Abstract
To clarify clinical characteristics of immune thrombocytopenia (ITP) subsets associated with autoimmune diseases (AIDs).
Five thousand five hundred twenty patients were reviewed retrospectively. One hundred four ITP patients were included for analysis. Clinical manifestations at first thrombocytopenic episode were recorded.
Systemic lupus erythematosus (SLE) and primary Sjogren syndrome (pSS) accounted for a large part in AIDs associated with secondary ITP. SLE-ITP, pSS-ITP, and primary ITP (pITP) patients were different in several aspects in clinical and immunological characteristics. A subgroup of patients in pITP patients with some obvious autoimmune features (defined as AIF-ITP) such as positive ANA but failing to meet the diagnosis criteria now used for a specific kind of connective tissue diseases were also different with other pITP patients in some immunological features, indicating the difference in the pathogenesis mechanism of those autoimmune featured ITP patients.
ITP patients were heterogeneous in clinical characteristics. Further study about the different pathogenesis of ITP subsets especially those AIF-ITP patients who only presented with thrombocytopenia will help us have a better understanding of pathogenesis of ITP and a better management of ITP patients.
Keywords: autoimmune diseases, clinical characteristics, immune thrombocytopenia
1. Introduction
Immune thrombocytopenia (ITP) is an acquired immune mediated disorder, defined as a peripheral blood platelet (PLT) count less than 100 × 109/L, which can be classified into secondary ITP and primary immune thrombocytopenia (pITP) based on if there is an underlying recognized diseases.[1] Pathogenesis of ITP is complex and still not very clear. Multiple mechanisms such as autoantibodies, complement, and T cells have been described in ITP, highlighting the heterogeneous nature of this disease.[2] Due to the heterogeneous nature of this disease, clinical features and the response to regular drug therapy such as glucocorticoid are different.[3] Though it is well known that ITP can be associated with different diseases or presented as sole manifestation, the specific difference between these different ITP subsets is still not clear. Lessons learned from secondary ITP suggest that pITP is also heterogeneous.[4] Different clinical characteristics and pathophysiology are still need to be evaluated in different subsets of ITP patients for a better management of ITP.
Autoimmune disease (AID) is one of the most common diseases complicated with secondary ITP. Prevalence of ITP has been reported to be ranging from 7% to 30% in systemic lupus erythematosus (SLE) patients, and clinical characteristics of ITP in SLE patients have been studied by several groups.[5,6] However, clinical characteristics of ITP in other AID such as Sjogren syndrome (SS) and the difference between ITP associated with different AID are still not very clear. In an endeavor to explain these questions, a retrospective study was undertaken to analyze basic clinical and immunological characteristics of ITP patients in rheumatology department.
2. Patients and methods
2.1. Patients
All available 5520 medical records of patients during 2012 to 2015 in rheumatology inpatient department of our hospital were reviewed. Approval by the ethics committee of The First Affiliated Hospital of Xiamen University was obtained for all procedures. Patients with diagnosis of thrombocytopenia, defined as a peripheral blood PLT count less than 100 × 109/L were enrolled for analysis. Two hundred twenty-six patients with diagnosis of thrombocytopenia were enrolled in this study. For those patients who were referred to our department several times, only the first encounter or the encounter with most comprehensive medical records was enrolled for analysis. After exclusion of the duplicate cases and those in which thrombocytopenia were caused by infection such as hepatitis C virus and drug, 85 patients in rheumatology department with diagnosis of ITP were analyzed. For analysis of clinical characteristics of pITP patients, another 19 primary ITP patients were further collected from hematology department. The diagnosis of connective disease was according to the American College of Rheumatology criteria.[7–11] The diagnosis of primary ITP was according to the International Working Group definition.[12]
2.2. Variables
Clinical data including detailed patients history, clinical manifestations, laboratory findings, and treatment strategy were obtained from patients’ medical records.
Basic data such as age, sex, and diagnosis were recorded. Clinical manifestations including symptoms related to connective tissue disease (CTD) such as skin rash, arthralgia, fever, and Raynaud phenomenon were recorded.
PLT number as well as white blood cell number and level of hemoglobin (HGB) at the onset of thrombocytopenia were recorded. Immunological characteristics such as autoantibody profile, complement level, and immunoglobulin level tested at the same time with PLT number test were recorded. Lymphocytes subsets such as T cells, B cells, and natural killer cells in peripheral blood were also recorded in some patients. Laboratory parameters such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine urea, albumin, and globulin (GLO) were also recorded.
