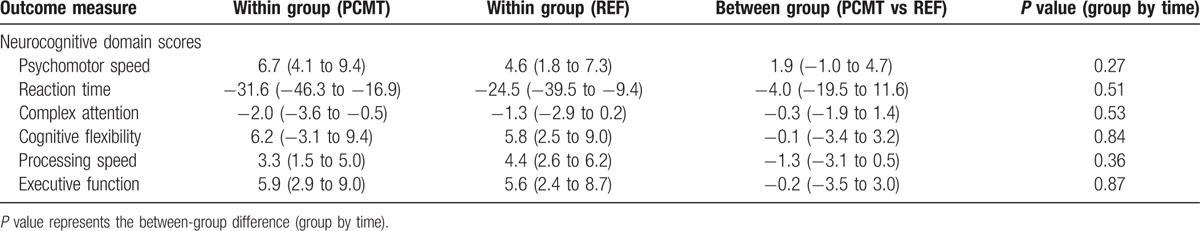
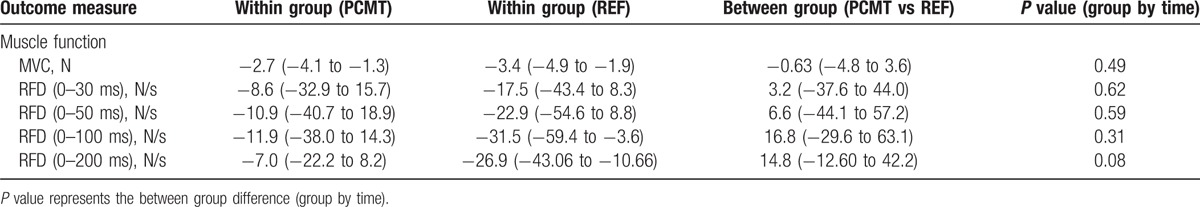
No between-group differences (least square means [95% confidence interval]) from baseline to follow-up could be detected in any of the neurocognitive domains as measured by the central nervous system vital signs neurocognitive assessment battery, for example, Psychomotoer Speed 1.9 (−1.0 to 4.7), Reaction Time −4.0 (−19.5 to 11.6), Complex Attention −0.3 (−1.9 to 1.4), and Executive Function −0.2 (−3.5 to 3.0). Similarly, we found no change in maximal voluntary isometric strength −0.63 (−4.8 to 3.6), or rate of force development 14.8 (−12.6 to 42.2) of the shoulder external rotators. Finally, test–retest reliability of maximal voluntary contraction and rate of force development shoulder external rotation showed high reliability at 0 to 30 ms, 0 to 50 ms, 0 to 100 ms, and 0 to 200 ms with ICCs at 0.95, 0.92, 0.93, 0.92, and 0.91, respectively.
Conclusion:
Ten weeks of PCMT did not improve neurocognitive or physical performance.
Keywords: brain, cognitive executive function, complex attention, pain, psychomotor speed, rate of force
1. Introduction
During the last decade epidemiological studies have mapped the prevalence of chronic musculoskeletal pain in the workforce, and approximately 25% of the population across 15 European countries report work-related neck- and shoulder pain.[1] While chronic pain has consequences in terms of reduced self-reported work ability,[2–6] such evaluations are often performed by questionnaire. By contrast, objective evaluation methods may be preferred to obtain more unbiased information. Such objective methods exist for both cognitive performance and physical function.
Neurocognitive performance and attention are negatively affected by chronic pain.[7–9] A recent study by Moore et al[9] found that the aspects of attention most affected by pain are those essential for the completion of complex tasks that require the processing of multiple cues and complex attention control. Furthermore, after investigating cognitive executive function and attention, Oosterman et al[7] concluded that in chronic pain conditions, sustained attention performance is diminished while mental flexibility, planning, and inhibition appear to remain intact. In addition, in patients with headache, diminished executive function and complex attention control have been reported[10] and a recent meta-analysis by Berryman et al[11] revealed small to moderate executive function performance impairment in people with chronic pain but the authors note a lack stringent control for possible influencing factors and methodology. Conversely, a study by Suhr[12] in 28 fibromyalgia patients, 27 chronic pain patients, and 21 controls revealed that differences in cognitive function were not apparent when controlling for fatigue, pain, and depression, which attests to the complexity of neuropsychological impairment in persons with pain.
