Supplemental Digital Content is available in the text
Keywords: EGFR, NSCLC, platinum–gemcitabine, targeted therapy
Abstract
Background:
Trials investigating the efficacy and safety of combining molecular targeted agent (MTA) with platinum–gemcitabine (PG) in first-line treatment of advanced non-small cell lung cancer (NSCLC) have shown inconsistent findings. This meta-analysis aimed to explore whether the addition of MTAs to PG in NSCLC could provide a survival benefit with a tolerable toxicity.
Methods:
Web of knowledge, PubMed, Ovid, Embase, and Cochrane Library were searched to identify relevant studies and extract data on overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and common grade 3 or 4 adverse events. Subgroup analyses were conducted on the basis of race and the type of MTA.
Results:
Twelve trials with a total of 6143 patients were included in this meta-analysis. Compared with PG chemotherapy, combination therapy of MTA with PG did not improve OS (hazard ratio [HR] = 0.96, 95% confidence interval [CI] = 0.90–1.01) but improved PFS (HR = 0.77, 95% CI = 0.66–0.89) and ORR (risk ratio [RR] = 1.33, 95% CI = 1.11–1.60). Subanalysis indicated that there was more incidence of grade 3 or 4 rash (RR = 11.20, 95% CI = 6.07–20.68), anemia (RR = 1.21, 95% CI = 1.01–1.46), diarrhea (RR = 2.62, 95% CI = 1.21–5.65), and anorexia (RR = 2.08, 95% CI = 1.12–3.88) in combining epidermal growth factor receptor targeted therapy group compared to PG group. An increased risk of grade 3 or 4 rash (RR = 5.08, 95% CI = 1.53–16.79), thrombocytopenia (RR = 1.50, 95% CI = 1.03–2.18), and hypertension (RR = 2.36, 95% CI = 1.05–5.32) was observed in sorafenib combination group.
Conclusion:
The combination of PG plus MTA was superior to PG alone in terms of PFS and ORR but not in OS. The combination chemotherapy also showed a higher frequency of grade 3 or higher toxic effects in patients with advanced NSCLC than PG chemotherapy.
1. Introduction
Non-small cell lung cancer (NSCLC), as the major cause of cancer-related death,[1] is often confirmed at the advanced stage when surgery is unsuitable.[2,3] For these patients, the first-line chemotherapy regimens mainly comprise platinum-based doublet with a combination of gemcitabine, docetaxel, vinorelbine, paclitaxel, or pemetrexed.[4] In particular, cisplatin–gemcitabine is widely used for its favorable efficacy and tolerable toxicity profile.[5–7] However, trials comparing the therapeutic effect of distinctive platinum-based chemotherapy have indicated that none of these cytotoxic chemotherapy could provide patients with significant survival benefit over other chemotherapies,[8] which implies that the standard cytotoxic chemotherapy has reached a therapeutic plateau,[4,9] and the alternative treatment strategies for those unresectable or metastatic NSCLC patients are urgently needed.
Numerous efforts have been made to develop the targeted therapies for NSCLC that can inhibit tumor cell invasion, growth and metastasis by blocking corresponding signal transduction pathways,[10] including gefitinib, erlotinib, and afatinib that are approved in the United States as the first-line treatment of NSCLC in patients with epidermal growth factor receptor (EGFR) mutation-positive, ceritinib approved for metastatic NSCLC patients with anaplastic lymphoma kinase positive, and nintedanib approved in second-line treatment for advanced adenocarcinoma patients in Europe. Alternatively, other trials that add molecular targeted agents (MTAs) to standard cytotoxic chemotherapy showed no significant improvements in overall survival (OS) when compared to the use of standard chemotherapy alone.[11–13] In addition to factors including development of resistance,[14] unselected histologic subtypes,[15] and racial differences among participants, one of the key factors leading to these resultant discrepancies among the studies is the inability to ascertain specific oncogenic drivers[16]; for example, many drugs still have no validated biomarkers to identify driver gene or locate specific subgroups of patients who are more likely to respond.[17] Moreover, because of the limited coverage of efficient testing facilities, gene test might not be a prior option for patients when they were assigned to certain treatment.[18,19] In Xue et al's[20] survey, the EGFR detection rate was only 9.6% in China. Consequently, MTAs are often used for unselected NSCLC patients regardless of the mutation status of the gene in the first-line stage. Therefore, it would be of value to investigate whether the addition of MTAs to standard cytotoxic chemotherapy in first-line treatment could provide unselected patients rather than specific small samples additional benefits.