Antinuclear antibodies (ANA) and anti-ds-DNA antibodies were determined by indirect immunofluorescence; anticardiolipin antibodies (ACA) and β2-glycoprotein 1 (β2-GP1) by a standard ELISA; antibodies to ds-DNA, SSA, SSB, Ro-52, RNP, RIB, AHA, Anua, CENPB, and Jo-1 by immunoblotting; complement 3 (C3), complement 4 (C4), IgG, IgA, IgM, and C reactive protein (CRP) by particular globin analyzer.
2.3. Statistical analysis
For 2 group comparisons of the binary data, either the Chi-squared test or Fisher exact test was used, and t test was used in comparison of continuous data. The resulting P values were corrected for multiple testing using the Benjamini–Hochberg method.[13] A P < 0.05 was considered significant. Continuous data are expressed as mean ± SD (standard deviation), and categorical data are expressed as positive “+” or negative “−.” All statistical calculations were done using SPSS21.0 software.
3. Results
3.1. SLE accounted for large part among autoimmune diseases associated with ITP
Prevalence of subsets of ITP patients secondary to different AIDs as well as primary ITP patients in rheumatology department of our hospital is shown in Table 1. Among the 85 patients with ITP, 33 (38.82%) patients were diagnosed as SLE, 16 (18.82%) patients were diagnosed as primary Sjogren syndrome (pSS), 2 (2.35%) patients were diagnosed as primary antiphospholipid syndrome (APS), 2 (2.35%) patients were diagnosed as dermatomyositis (DM), 1 (1.18%) patient was diagnosed as systemic sclerosis (SSc), 4 (4.71%) patients were diagnosed as undifferentiated connective tissue disease (UCTD), 3 (3.53%) patients were diagnosed as rheumatoid arthritis (RA), 24 (28.24%) patients were diagnosed as primary ITP.
Table 1.
Prevalence of different subsets of ITP patients associated with autoimmune diseases.
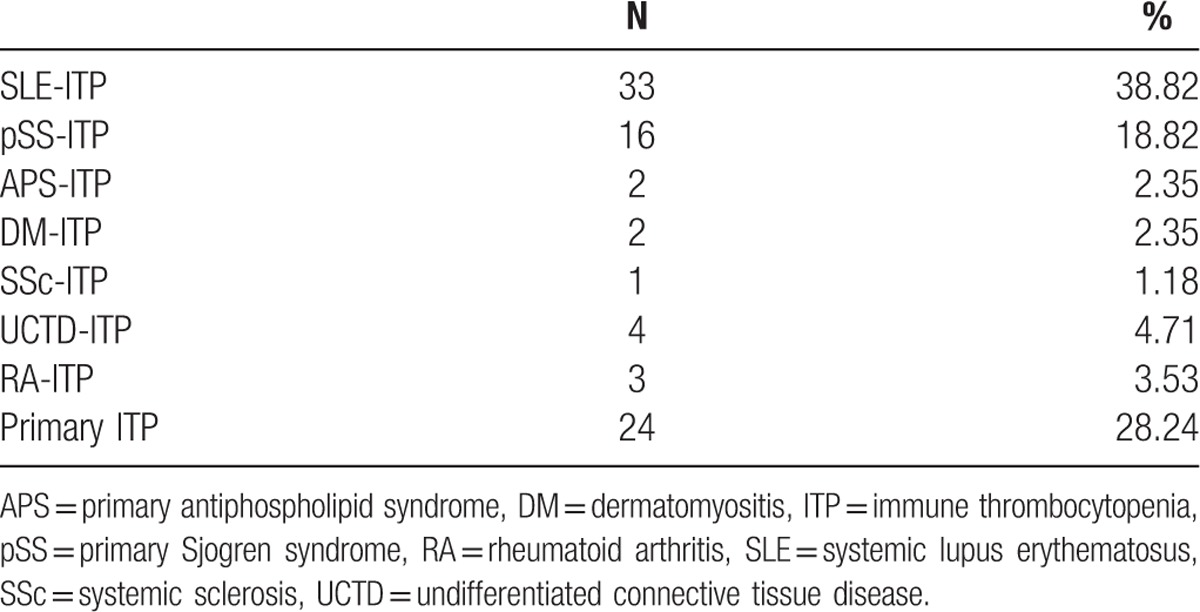
SLE-ITP might be most common in secondary ITP patients associated with AIDs. Patients with some characteristics of CTDs such as positive ANA and arthritis but failing to been classified into any specific CTDs or UCTD and diagnosed as primary ITP also accounted for a large part of those ITP patients in rheumatology department.