To our knowledge, neurocognitive impairment due to chronic musculoskeletal pain has yet to be investigated in the working population among those specifically performing specialized and repetitive movement tasks (e.g., laboratory technicians). It could be speculated that a decline in psychomotor speed could negatively affect cognitive abilities including psychomotor speed and complex attention tasks, as people with chronic pain often exhibit decreased rate of force development (RFD) and neural drive to the painful muscles. Exercise intervention studies compiled in several reviews[13–15] show promising effects on cognitive performance in the domains of processing speed, memory, and executive function in healthy older adults but the research in healthy work-able women is to our knowledge scarce. However, the effects of mindfulness meditation interventions on cognitive flexibility, attentional focus, and emotional regulation, which are important elements of overall cognitive function, have been reported showing positive correlations with meditation practice in both adolescents[16] and adults.[17]
Physical function is affected by chronic pain. Andersen et al[18] found an 18% lowered maximal voluntary force production whereas RFD (i.e., rate of rise in contractile force at the onset of contraction) was reduced by 54% in women with chronic trapezius myalgia compared with pain-free controls. Andersen et al[18] suggested that RFD is a more sensitive measure of mechanical muscle performance in painful conditions than maximal muscle strength. In support of these findings Ervilha et al[19] reported that the initial (100 ms) agonistic electromyography burst activity was particularly suppressed during dynamic contractions in conditions of upper limb muscle pain. The effect of muscle pain on the ability to produce muscular force rapidly is predominantly caused by neural mechanisms rather than muscular atrophy, because average trapezius muscle mass and fiber size do not seem to differ between women with and without chronic neck pain.[18] In occupational settings, such neuromuscular changes are likely to influence the worker's motor strategy and compensatory activation of agonistic and antagonistic muscles to fulfill specific job demands. Concurrently, modification in neuro-mechanical function may contribute to aggravation or spreading of pain accompanied by an escalating imbalance between work demands and individual musculoskeletal resources, consequently affecting work participation, and overall quality of life.[18]
A multitude of physiological parameters are known to influence RFD in pain-free individuals, including efferent neural drive to the muscles encompassing both motor neuron recruitment firing frequency, muscle fiber size and composition, and viscoelastic properties of the muscle-tendon complex.[18–24] Additionally, RFD obtained in various time intervals is affected by different physiological parameters, where early-phase RFD (<40 ms from onset of contraction) is mainly affected by intrinsic contractile properties of the muscle whereas late-phase RFD is more reliant on maximal voluntary contraction (MVC).[25] Several studies have shown promising and effective reductions in upper limb musculoskeletal pain in response to specific strength training performed at the workplace.[26–29] With a biomechanical approach to pain-relief, several studies have reported increased RFD paralleled by an augmented rate of electromyography rise in chronically painful neck/shoulder muscles following interventions of progressive strength training.[18,30,31] Thus, it seems that progressive strength training has the ability to counteract the observed inhibition in initial activation of painful muscles, consequently leading to increased RFD.
The aim of the present study was to evaluate the effect of a multifaceted intervention strategy with physical-, cognitive-, and mindfulness training elements versus a reference group on neurocognitive performance, MVC and RFD in female laboratory technicians with chronic upper limb pain. We hypothesize between-group neurocognitive performance improvements and increased RFD following the intervention period, as pain may inhibit motor output and neurocognitive function.[9,32–36]
2. Methods
2.1. Setting
During spring/summer of 2014 our research team conducted a randomized controlled intervention trial at a large pharmaceutical company in Copenhagen, Denmark. Briefly, we performed a single-blinded trial with allocation concealment, in a 2-armed parallel group format among female laboratory technicians. The participants were parallel-assigned to receive either a 10-week physical-cognitive-mindfulness training intervention or continue following already implemented company guidelines for reducing work-related musculoskeletal pain of the upper extremity and low back. The present article presents tertiary outcomes obtained during the study period. Primary and secondary outcomes on musculoskeletal pain and work related stress have previously been reported.[37]
2.2. Ethics and trial registration
Prior participant enrolment we registered the study in the ClinicaltTrials.gov register: “Implementation of physical exercise at the Workplace (IRMA09) – Laboratory technicians,” registration no. NCT02047669. Ethical approval was obtained from The Danish National Committee on Biomedical Research Ethics (The local ethical committee of Frederiksberg and Copenhagen; H-3-2010-062) as part of the research program “Implementation of physical exercise at the workplace (IRMA).” This study, as well as the previously published studies describing the protocol,[38] primary- and secondary[37] outcomes adhere to the criteria of the revised Consolidated Standards of Reporting Trials 2010 statement for reporting randomized trials. All experimental conditions conformed to The Declaration of Helsinki.
2.3. Participants
At the time of enrolment, the eligible participants (n = 112) all had chronic musculoskeletal pain in 1 or more of the following regions: upper back, lower back, neck, shoulders, elbows, or hands/wrists. To fulfill the definition of chronic pain all following criteria were met for at least 1 of these body regions: pain intensity of ≥3 (0–10 Visual Analogue Scale [VAS] supported by drawings from the Nordic Questionnaire[39]) during the last week, pain frequency of ≥3 days during the last week, pain lasting at least 3 months. Participants with typical exclusion criteria, for example, severe hypertension (>160/100), were allowed to participate in the less strenuous part of the training intervention if their general practitioner provided consent. We excluded participants with life-threatening diseases, and pregnancy was considered a contraindication to the training. Figure 1 shows participant flow through the study.
Figure 1.