Most published meta-analyses that evaluate the antitumor activity from the combination of MTAs included articles with no restrictions on basic therapies.[21–24] This might lead to biased conclusions as a result of interaction between histology and the use of third-generation agents. In squamous NSCLC patients, superiority of gemcitabine has been observed in comparison to cisplatin–pemetrexed.[25] Conversely, there is a superior efficacy for cisplatin–pemetrexed chemotherapy in nonsquamous NSCLC patients.[26] Grossi et al's[8] study has indicated that gemcitabine-based chemotherapy has an 8% decrease in the risk for immediate progression when compared to paclitaxel. Furthermore, patients who received treatment with cisplatin and gemcitabine were more likely to suffer grade 3, 4, or 5 renal toxicity in comparison to those treated with cisplatin plus paclitaxel,[27] which suggested that these doublets were different in toxicity profiles. Therefore, unified approach among articles is needed to answer the question that whether the addition of MTA to basic therapy in first-line treatment of advanced NSCLC could provide the efficiency with a tolerable toxicity. Among all these basic therapies, platinum–gemcitabine (PG) chemotherapy, which has been recommended by National Comprehensive Cancer Network guidelines as one of the systemic therapy options for advanced NSCLC with a PS of 0 to 2 in first-line treatment,[4] has been widely used in combination with MTAs for advanced NSCLC in phase 2 or 3 clinical trials. Although PG plus MTAs treatment may achieve better clinical outcomes than PG alone for NSCLC in theory,[28–30] results are still controversial between different trials.[28,29] Thus, we present this meta-analysis.
2. Materials and methods
Ethical approval was not necessary, because all publications included in this study were published officially.
2.1. Search strategy
Web of knowledge, PubMed, Ovid, Embase, and Cochrane Library databases were searched to identify relevant trials using the following:
Web of knowledge, PubMed, Ovid, Embase, and Cochrane Library databases were searched to identify relevant trials using the following key words: Gemcitabine AND (Cisplatin OR Carboplatin OR Platinum) AND (“Non-Small Cell Lung Carcinomas” OR “Non-Small Cell Lung Cancer”) AND MTAs (Necitumumab OR Trastuzumab OR Bevacizumab OR Avastin OR Sunitinib OR Sutent OR Sorafenib OR Nexavar OR Pazopanib OR Votrient OR Cediranib OR Recentin OR Axitinib OR Erlotinib OR Gefitinib OR Cetuximab OR Panitumumab OR Lapatinib OR Vandetanib OR Zactima). We also manually reviewed the abstracts and virtual meeting presentations from the American Society of Clinical Oncology and the European Society for Medical Oncology congresses held between January 2000 and May 1, 2016. In addition, reference lists of retrieved articles and reviews[17,31–34] were also reviewed to find other potentially relevant articles. When more than 1 publication was identified for the same clinical trial, the most recent or complete report of that trial was used.
2.2. Selection criteria
We included studies that met all the following criteria: the study was a first-line randomized controlled trial (RCT) in patients with histologically or cytological confirmed NSCLC; the treatment group was PG plus MTAs, while the control group was PG plus placebo or PG alone; the number of patients and evaluation of therapeutic effects or safety were presented or could be extracted; and the sample size in each group was not less than 5. Trials included concurrent radiotherapy or any drugs other than MTAs and PG were excluded.