3.2. Clinical characteristics were different in subsets of ITP patients
As SLE-ITP, pSS-ITP, and primary ITP (pITP) accounted for the major part of ITP patients in rheumatology department, clinical characteristics of these 3 groups were further analyzed. For a better analysis of primary ITP patients, 19 more primary ITP were collected from hematology department.
Basic clinical characteristics of these 3 ITP groups are shown in Table 2. Patients in pSS-ITP group were older than SLE-ITP and pITP group, however only significant difference was detected between pSS-ITP and SLE-ITP group. The percentage of female patients was similar in these 3 groups. Symptoms related to CTD such as arthritis, dry mouth, dry eye, and skin rash were rarely seen in pITP patients compared with SLE-ITP and pSS-ITP patients. Dry eye was more specific to pSS-ITP group. Skin rash was more common in SLE-ITP group. Hemorrhagic manifestations such as mucosal bleeding were more common in pITP group, however only significant difference was detected between SLE-ITP and pITP group. Renal disease was found in 21.20% in SLE-ITP group, as no patient with renal disease was found in pSS-ITP and pITP group. Other symptoms including fever, oral ulcers, Raynaud phenomenon, swelling, and weakness were all less common in all these 3 ITP group. Interstitial lung disease (ILD) and thyroid abnormalities were presented in all 3 groups with no significant difference in prevalence.
Table 2.
Clinical characteristics in SLE-ITP, pSS-ITP, and pITP patients.
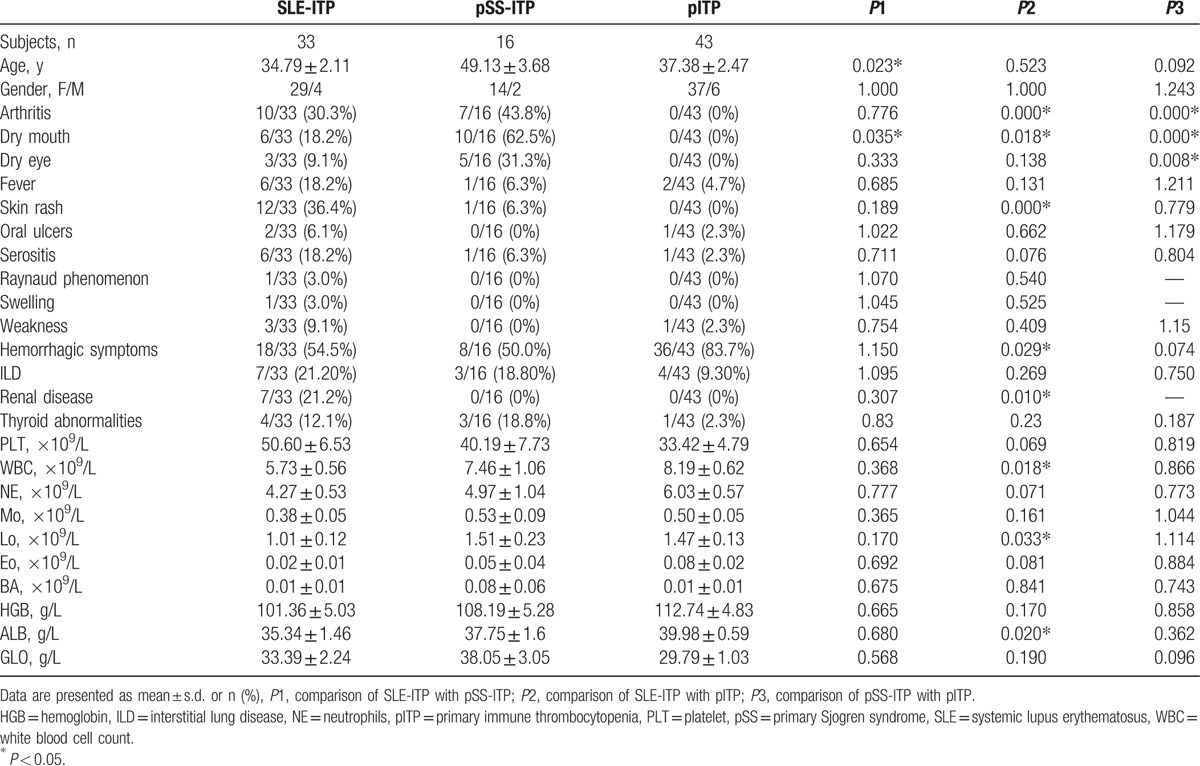
Number of PLT in peripheral blood in pITP group was lower than SLE-ITP and pSS-ITP group, which may relate to the more common hemorrhagic manifestations in pITP patients, though no significant difference was detected. Laboratory findings including white blood cell count (WBC) and lymphocytes (Lo) number in peripheral blood in SLE-ITP group were significant lower than pITP group. Level of albumin in serum was significant lower in SLE-ITP group compared with pITP group. No significant difference was detected in levels of neutrophils (NE), monocytes (Mo), basophils (Bo), eosinophil (Eo), HGB, and GLO between these 3 groups.