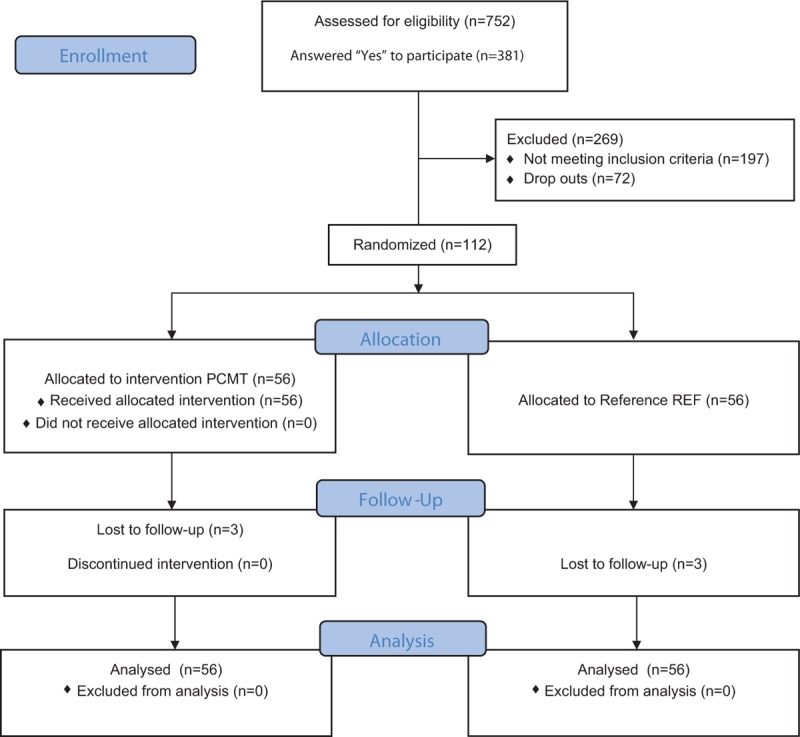
CONSORT flow diagram showing the flow of participants through the study.
2.4. Participant allocation
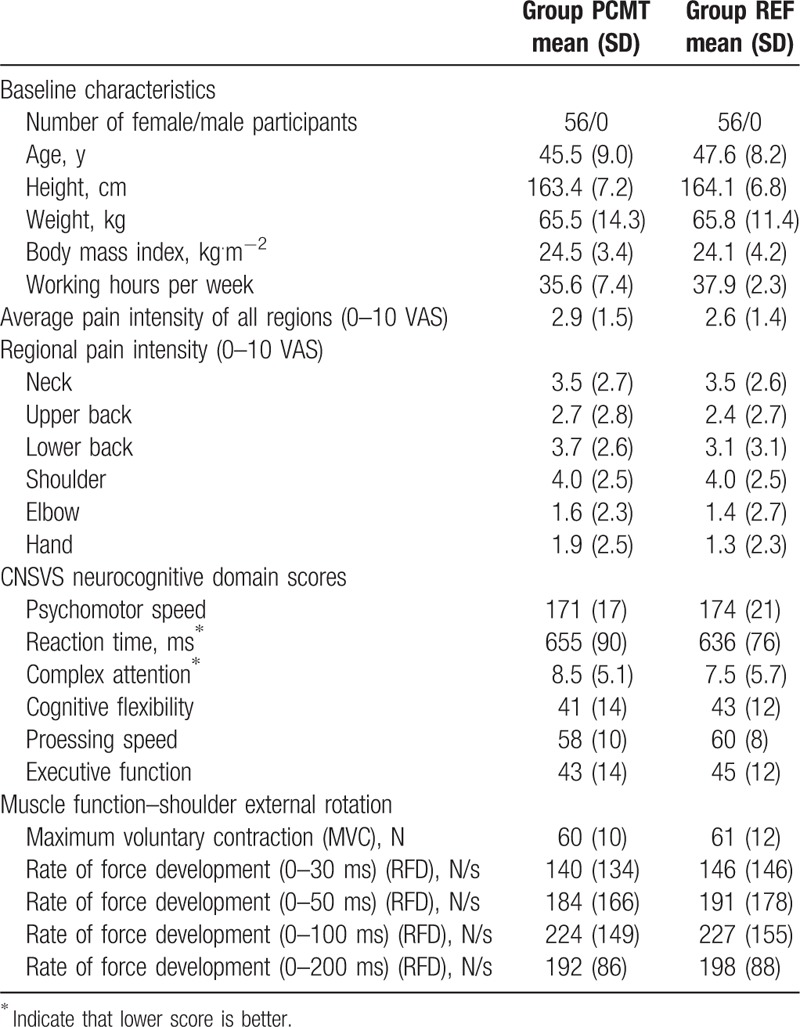
To allocate the participants, a random numbers table in the SAS statistical software was generated, thus allowing a randomized distribution of the laboratory technicians to either a Physical-Cognitive-Mindfulness training (PCMT) intervention group or a reference group (REF). This allocation was performed after the participants had answered a baseline questionnaire providing information on descriptive characteristics and perceived level of musculoskeletal pain (Table 1). Furthermore, participants were informed that it was unknown which treatment model would work best for reducing pain and they were instructed not to reveal their particular intervention to colleagues or study assessors. Following group allocation each participant was invited to complete a test battery consisting of an extensive clinical examination by a specialized physical therapist, a neurocognitive assessment, and a series of physical function measurements of the shoulder external rotator complex. The entire test battery lasted approximately 90 minutes and all assessors were blinded to participant allocation. Upon conclusion of the 10-week intervention the participants were asked again to fill out the questionnaire on musculoskeletal pain and complete the test battery for follow-up measurements. All participants were at the time of enrolment in the 25th to 74th percentile in the 6 domains of neurocognitive function, age- and gender matched for normative scores, which according to the Central Nervous System Vital Signs (CNSVS) guidelines are described as average with normal function and capacity.[40]
Table 1.