2.3. Data extraction and quality assessment
Data extraction was performed by 2 investigators independently, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement.[35] The following information was extracted from each study: first author, published date, study design, sample size, race, median age, male percentage, stage of disease, histological type, Eastern Cooperative Oncology Group performance status (ECOG PS), treatment regimen, hazard ratio (HR) with 95% confidence interval (CI) of OS and progression-free survival (PFS), objective response rate (ORR), and the rate of 3 to 4 grade adverse effects if given by more than 3 articles. HR and 95% CI of OS and PFS were approximated from Kaplan–Meier curves using the method described by Tierney et al[36] if necessary.
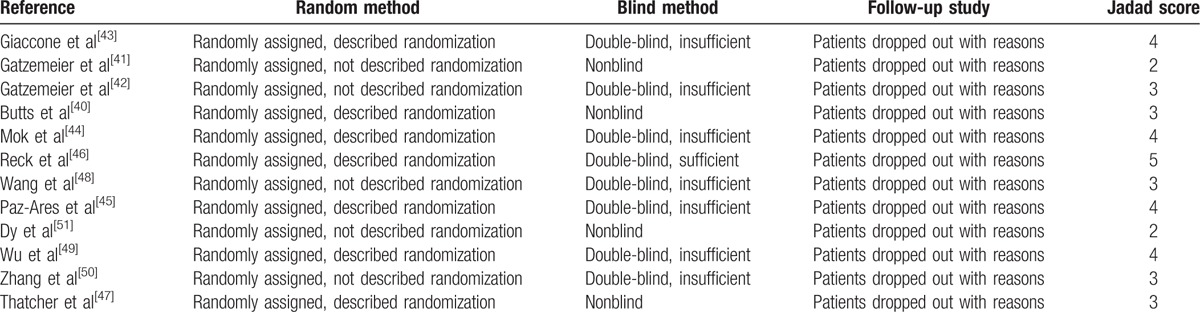
The quality of studies was evaluated by 2 investigators with Jadad et al[37] score. Score ≥3 was set as high quality. Disagreements between reviewers were settled by consensus or asking another expert.
2.4. Statistical analysis
This meta-analysis was conducted by Review Manager (RevMan; ver. 5.0; Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008). The OS and PFS were treated as time-to-event variables, and expressed as HR with 95% CI for each study. For studies reported stratified risk estimates by dosage, we combined these estimates using a random-effects model to obtain pooled estimates for OS and PFS. ORR and incidence of 3 to 4 grade adverse events (AEs) were treated as dichotomous variables and expressed as risk ratio (RR) with 95% CI for each study. The χ2-based Q test was used to test the statistical heterogeneity, and the I2 statistic was used to quantify the percentage of total variation across trials attributable to statistical heterogeneity across trials.[38] The fixed-effects model (Mantel–Haenszel method) was initially used. If the P value was less than 0.1, the assumption of homogeneity was deemed invalid; in this case, we reported summary estimates from the random-effects models (DerSimonian and Laird method). Otherwise, the fixed-effects model was reported. Forest plots were used to display the results. Subgroup analysis was performed on the basis of the type of MTAs and race for all end-points. Pooled results of subset analysis were reported when more than 3 articles were included in the model. Sensitivity analyses were performed. The probability of publication bias was assessed using funnel plots and Egger et al[39] test. Two-sided P < 0.05 was considered as statistically significant.
3. Results
3.1. Eligible studies
A total of 1476 potentially relevant studies were reviewed. Fig. 1 shows the selection process and reasons for exclusion. Ultimately, 12 first-line RCTs[40–51] with 6143 patients met the criteria for inclusion (Supply table 1 shows the dosage schedule of the included studies). Those patients participated in the targeted-trial group from distinctive studies received PG chemotherapy plus one of the following MTAs: gefitinib; trastuzumab; erlotinib; cetuximab; bevacizumab; sorafenib, cediranib, and necitumumab.
Figure 1.