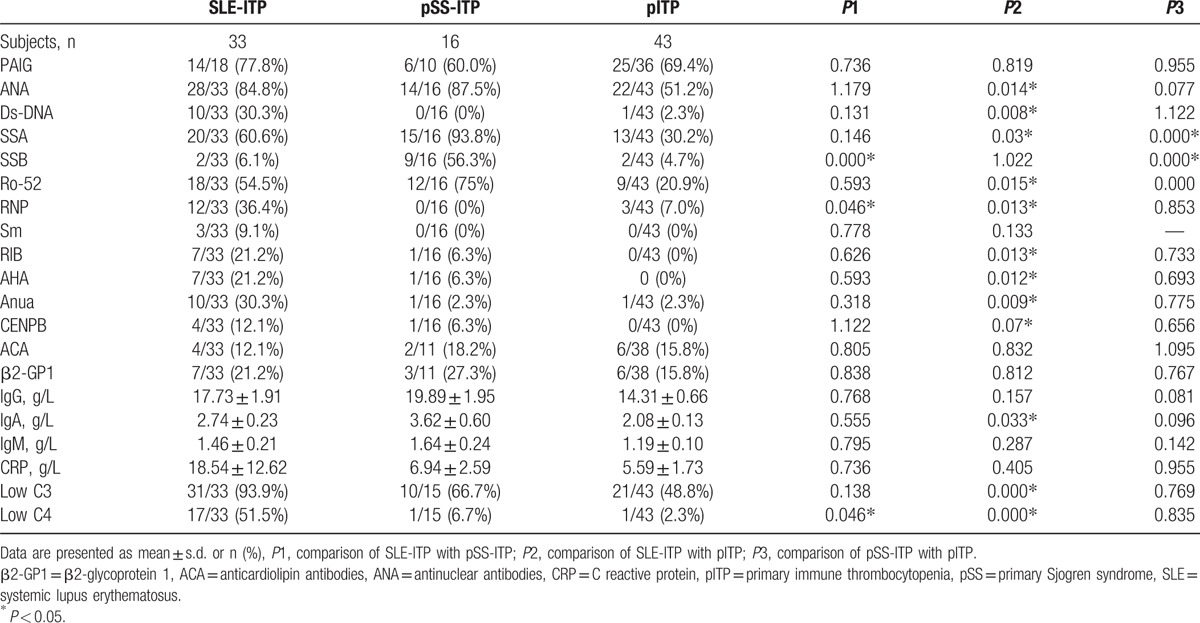
Immunological characteristics of these 3 ITP groups are shown in Table 3. PLT associated immunoglobulin was found in 77.80% patients in SLE-ITP group, 60.00% patients in pSS-ITP group, and 69.40% patients in pITP group. Positive ANA were found in a large part of these ITP patients even in pITP group. However, prevalence of positive ANA was significant lower in pITP group compared with SLE-ITP group. Autoantibody to ds-DNA and RNP were more specific to SLE-ITP group, and anti-SSA and anti-SSB were more common in pSS-ITP group. Anti-Smith autoantibody was only found in SLE-ITP patient, though no significant difference was detected which might due to relatively small sample size. Prevalence of other autoantibodies such as Ro-52, RIB, AHA, Anua, CENPB were significant higher in SLE-ITP group than pITP group, though no significant difference was detected between SLE-ITP and pSS-ITP group or between pSS-ITP group and pITP group. Level of IgA was significant lower in pITP group than SLE-ITP group. Decreased level of C3 in serum was presented in a large part of ITP patients in all 3 groups, and this was more common in SLE-ITP group. Decreased level of C4 in serum was mainly presented in SLE-ITP group, which was rarely seen in pSS-ITP and pITP groups. Prevalence of anticardiolipin (ACA) antibody, β2-GP1, level of IgG, IgM, and CRP were similar in the 3 groups.
Table 3.
Immunological characteristics in SLE-ITP, pSS-ITP, and pITP patients.