Participant characteristics, neurocognitive performance, and muscle function at baseline after group allocation; presented as mean (SD).
2.5. Neurocognitive assessment
Neurocognitive function was assessed by the CNSVS (CNS Vital Signs, Morrisville, NC) computerized test battery, which has been shown to be a stable and reliable assessment of cognitive function with high validity.[41] The 5 included subtests were (in administered order): Symbol Digit Coding test, Shifting Attention Test, Finger Tapping Test, Stroop Test, and Continuous Performance Test. The tests themselves are online versions of standard measures of cognitive task performance and have previously been described and tested for reliability and validity in the literature.[41] The test runs were highly consistently delivered: the test battery is automatically presented to the participants with written instructions and a short practice round immediately followed by the actual tests in the selected battery. Time to completion of the full test battery was between 20 and 30 minutes depending on how long the participants needed to read the instructions. The CNSVS can be administered in a variety of languages, including Danish, which was the setting during this trial. Briefly, the subtests provided domain measures of: Psychomotor Speed, Reaction Time (measured in milliseconds), Complex Attention, Cognitive Flexibility, Processing Speed, and Executive Function. The scores of each of the domains were calculated either as differences between correct and erroneous responses within each subtest, or summation of the responses from 2 or more subtests according to the specifications of domain calculations of the CNSVS.[40,41] For reference, each domain indicates the following: psychomotor speed is a measure of how well a subject perceives, attends, responds to complex visual-perceptual information, and performs simple fine motor coordination. It is an expression of the ability to perform simple motor skills and dexterity through cognitive functions (e.g., use of precision instruments or tools) and performing mental and physical coordination (e.g., driving a car or playing a musical instrument). Reaction time is a measure of how quickly the subject can react to a simple and increasingly complex direction set. Examples of this include driving a car, attending to conversation, and responding to a set of simple instructions. Complex attention is defined as the ability to track and respond to a variety of stimuli over lengthy periods of time and/or perform complex mental tasks requiring vigilance. Cognitive flexibility describes how well the person is able to adapt to rapidly changing and increasingly complex sets of directions and/or to manipulate the information. This can be exemplified as reasoning skill, switching tasks, decision-making, impulse control, and strategy formation. Processing speed is how well a person recognizes and processes information (e.g., fine motor coordination or visual-perceptual ability). Executive function is defined as how well a person recognizes rules, categories and performs in rapid decision-making tasks. In everyday life executive function is the ability to sequence and manage multiple tasks simultaneously and the ability to quickly respond to changing sets of instructions.[40]
2.6. Physical function
MVC and RFD of the shoulder external rotators were assessed during concurrent isometric external rotation of the gleno-humeral joint using a custom-built dynamometer with 2 strain gauge load cells (KIS-2, 1 KN, Vishay Transducers Systems, Germany). Participants were seated upright in a chair with the elbow of their dominant arm flexed at 90° while applying outward-directed force to a vertical oriented handlebar (dynamometer setting) in front of them, thus creating external rotation of the gleno-humeral joint. The anterior part of the forearm was supported by the dynamometer setting and allowed the participants to brace against during the isometric MVC. The participants performed 3 MVCs separated by a 30 seconds rest period, and were instructed to apply force to the dynamometer as fast and forcefully as possible. Online feedback and verbal encouragement was given during the MVCs. The MVC trial with the highest peak force was selected for further statistical evaluation. RFD was determined during 4 time intervals (0–30, 0–50, 0–100, and 0–200 ms from onset of contraction). The attempt yielding the highest RFD in the respective time interval was selected for statistical analysis. Raw MVC force signals were sampled at 1000 Hz and subsequently low pass filtered (15 Hz cut-off frequency, fourth-order zero-lag Butterworth filter) using a custom-made MatLab program (MathWorks).
2.7. Test–retest reliability
On a separate occasion, our research team also performed test–retest reliability measures of the physical function tests in our lab. Participants (n = 32; 15 men and 17 women; mean [standard deviation]; age: 32 years (8.7), height: 174.0 cm (9.0), weight: 69.5 kg (13.6), BMI: 22.7 (2.5)) performed the dynamometer testing of the shoulder external rotation on 2 separate occasions, 1 week apart, in accordance with the testing protocol used in the present study. All participants were instructed not to perform any exercises for 48 hours leading up to the reliability measures and were also asked not to engage in any new physical activities between the first and second test rounds. Lastly, all participants were instructed to be adequately hydrated and well rested before testing on both occasions.