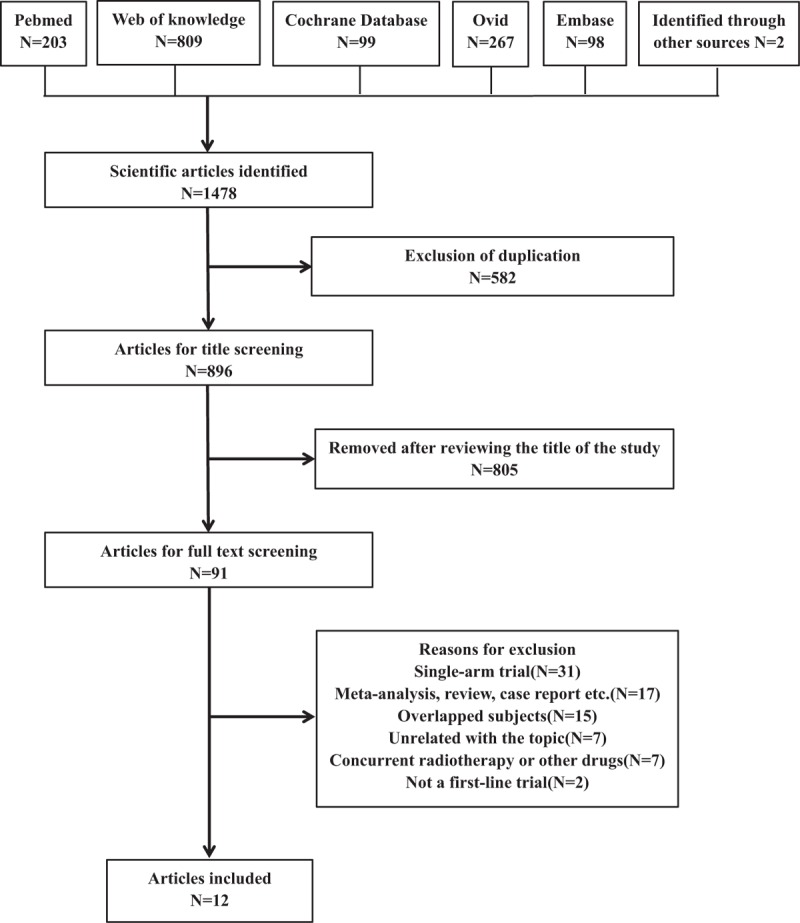
Search strategy and flowchart for this meta-analysis.
These trials represented 6 phase III studies, 4 phase II studies, and 2 RCTs. The characteristics of each trial are summarized in Table 1. Clinical characteristics were matched for age, male percentage, race, ECOG PS, stage of disease, and histology between experimental and control groups in each study. More than 94% patients had good ECOG PS (0 or 1) and approximately 80% patients were recruited at stage IV NSCLC. The quality of included studies was shown in Table 2. All included trials involved randomized treatment allocation. Ten of 12 (83.3%) studies were defined as high-quality studies, whereas the remaining 2 were open label trials with score 2.
Table 1.
Characteristics of included studies in the meta-analysis.
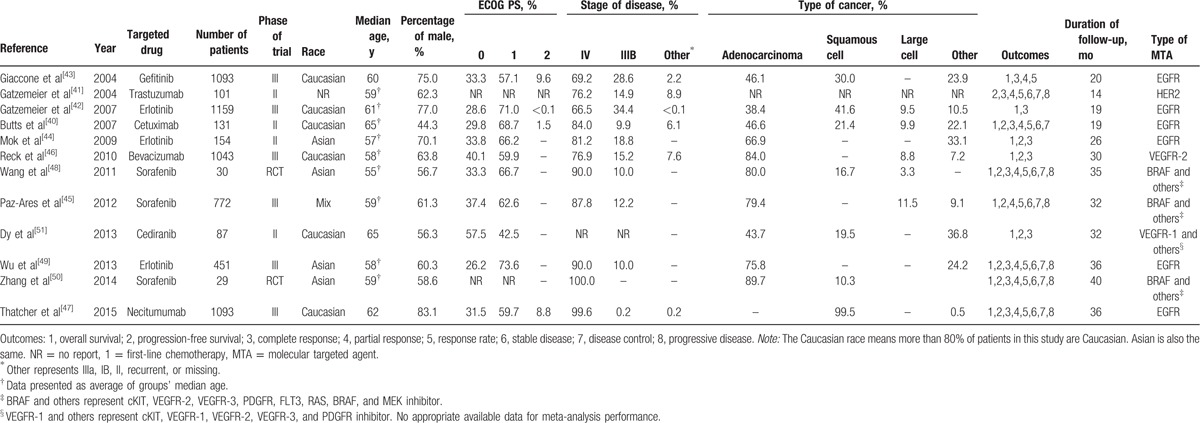
Table 2.