3.3. Heterogeneity existed in primary ITP patients
Among those pITP patients who did not meet criteria for diagnosis of any kind of CTDs or UCTD, a subset of patients with more obvious AIF-ITP such as positive ANA or other specific autoantibodies can be easily distinguished from other pITP patients, whether these autoimmune featured pITP patients were different from other pITP patients was unknown. In order to answer this question, we further analyzed clinical characteristics in pITP patients with positive ANA or positive autoantibodies common presented in CTDs including SSA, SSB, Ro-52, ds-DNA, Smith, RIB, AHA, Anua, RNP, CENPB, ACA, and β2-GP1, which was defined as autoimmune featured pITP (AIF-ITP) group in analysis.
Basic clinical characteristics of AIF-ITP and pITP group are shown in Table 4. No significant difference was detected in these basic clinical characteristics such as age, gender, symptoms related to CTDs and other laboratory findings between AIF-ITP and pITP group.
Table 4.
Clinical characteristics in AIF-ITP and pITP patients.
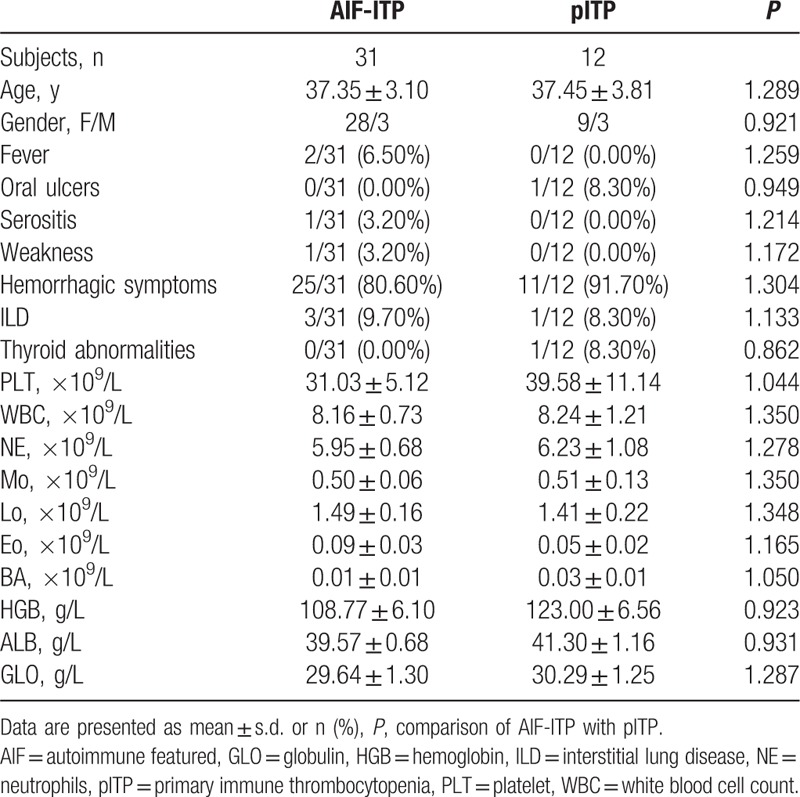
Immunological characteristics of AIF-ITP and pITP are shown in Table 5. Prevalence of positive autoantibodies was significant higher in AIF-ITP group than pITP group, which was due to the way we define the group. Positive ANA was presented in 71% of AIF-ITP group. Autoantibodies related with Sjogren syndrome including anti-SSA and anti-Ro-52 were common in AIF-ITP group. Other autoantibodies related to SLE or SSc patients were rarely seen in AIF-ITP patients. Among those AIF-ITP patients with positive ANA, 11 (50%) patients had no autoantibodies against extractable nuclear antigen (ENA) been detected.
Table 5.
Immunological characteristics in AIF-ITP and pITP patients.