2.8. Interventions
The intervention design has previously been described.[37,38] In short, we introduced a multifactorial 10-week intervention to the PCMT group consisting of joint mobility exercises focusing on precise motor control, 4 different strength training exercises with elastic bands, cognitive behavioral therapy in which education and counseling about fear of movement, the positive effects of movement, as well as de-catastrophizing pain were the main focus areas, and mindfulness group-training. Joint mobility/precise motor control training, strength training using elastic resistance bands, cognitive behavioral therapy, focusing on fear avoidance behavior and pain education, were grouped together and administered in brief 20 minutes sessions 4 times per week by a professional trainer with a relevant background. The elastic resistance exercises primarily consisted of 1 to 2 sets of approximately 10 reps using a resistance band equaling 15 to 20RM of 3 to 4 different upper extremity exercises (e.g., shoulder lateral raise, shoulder external rotation, and wrist extension). The primary emphasis during the physical training was on the precise motor control exercises and the behavioral aspects. Precise joint mobility and motor control exercises included various shoulder, neck, elbow, and wrist rotations in more or less challenging body positions. Further, the instructor was also encouraged to include exercises for other regions (e.g., hip, sacro-illiac joint, and low back) to all, or individual participants, if there was a specific need. Examples of both the elastic resistance exercises and the precise joint mobility/motor control exercises have previously been published.[38] Mindfulness sessions were kept separate from the physical training and were administered once weekly by a psychologist specialized in stress and yoga. To address possible confounding factors, we tracked (i.e., asked by questionnaire at baseline and follow-up): number of days using pain medication within the last week, number of treatment sessions (e.g., by a medical doctor, physical therapist, or other types of health personnel) for pain in the back, neck, shoulders, elbows, or hands/wrists within the last month. The REF group served as a control group adhering to on-going company initiatives as described elsewhere.[38] Primary outcome results have also previously been reported.[37]
2.9. Statistics
All statistical analyses were performed in accordance with the intention-to-treat principle by including all subjects in the analysis regardless of actual participation or dropout. Missing values were not imputed but handled by the statistical software. Between-group differences of neurocognitive- and musculoskeletal function at follow-up were determined by a linear mixed model using Proc Mixed of the SAS statistical software, version 9.2 (SAS institute, Cary, NC). Fixed factors were treatment, time and treatment by time interaction. Analyses of neurocognitive- and physical function were controlled for neurocognitive performance and physical function at baseline, respectively. A priori power analysis based on previous measurements reveals that 27 participants of each group for 95% power, type I error probability of 5% is sufficient to test the null-hypothesis of equality (alpha = 0.05, beta = 0.05). Given an estimated 10% dropout rate we aimed at recruiting at least 30 participants for each group. Results are reported as between-group least square means (95% confidence interval). We accepted P values less than 0.05 as statistically significant. Baseline and descriptive results are reported as mean (standard deviation).
3. Results
Test–retest reliability measures of the custom-made dynamometer setup on the shoulder external rotation showed high reliability for MVC and RFD at 0 to 30 ms, 0 to 50 ms, 0 to 100 ms, and 0 to 200 ms with an ICC at 0.95, 0.92, 0.93, 0.92, and 0.91, respectively.
As previously reported, 3 participants of the intervention study from each group dropped out of without providing information as to why. Consequently, 53 participants from each group completed the follow-up testing and questionnaire on musculoskeletal pain.[37]
There were no between-group changes at 10-week follow-up on any of the neurocognitive domains. Table 2 summarizes the changes within- and between groups of each domain.
Table 2.
Between- and within group differences at 10-week follow-up on neurocognitive domain scores; presented as least square mean (95% confidence interval).
There were no between-group changes at 10-week follow-up on either MVC or RFD at 0 to 30 ms, 0 to 50 ms, 0 to 100 ms, or 0 to 200 ms. Table 3 summarizes the results on physical function of the shoulder external rotators for each group and between groups at 10-week follow-up.
Table 3.
Between- and within group differences at 10-week follow-up on muscle function (maximum voluntary contraction [N] and rate of force development [N/s]) of the shoulder external rotator; presented as least square mean (95% confidence interval).
4. Discussion
The present study demonstrates that neither neurocognitive performance, as measured by the CNSVS test battery of neurocognitive function, nor physical function measured by MVC and RFD of the shoulder external rotators was improved by a 10-week physical-cognitive-mindfulness training intervention at a company worksite compared with a reference group following the company's ongoing health initiatives. Primary outcomes on musculoskeletal pain and stress have previously been reported[37] but as those parameters are a part of discussing the results of the present study, they are included here as reference. In brief, average pain of the 6 regions between groups at 10-week follow-up was −1.0 (−1.4 to −0.6) (P <0.0001). The PCMT and REF groups decrease in pain was 52% and 15% respectively. Stress score was not different at 10-week follow-up between groups (P = 0.16). Finally, an explorative dose–response analysis showed that pain decreased on average 0.6 points (0–10 VAS) per average weekly attended physical-cognitive training session, whereas pain increased per weekly attended mindfulness session by 0.15 (0–10 VAS).[37]