Jadad score of included studies.
3.2. Efficacy
3.2.1. Overall survival analysis
Eleven eligible trials[40,42–47,49–51] were included in the analysis of OS. The heterogeneity of therapeutic effect was not significant (P = 0.23, I2 = 22.80%). The pooled analysis from a fixed effect model did not demonstrate a significant difference between PG plus MTA group and PG group, although the overall trend favored the use of MTAs (HR = 0.96, 95% CI = 0.90–1.01; Fig. 2). In the subgroup analysis, there were also no significant differences in those who treated with EGFR inhibitor (HR = 0.95, 95% CI = 0.89–1.02), those who treated with BRAF inhibitor, and others (HR = 0.99, 95% CI = 0.84–1.16); Caucasian patients (HR = 0.96, 95% CI = 0.90–1.03); and Asian patients (HR = 0.86, 95% CI = 0.72–1.04), respectively.
Figure 2.

The forest plot of the meta-analysis based on the type of molecular targeted agent (MTA) and race for the overall survival (A and B), progression-free survival (C and D), and overall response rate (E and F) of platinum–gemcitabine (PG) plus MTA and PG for non-small cell lung cancer.
3.2.2. Progression-free survival analysis
Total 10 selected studies[40,41,44–47,49–51] described the PFS analysis of patients with NSCLC. The heterogeneity test indicated that a random effect model should be selected (P < 0.01, I2 = 73.60%). The pooled results indicated that the combination of MTA with PG chemotherapy could prolong PFS when compared to chemotherapy alone (HR = 0.77, 95% CI = 0.66–0.89; Fig. 2). In the subgroup analyses, a significant benefit on PFS was found for patients who followed the treatment with EGFR inhibitor (HR = 0.69, 95% CI = 0.52–0.90), patients who followed the treatment with BRAF inhibitor (HR = 0.83, 95% CI = 0.71–0.97), Caucasian patients (HR = 0.82, 95% CI = 0.75–0.89), and Asian patients (HR = 0.57, 95% CI = 0.45–0.73).
3.2.3. Objective response rate
Eleven trials[40–44,46,47,49–51] presented data on ORR. A significant heterogeneity among studies was detected (P < 0.01, I2 = 76.20%), and combined RR was calculated using the random effect model. The odds of response were significantly higher for patients in the chemotherapy plus MTA compared to chemotherapy alone (RR = 1.33, 95% CI = 1.11–1.60; Fig. 2). Subgroup analysis results of ORRs indicated that patients treated with the combination of EGFR inhibitor with PG chemotherapy may predict a better ORR (RR = 1.29, 95% CI = 1.04–1.60) than PG chemotherapy alone. Subgroup analysis based on race also showed a significant difference in both the Caucasian patients (RR = 1.20, 95% CI = 1.00–1.45) and the Asian patients (RR = 1.90, 95% CI = 1.38–2.61).