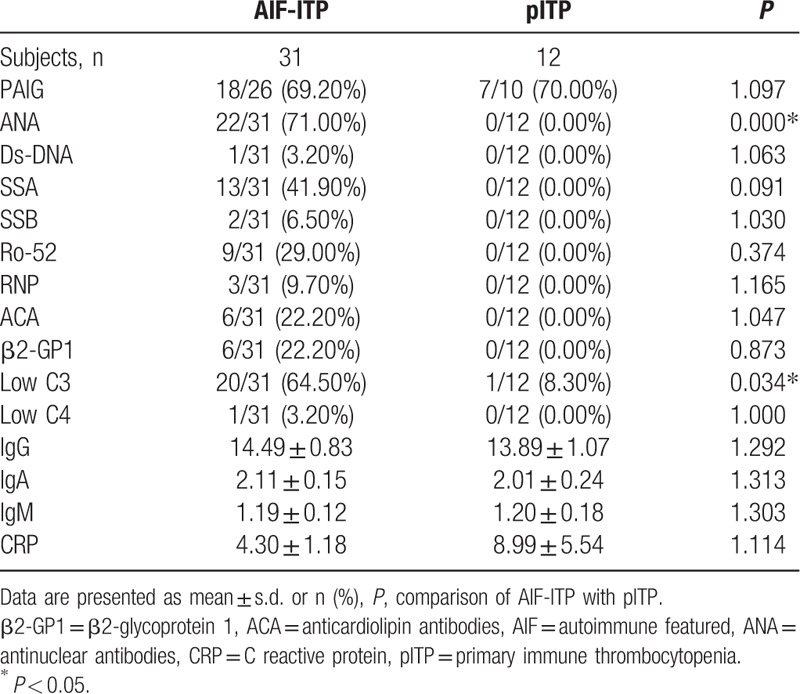
Low level of C3 was presented in 64.50% in AIF-ITP patients, while only 1 (8.30%) in pITP group presented with low C3, indicating role of C3 in the pathogenesis mechanism of AIF-ITP patients instead of pITP patients. Low level of C4 was rarely seen in both AIF-ITP and pITP group. No significant difference was detected in level of IgG, IgA, IgM, and CRP between AIF-ITP and pITP patients.
3.4. Correlation of different immunological variables with PLT
Level of C3, C4, and IgG were usually used as markers for the evaluation of disease activity in several AIDs such as SLE and pSS. We further analyzed the correlation of C3 (Fig. 1A), C4 (Fig. 1B), and IgG (Fig. 1C) with number of PLT in CTD-ITP and AIF-ITP patients. However, no significant correlation was found between C3, C4, and IgG with PLT, nor within any subgroups of ITP (data not shown here). Lymphocyte subsets including T cells, B cells, and NK cells were tested in few of CTD-ITP and AIF-ITP patients. Though the percentage of B cells in peripheral blood lymphocytes was only tested in 7 patients, a significant negative correlation was found between B cells percentage and PLT, indicating the major role of B cells in the pathogenesis of ITP.
Figure 1.
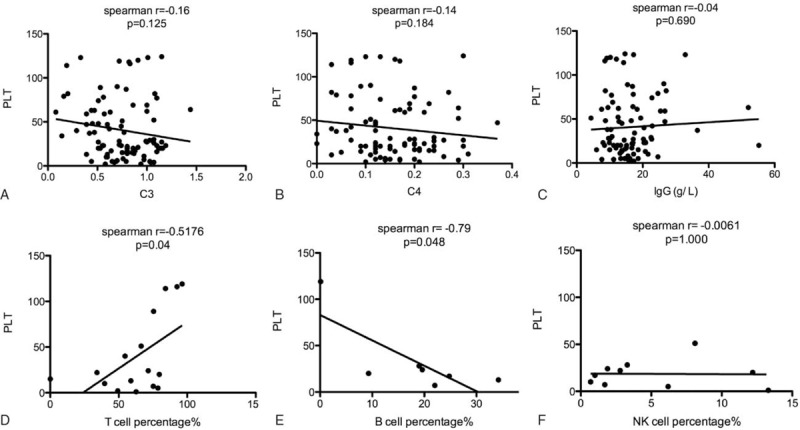
Correlation of different immunological variables with PLT. (A) The correlation between C3 level and PLT in ITP patients was determined using Spearman correlation coefficients, n = 85. (B) The correlation between C4 level and PLT in ITP patients was determined using Spearman correlation coefficients, n = 85. (C) The correlation between IgG level and PLT in ITP patients was determined using Spearman correlation coefficients, n = 85. (D) The correlation between T cell percentage in peripheral blood lymphocytes and PLT in ITP patients was determined using Spearman correlation coefficients, n = 16. (E) The correlation between B cell percentage in peripheral blood lymphocytes and PLT in ITP patients was determined using Spearman correlation coefficients, n = 7. (F) The correlation between NK cell percentage in peripheral blood lymphocytes and PLT in ITP patients was determined using Spearman correlation coefficients, n = 10.
4. Discussion
ITP was a common manifestation complicated with AIDs. Though it was well known that immune factors were involved in the pathogenesis of thrombocytopenia, it was recently recognized that diversity existed in ITP in both pathogenesis and clinical characteristics.[4] Our study was the first study to analyze the different clinical characteristics among different subsets of ITP.