The lack of improvement in MVC and RFD is in contrast to other interventions and investigations using strength training to reduce chronic and nonchronic musculoskeletal pain.[18,25,26,30,31] A possible explanation for the lack of improvement in RFD in the present study may be attributed to a relatively low intensity of the physical training. That is, strength training is per definition training that increases muscle strength, but we did not find any change in muscle strength as result of the present training regimen. As described previously by our research team,[37,38] the intervention did in fact have physical training elements but focused mostly on motor control training and precise joint mobility. It is therefore reasonable to speculate that the mechanical load placed on the body during training was not sufficient to cause adaptations in RFD, as there is a minimal load necessary to increase neural drive and stimulate type II fiber hypertrophy.[20,42] As an example, Andersen et al[18] found improvements in RFD of the trapezius muscle following high-intensity progressive resistance training and Jay et al[30] have likewise reported increases in RFD and to a lesser extent increases in maximal strength following short duration progressive resistance training using elastic bands. Both of these studies utilized a progressive resistance training model with higher work intensities, which is a noteworthy contrast to the present study and therefore a reasonable explanation for the observed lack of improvement in RFD. Regardless of the lack of changes in physical function our research team has previously reported a 52% (average of 6 body regions) decrease in musculoskeletal pain in the same population of female laboratory technicians.[37] Based on the aforementioned research on RFD of chronically painful muscles, we expected to see an increase in RFD following the intervention period, as pain suggestively could inhibit motor output.[9,32–36] However, this was not the case. Musculoskeletal pain may not have been a limiting factor for muscle strength in the present population of laboratory technicians, and the present results suggest that pain reductions may not always lead to increased RFD. This is in contrast to the findings by Andersen et al[31] showing a significant correlation between pain reductions and increases in RFD. In our previous study reporting the changes in pain following the intervention, the average pain (0–10 VAS) of the 6 body regions was 2.9 and 2.6 for the PCMT and REF groups respectively, with pain scores up to 3.5 for the neck and 4.0 for the shoulders before intervention commencement.[37] These pain levels are similar to other studies demonstrating changes in RFD following resistance training,[18,30,31] indicating that the female laboratory technicians in the present study had sufficient chronic musculoskeletal pain for us to expect increases in RFD.
Similar to the lack of changes in physical function, we did not observe any changes in neurocognitive performance as tested by the CNSVS neurocognitive assessment software. This is in contrast to previous findings, which generally show pain subtly affecting some but not all domains of cognitive function either in a direct or indirect manner.[7–10] For example, a review by Moriarty et al[43] states that neurocognitive impairment is commonly associated with the pain experience being a severe obstacle to daily activities and rehabilitation strategies, especially in the chronic pain population. The literature surrounding chronic pain and cognitive performance impairment is however not conclusive. As mentioned previously, Oosterman et al[7] found only diminished sustained attention performance while mental flexibility, planning, and inhibition appeared to remain intact when investigating cognitive executive function and attention and Suhr[12] found that although fibromyalgia patients had more memory complaints and reported more fatigue, pain, and depression, they did not differ from healthy controls in cognitive performance. These observations on cognitive performance and pain are noteworthy and should be taken into account. In the present study, it is very likely that the intensity of pain experienced by the participants was not severe enough to affect neurocognitive performance significantly. Furthermore, the participants were not cognitively impaired at baseline, which makes it difficult to improve further. Pain has previously been shown to modulate cerebral activity during cognitive performance tasks, but uniformity of the modulation is divergent. Rémy et al[44] found that a positive modulation effect on thermo-nociception (thermal hot stimulation 46–49°C) was observed in the midcingulate and the dorsomedial prefrontal cortex. Conversely, a negative modulation effect was observed in perigenual cingulate cortex, insula, and medial thalamus.[44] We do not know which brain regions were active during the neurocognitive assessment in the present study, nor do we know what kind of brain activity chronic musculoskeletal pain of the magnitude reported by the laboratory technicians, elicits. Consequently, it can be speculated that overall modulatory brain activity did not change due to an insufficient pain sensation. This can be supported by the degree of pain induced to detect cortical activity changes by Rémy et al.[44] In their study, the participants reported the hot stimulus (46–49°C), inducing pain of more than 6 on a 0 to 10 VAS scale, which is much higher than the musculoskeletal pain reported by our participants. It may therefore be speculated that the laboratory technicians were not experiencing sufficiently intense pain to affect cognitive performance, which explains the lack of change in neurocognitive performance.
4.1. Strengths and limitations
The present study contains both strengths and limitations. The randomized controlled design with parallel assigned, concealed allocation, and blinded examiners reduced the risk of systematic bias and is therefore a major strength. Further, the limited number of drop-outs and the intention-to-treat analysis, which inherently accounts for missing values, are also noteworthy strengths. Finally, our dynamometer setup was highly reliable for testing physical function and was performed by the same test leader, which both contribute to the strengths of this study. However, an important limitation was the inability to blind participants to the treatments as well as the intervention consisted of several different elements. Participant outcome expectations are a limitation in intervention trials but in the present study we informed participants before group allocation that we did not know which treatment would work the best and whichever treatment at the end of the trial that proved to be the most effective in reducing musculoskeletal pain would be offered to the participants of the group not having received that particular treatment during the intervention. Finally, the seemingly nonconsensus about how cognitive function, and what domains to be included in the evaluation hereof, should be mentioned. For instance, Veldhuijzen et al[45] used only 2 single tests (the Stroop Color-Word Test and the Multi-Source Interference Test) to evaluate cognitive decline in fibromyalgia patients versus health controls, which lead them to conclude that cognitive inhibition remained intact but a decline in mental processing and/or psychomotor speed was evidenced. Clearly, a uniform definition of what is included as domains in cognitive processing is undetermined and constitutes a limitation to the present study as the CNSVS test battery does include psychomotor speed as a domain of cognitive function and processing ability.