3.3. Safety
All of the 12 articles presented data on grade 3 or 4 AEs. Based on the available data, the common toxicities related to PG plus MTA therapy included anemia, leukopenia, neutropenia, thrombocytopenia, hypertension, diarrhea, anorexia, nausea, vomiting, asthenia, fatigue, and rash. The results of these AEs were presented in Table 3. For rash, patients in the PG plus MTA group were at much greater risk (RR = 9.75, 95% CI = 5.67–16.76; Fig. 3) than patients in the PG group in a fixed effect model, without significant heterogeneity (P = 0.85, I2 < 0.01%). In a subset analysis of Asian patients, RR = 7.42, 95% CI = 2.03 to 27.19. In Caucasian patients, the results favored the chemotherapy of PG alone obviously (RR = 11.42, 95% CI = 5.92–22.05). For anemia, overall analysis showed no significant difference between PG plus MTA groups versus PG group, while the addition of MTAs could increase the risk in patients treated with EGFR inhibitor (RR = 1.21, 95% CI = 1.01–1.46). For thrombocytopenia, overall analysis did not demonstrate a difference. In the subgroup analysis, we found that patients treated with BRAF inhibitor were more likely to have thrombocytopenia (RR = 1.50, 95% CI = 1.03–2.18). For hypertension, the overall RR was 2.87 (95% CI = 1.68–4.90) which indicated that the combination of MTA with PG chemotherapy could increase the probability of hypertension. Subgroup analysis of BRAF inhibitor on hypertension showed similar results (RR = 2.36, 95% CI = 1.05–5.32). For diarrhea, effects of grades 3 and 4 toxicities were more frequent in the group treated with MTAs (RR = 3.23, 95% CI = 2.17–4.80). Subgroup analysis based on race and type of MTA showed that both the Caucasian patients (RR = 3.24, 95% CI = 1.35–7.75) and patients treated with EGFR inhibitor (RR = 2.62, 95% CI = 1.21–5.65) resulted in significantly increased risk of diarrhea. For anorexia, significant difference were discovered in both overall results (RR = 1.74, 95% CI = 1.10–2.77) and subset results (Caucasian: RR = 2.16, 95% CI = 1.14–4.09; EGFR: RR = 2.08, 95% CI = 1.12–3.88). No significant differences were observed in the rates of other toxicities including leukopenia, neutropenia, nausea, vomiting, asthenia, and fatigue.
Table 3.
Grade 3 or 4 AEs occurred in included trials.
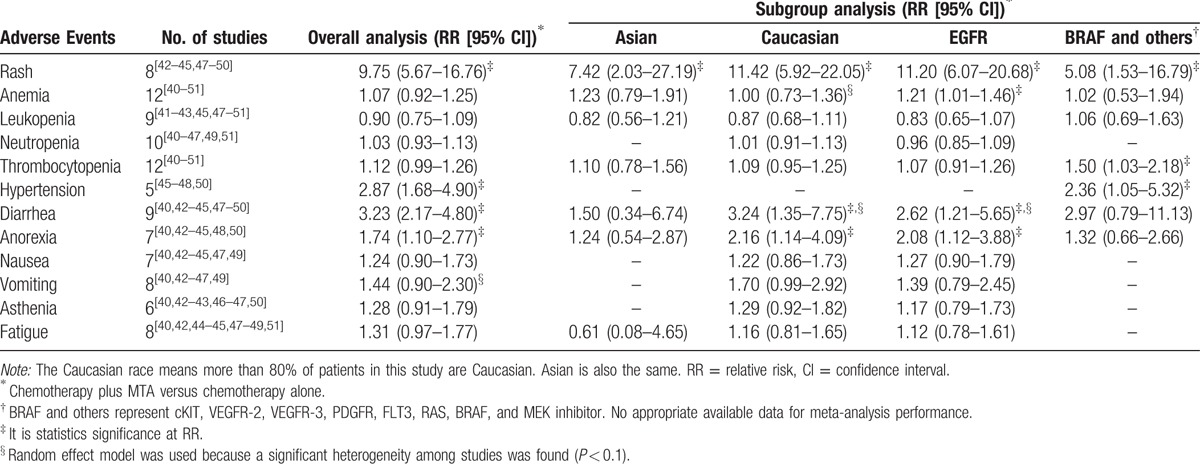
Figure 3.