ITP was common in SLE patients, ranging from 7% to 30% in large reported series.[14,15] ITP in SLE patients defined a subgroup of patients with higher morbidity and is thus a major complication of SLE.[5] In these SLE-ITP patients, low baseline C3 levels seemed to correlate with an increased risk of relapse in thrombocytopenia in patients with SLE, and predict subsequent C3 measurements.[16] Besides the characteristics of SLE-ITP patients compared with other SLE patients without ITP, our study explored more SLE-ITP-specific characteristics compared with other ITP subsets. In our study, we found that SLE-ITP might be most common in ITP patients in rheumatology department. Compared with other ITP patients such as pSS-ITP and pITP patients, autoantibodies specific to SLE such as ds-DNA and were also specific to SLE-ITP patients. Skin rash such as malar rash and discoid rash were also more common in SLE-ITP patients. Level of lymphocytes in peripheral blood was much lower in SLE-ITP patients. Level of C3 and C4 were significant lower in SLE-ITP patients, especially C4. Low level of C4 was presented in more than half of SLE-ITP patients, while low level of C4 was rarely seen in other ITP subsets, indicating the specific role of C4 in the pathogenesis of SLE-ITP compared with other ITP subsets.
ITP in Sjogren syndrome has been reported in several case reports.[17,18] Intravenous immunoglobulin and low-dose rituximab has been reported to be effective in pSS-ITP.[19,20] However, the specific clinical characteristics of pSS-ITP patients were still not very clear. Our study found that pSS-ITP was second common in CTD associated secondary ITP, accounting for about 18.82% in thrombocytopenia patients in rheumatology department. Compared with SLE-ITP, pSS-ITP patients were significant older. Prevalence of symptoms associated with pSS such as dry mouth and autoantibodies associated with pSS such as SSA was significant higher in pSS-ITP patients.
Primary immune thrombocytopenia (pITP) is an acquired immune-mediated disorder characterized by isolated thrombocytopenia, defined as the absence of any obvious initiating and/or underlying cause of the thrombocytopenia.[1] Until recently, the abbreviation ITP stood for idiopathic thrombocytopenic purpura, but current awareness relating to the immune-mediated nature of the disease. Immune factors caused by infection, cancer, and AIDs were proved to be involved in the pathogenesis of ITP. Different causes of immune abnormalities gave rise to the diversity of ITP patients.[4] Autoimmune-mediated thrombocytopenia was common associated with CTDs. However, current classification criteria do not allow a CTD designation for thrombocytopenia in isolation. There was a subset of patients in pITP patients who have some AIF-ITP such as positive ANA who did not meet classification criteria for any CTDs or UCTD. In our study, we classified these patients as autoimmune-featured thrombocytopenia (AIF-ITP) and analyzed the clinical characteristics of this group. We found that compared with other pITP patients, decreased level of C3 was significant higher in AIF-ITP patient, indicating the important role of complement system in the pathogenesis of AIF-ITP patients instead of other pITP patients. Anti-SSA was the major autoantibody found in AIF-ITP patients, with prevalence of 41.90%. However, among those AIF-ITP patients with positive ANA, about 50% patients had no autoantibodies against ENA been detected, indicating other unknown autoantibodies which may be specific to ITP in those patients. Study of those patients may provide an opportunity to further our understanding of tissue-specific autoantibodies and the pathogenesis of autoimmune thrombocytopenia.
Though complement and autoantibodies were proved to be involved in the pathogenesis of ITP, our study found no correlation between level of C3, C4, and IgG in CTD-ITP and AIF-ITP patients, indicating the complex mechanism in ITP pathogenesis. A significant negative correlation was found between PLT and B cells percentage, confirming the important role of B cells in the pathogenesis of autoimmune thrombocytopenia.
This is the first study describing the different clinical characteristics of ITP subsets, especially those autoimmune-featured ITP patients in primary ITP patients. However, this study has several limitations. Firstly, it is a retrospective study conducted in only 1 institute, and the study sample might not be enough to detect some potential difference between groups. Secondly, the lack of certain clinical information of some patients may lead to the bias of this study. Thirdly, as we only included ITP patients in the inpatient department, the difference between patients in outpatient and inpatient department may also contribute to the bias of this study.
5. Conclusion
Our study showed that clinical characteristics were different among ITP subsets, indicating the different pathogenesis of ITP subsets. Diversity also existed in pITP patients according to current criteria. There was a subset of patients in pITP patients with some features of autoimmune such as positive ANA in who thrombocytopenia was the sole manifestation, who did not meet the diagnosis criteria for any CTDs. Those autoimmune-featured thrombocytopenia (AIF-ITP) were significant different with other pITP patients in some clinical characteristics. Study of the different pathogenesis of ITP subsets especially those AIF-ITP patients who only presented with thrombocytopenia will help us understand the pathogenesis of ITP and have a better management of ITP patients.