5. Conclusion
A 10-week physical-cognitive-mindfulness training intervention did not improve maximal strength or RFD of chronically painful muscles. Neither did neurocognitive performance change over the intervention period. These findings are contradictory to previous findings, both in the field of musculoskeletal pain rehabilitation and neurocognitive function. Interestingly, RFD can remain unaltered even with significant decreases in pain. Furthermore, testing pain-derived impairment of neurocognitive function may lack validity without monitoring different brain regions, as the modulation of cognitive performance is not uniform in the cortex. In the present study, however, pain intensity may not have been sufficiently severe to limit neurocognitive function. Further exploration of neurocognitive impairment and musculoskeletal pain is therefore warranted.
Footnotes
Abbreviations: CNSVS = central nervous system vital signs, EMG = electromyography, MVC = maximal voluntary contraction, RFD = rate of force development, VAS = visual analog scale.
The authors have no conflicts of interest to disclose.
References
- [1].Bongers PM, Ijmker S, van den Heuvel S, et al. Epidemiology of work related neck and upper limb problems: psychosocial and personal risk factors (Part I) and effective interventions from a bio behavioural perspective (Part II). J Occup Rehabil 2006;16:272–95. [DOI] [PubMed] [Google Scholar]
- [2].Oberlinner C, Yong M, Nasterlack M, et al. Combined effect of back pain and stress on work ability. Occup Med Oxf Engl 2015;65:147–53. [DOI] [PubMed] [Google Scholar]
- [3].Sundstrup E, Jakobsen MD, Brandt M, et al. Workplace strength training prevents deterioration of work ability among workers with chronic pain and work disability: a randomized controlled trial. Scand J Work Environ Health 2014;40:244–51. [DOI] [PubMed] [Google Scholar]
- [4].Kettunen O, Vuorimaa T, Vasankari T. 12-mo intervention of physical exercise improved work ability, especially in subjects with low baseline work ability. Int J Environ Res Public Health 2014;11:3859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].de Boer AGEM, Burdorf A, van Duivenbooden C, et al. The effect of individual counselling and education on work ability and disability pension: a prospective intervention study in the construction industry. Occup Environ Med 2007;64:792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Vries HJ, Reneman MF, Groothoff JW, et al. Workers who stay at work despite chronic nonspecific musculoskeletal pain: do they differ from workers with sick leave? J Occup Rehabil 2012;22:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oosterman J, Derksen LC, van Wijck AJM, et al. Executive and attentional functions in chronic pain: does performance decrease with increasing task load? Pain Res Manag 2012;17:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Keogh E, Moore DJ, Duggan GB, et al. The disruptive effects of pain on complex cognitive performance and executive control. PLoS One 2013;8:e83272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Moore DJ, Keogh E, Eccleston C. The effect of threat on attentional interruption by pain. Pain 2013;154:82–8. [DOI] [PubMed] [Google Scholar]
- [10].Moore DJ, Keogh E, Eccleston C. Headache impairs attentional performance. Pain 2013;154:1840–5. [DOI] [PubMed] [Google Scholar]
- [11].Berryman C, Stanton TR, Bowering KJ, et al. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev 2014;34:563–79. [DOI] [PubMed] [Google Scholar]
- [12].Suhr JA. Neuropsychological impairment in fibromyalgia: relation to depression, fatigue, and pain. J Psychosom Res 2003;55:321–9. [DOI] [PubMed] [Google Scholar]
- [13].Angevaren M, Aufdemkampe G, Verhaar HJJ, et al. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 2008;CD005381. [DOI] [PubMed] [Google Scholar]
- [14].Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med 2010;72:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Snowden M, Steinman L, Mochan K, et al. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc 2011;59:704–16. [DOI] [PubMed] [Google Scholar]
- [16].Sanger KL, Dorjee D. Mindfulness training for adolescents: a neurodevelopmental perspective on investigating modifications in attention and emotion regulation using event-related brain potentials. Cogn Affect Behav Neurosci 2015;15:696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Conscious Cogn 2009;18:176–86. [DOI] [PubMed] [Google Scholar]
- [18].Andersen LL, Holtermann A, Jørgensen MB, et al. Rapid muscle activation and force capacity in conditions of chronic musculoskeletal pain. Clin Biomech Bristol Avon 2008;23:1237–42. [DOI] [PubMed] [Google Scholar]
- [19].Ervilha UF, Arendt-Nielsen L, Duarte M, et al. Effect of load level and muscle pain intensity on the motor control of elbow-flexion movements. Eur J Appl Physiol 2004;92:168–75. [DOI] [PubMed] [Google Scholar]
- [20].Aagaard P. Training-induced changes in neural function. Exerc Sport Sci Rev 2003;31:61–7. [DOI] [PubMed] [Google Scholar]
- [21].Suetta C, Aagaard P, Rosted A, et al. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol (1985) 2004;97:1954–61. [DOI] [PubMed] [Google Scholar]