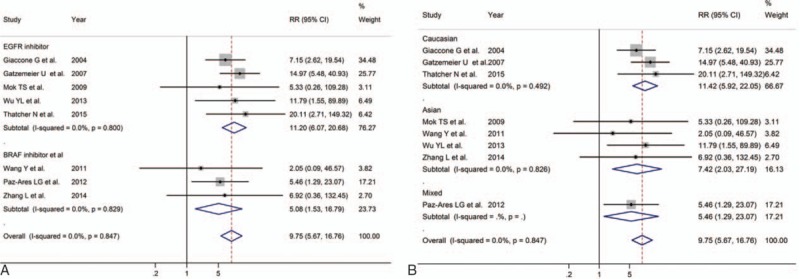
The forest plot of the meta-analysis for the 3 to 4 grade rash based on the type of molecular targeted agent (A) and race (B) of platinum–gemcitabine (PG) plus MTA and PG for non-small cell lung cancer.
3.4. Publication bias
No publication bias for OS, PFS, and ORR were found according to the Egger test—OS: P = 0.58, PFS: P = 0.86, and ORR: P = 0.30, respectively (Fig. 4).
Figure 4.
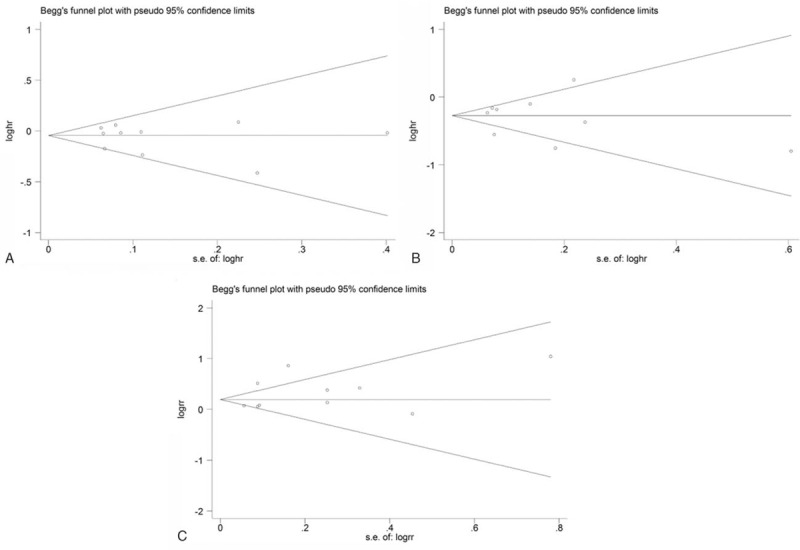
Begg funnel plot for the bias test of publication for the overall survival (A), progression-free survival (B), and overall response rate (C). Each point represents a separate study for the indicated association.
3.5. Sensitivity analysis
The sensitivity analyses demonstrated that no single trial could remarkably alter the pooled results for PFS, OS, and ORR.
4. Discussion
The present study explored the efficacy and safety of combining MTA with PG over PG therapy alone in first-line treatment of advanced NSCLC. The main findings are as follows: (first) the addition of targeted therapy with PG therapy showed a general benefit of improvements of PFS and ORR although the improvement did not translate into statistically significant prolonged OS. Moreover, MTA group showed trends toward increased survival in terms of OS, PFS, and ORR in the subgroup analysis based on race and type of MTAs. (Second) More incidences of grade 3 or higher toxic effects have been found in patients treated with PG plus MTA compared to those treated with PG alone. In the subgroup analysis, the addition of MTAs could increase the likelihood of rash, anemia, diarrhea, and anorexia in patients treated with EGFR inhibitor and increase the risk of rash, thrombocytopenia, and hypertension in patients treated with BRAF inhibitor. What is more, Caucasian patients were more likely to have grade 3 or higher diarrhea and anorexia when treated with the combination of MTA and PG while Asian patients did not.