Footnotes
Abbreviations: β2-GP1 = β2-glycoprotein 1, ACA = anticardiolipin antibodies, AID = autoimmune disease, AIF-ITP = autoimmune features, ANA = antinuclear antibodies, Bo = basophils, C3 = complement 3, C4 = complement 4, CRP = C reactive protein, CTDs = connective tissue diseases, DM = dermatomyositis, ENA = extractable nuclear antigen, Eo = eosinophil, GLO = globulin, HGB = hemoglobin, ILD = interstitial lung disease, ITP = immune thrombocytopenia, Lo = lymphocytes, Mo = monocytes, NE = neutrophils, pITP = primary ITP, PLT = platelet, pSS = primary Sjogren syndrome, RA = rheumatoid arthritis, SLE = systemic lupus erythematosus, SSc = systemic sclerosis, UCTD = undifferentiated connective tissue disease, WBC = white blood cell count.
Funding: The work was partially supported by NSFC (Natural Science Foundation of China) grant 81273285 to Dr. Guixiu Shi and NSFC (Natural Science Foundation of China) grant 81501407 to Dr. Yuan Liu.
The authors have no conflicts of interest to disclose.
References
- [1].Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168–86. [DOI] [PubMed] [Google Scholar]
- [2].Cines DB, McMillan R. Pathogenesis of chronic immune thrombocytopenic purpura. Curr Opin Hematol 2007;14:511–4. [DOI] [PubMed] [Google Scholar]
- [3].Provan D, Newland AC. Current management of primary immune thrombocytopenia. Adv Ther 2015;32:875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood 2009;113:6511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ziakas PD, Giannouli S, Zintzaras E, et al. Lupus thrombocytopenia: clinical implications and prognostic significance. Ann Rheum Dis 2005;64:1366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jallouli M, Frigui M, Marzouk S, et al. Clinical implications and prognostic significance of thrombocytopenia in Tunisian patients with systemic lupus erythematosus. Lupus 2012;21:682–7. [DOI] [PubMed] [Google Scholar]
- [7].Silman AJ. The 1987 revised American Rheumatism Association criteria for rheumatoid arthritis. Br J Rheumatol 1988;27:341–3. [DOI] [PubMed] [Google Scholar]
- [8].Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum 2001;44:735–6. [DOI] [PubMed] [Google Scholar]
- [9].Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. [DOI] [PubMed] [Google Scholar]
- [10].Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- [11].Vitali C, Bombardieri S, Moutsopoulos HM, et al. Assessment of the European classification criteria for Sjogren's syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjogren's Syndrome. Ann Rheum Dis 1996;55:116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117:4190–207. [DOI] [PubMed] [Google Scholar]
- [13].Ferreira JA. The Benjamini-Hochberg method in the case of discrete test statistics. Int J Biostat 2007;3: Article 11. [DOI] [PubMed] [Google Scholar]
- [14].Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine 1999;78:167–75. [DOI] [PubMed] [Google Scholar]
- [15].Mok CC, Lee KW, Ho CT, et al. A prospective study of survival and prognostic indicators of systemic lupus erythematosus in a southern Chinese population. Rheumatology 2000;39:399–406. [DOI] [PubMed] [Google Scholar]
- [16].Ziakas PD, Poulou LS, Giannouli S, et al. Thrombocytopenia in lupus: baseline C3 as an independent risk factor for relapse. Ann Rheum Dis 2007;66:130–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Klepfish A, Friedman J, Schechter Y, et al. Autoimmune neutropenia, thrombocytopenia and Coombs positivity in a patient with primary Sjogren's syndrome. Rheumatology 2001;40:948–9. [DOI] [PubMed] [Google Scholar]
- [18].Tachibana J, Sugai S, Yoshioka R, et al. [Four patients with thrombocytopenia associated with Sjogren's syndrome]. Nihon Naika Gakkai Zasshi 1988;77:1697–703. [DOI] [PubMed] [Google Scholar]
- [19].Choung BS, Yoo WH. Successful treatment with intravenous immunoglobulin of severe thrombocytopenia complicated in primary Sjogren's syndrome. Rheumatol Int 2012;32:1353–5. [DOI] [PubMed] [Google Scholar]
- [20].Zhou L, Xin XF, Wu HX. [The efficacy and safety of low-dose rituximab in treatment of primary Sjogren's syndrome with thrombocytopenia]. Zhonghua Nei Ke Za Zhi 2012;51:37–41. [PubMed] [Google Scholar]