- [22].Kamaleri Y, Natvig B, Ihlebaek CM, et al. Number of pain sites is associated with demographic, lifestyle, and health-related factors in the general population. Eur J Pain Lond Engl 2008;12:742–8. [DOI] [PubMed] [Google Scholar]
- [23].Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol 1998;513(Pt 1):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Andersen LL, Andersen JL, Magnusson SP, et al. Neuromuscular adaptations to detraining following resistance training in previously untrained subjects. Eur J Appl Physiol 2005;93:511–8. [DOI] [PubMed] [Google Scholar]
- [25].Aagaard P, Simonsen EB, Andersen JL, et al. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol (1985) 2002;93:1318–26. [DOI] [PubMed] [Google Scholar]
- [26].Jay K, Frisch D, Hansen K, et al. Kettlebell training for musculoskeletal and cardiovascular health: a randomized controlled trial. Scand J Work Env Health 2011;37:1–8. [DOI] [PubMed] [Google Scholar]
- [27].Andersen LL, Andersen CH, Sundstrup E, et al. Central adaptation of pain perception in response to rehabilitation of musculoskeletal pain: randomized controlled trial. Pain Physician. 2012;15:385–394. [PubMed] [Google Scholar]
- [28].Jakobsen MD, Sundstrup E, Brandt M, et al. Effect of workplace- versus home-based physical exercise on musculoskeletal pain among healthcare workers: a cluster randomized controlled trial. Scand J Work Environ Health 2015;41:153–63. [DOI] [PubMed] [Google Scholar]
- [29].Sundstrup E, Jakobsen MD, Andersen CH, et al. Effect of two contrasting interventions on upper limb chronic pain and disability: a randomized controlled trial. Pain Physician 2014;17:145–54. [PubMed] [Google Scholar]
- [30].Jay K, Schraefel M, Andersen CH, et al. Effect of brief daily resistance training on rapid force development in painful neck and shoulder muscles: Randomized controlled trial. Clin Physiol Funct Imaging 2012;33:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Andersen LL, Andersen JL, Suetta C, et al. Effect of contrasting physical exercise interventions on rapid force capacity of chronically painful muscles. J Appl Physiol (1985) 2009;107:1413–9. [DOI] [PubMed] [Google Scholar]
- [32].Nijs J, Daenen L, Cras P, et al. Nociception affects motor output: a review on sensory-motor interaction with focus on clinical implications. Clin J Pain 2012;28:175–81. [DOI] [PubMed] [Google Scholar]
- [33].Urban PP, Solinski M, Best C, et al. Different short-term modulation of cortical motor output to distal and proximal upper-limb muscles during painful sensory nerve stimulation. Muscle Nerve 2004;29:663–9. [DOI] [PubMed] [Google Scholar]
- [34].Carleton RN, Asmundson GJG, Collimore KC, et al. Strategic and automatic threat processing in chronic musculoskeletal pain: a startle probe investigation. Cogn Behav Ther 2006;35:236–47. [DOI] [PubMed] [Google Scholar]
- [35].Diederichsen LP, Winther A, Dyhre-Poulsen P, et al. The influence of experimentally induced pain on shoulder muscle activity. Exp Brain Res 2009;194:329–37. [DOI] [PubMed] [Google Scholar]
- [36].Arendt-Nielsen L, Graven-Nielsen T. Muscle pain: sensory implications and interaction with motor control. Clin J Pain 2008;24:291–8. [DOI] [PubMed] [Google Scholar]
- [37].Jay K, Brandt M, Hansen K, et al. Effect of individually tailored biopsychosocial workplace interventions on chronic musculoskeletal pain and stress among laboratory technicians: randomized controlled trial. Pain Physician 2015;18:459–71. [PubMed] [Google Scholar]
- [38].Jay K, Brandt M, Sundstrup E, et al. Effect of individually tailored biopsychosocial workplace interventions on chronic musculoskeletal pain, stress and work ability among laboratory technicians: randomized controlled trial protocol. BMC Musculoskelet Disord 2014;15:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kuorinka I, Jonsson B, Kilbom A, et al. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon 1987;18:233–7. [DOI] [PubMed] [Google Scholar]
- [40].Brief CNS VS Interpretation Guide 2014 [Internet]. CNSVS. [cited Jul 6, 2015]. Available at: http://www.cnsvs.com/WhitePapers/CNSVS-BriefInterpretationGuide.pdf (accessed January 10, 2016). [Google Scholar]
- [41].Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol 2006;21:623–43. [DOI] [PubMed] [Google Scholar]
- [42].Häkkinen K, Komi PV, Alén M. Effect of explosive type strength training on isometric force- and relaxation-time, electromyographic and muscle fibre characteristics of leg extensor muscles. Acta Physiol Scand 1985;125:587–600. [DOI] [PubMed] [Google Scholar]
- [43].Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol 2011;93:385–404. [DOI] [PubMed] [Google Scholar]
- [44].Rémy F, Frankenstein UN, Mincic A, et al. Pain modulates cerebral activity during cognitive performance. NeuroImage 2003;19:655–64. [DOI] [PubMed] [Google Scholar]
- [45].Veldhuijzen DS, Sondaal SFV, Oosterman JM. Intact cognitive inhibition in patients with fibromyalgia but evidence of declined processing speed. J Pain 2012;13:507–15. [DOI] [PubMed] [Google Scholar]