Whether the benefit of first-line targeted therapy may be best defined by PFS is still inconclusive, while OS is widely acknowledged as the main index to evaluate efficacy in the treatment of advanced NSCLC. In the present study, the results of PFS favored the use of MTA while the results of OS were less promising. The reasons for the inconsistency between OS and PFS deserve further investigating. One possible explanation is that mono targeted therapy eventually leads to the progression of resistance.[52] Although certain strategies such as the combination of cetuximab and afatinib have shown promising effect in combating resistance[53] for advanced NSCLC, other combination strategies that targets specific molecule or new multitargets medicines have been found to cause more AEs[54] rather than significantly improve the efficacy. Another possible explanation is that while PFS attempt to directly measure treatment efficacy, the interactions in follow-up treatment regimens between groups could confound the OS for patients included in the first-line PG treatment group usually receive MTAs for second-line treatment after progression. Thatcher et al's[55] meta-analysis found that angiogenesis inhibitors resulted in significant improvement in OS in second-line and subsequent settings but not in the first-line settings, compared with nonangiogenesis inhibitors. Moreover, the significant benefit has been found in OS (HR = 0.94, 95% CI = 0.88–1.00) when 2 articles following patients for less than 20 months were excluded, which might imply a limited duration of follow-up have a confounding effect on OS.
With regard to the targeted agents used, the combined therapy of MTAs differed between the included studies, but all studies used PG doublet therapy, and combined targeted agents included EGFR, HER2, VEGFR-1, VEGFR-2, and BRAF, respectively. Subgroup meta-analysis stratified for each involved pathway was carried out. Among these, 1 subgroup meta-analysis investigating the EGFR inhibitor revealed a significant benefit in PFS with 4 articles involved and ORR with 6 articles involved than PG group which was consistent with previous studies[56–58] in first-line or second-line settings. As for toxicity profile, there were more incidence of grade 3 or 4 rash, anemia, diarrhea, and anorexia in combining EGFR targeted therapy group compared to PG group. Equivalent frequencies were observed between the 2 groups with respect to the risk of grade 3 or 4 leukopenia, neutropenia, thrombocytopenia, nausea, vomiting, asthenia, and fatigue. OuYang et al's[56] study also found more frequent diarrhea and anorexia in the combined regimen arm but missed the differences in rash and anemia. Another subgroup meta-analysis investigating sorafenib, which is a small inhibitor of several tyrosine protein kinases, such as vascular endothelial growth factor receptor, PDGFR, and Raf family kinases (more avidly C-Raf than B-Raf),[59] showed a significant benefit in PFS with 3 articles involved, but not in OS with 3 articles involved or ORR with 2 articles involved. An increased risk of grade 3 or 4 rash, thrombocytopenia, and hypertension was observed in sorafenib combination group. There is not any difference between 2 groups in anemia, leukopenia, diarrhea, and anorexia. Besides rash and hypertension, Wang et al's[60] study discovered a grade 3 or greater sorafenib-related AEs included fatigue, diarrhea, oral mucositis and hand-foot skin reaction, toxicity profile of thrombocytopenia was not presented in Wang meta. Since our study well balanced basic therapies which might be a potential confounding factor between experimental and control groups, AEs found in this study should be considered in choice of treatment schemes. In addition, the significantly higher incidence of rash we found in the group using MTA combined with PG than that of the group using PG challenges how we set blinding in clinical trials.
Our meta-analysis showed a high heterogeneity level for PFS (I2 = 73.6%, P < 0.01) and ORR (I2 = 76.2%, P < 0.01). In order to explore the heterogeneity, subgroup analysis was performed according to the classifications of ethnicity and type of MTAs. For PFS, high heterogeneity level has been detected in the EGFR inhibitor subgroup (I2 = 86.5%, P < 0.01). When we further included articles that predominantly enrolled Asian populations only in this subgroup, the level of heterogeneity decreased (I2 = 0.7%, P = 0.32). For PFS in Caucasian dominant populations, the heterogeneity also disappeared (I2 < 0.1%, P = 0.72). Similar results appeared for ORR, where the P value with heterogeneity test was 0.11 for Asian dominant population and 0.76 for Caucasian dominant population. Therefore, ethnicity could be the main reason for the heterogeneity. Our results were consistent with previous researches[61–63] which demonstrated that ethnicity could be a major factor that influences the survival outcome from EGFR-tyrosine-kinase inhibitors (TKIs) therapy. Notably, in the subanalysis of PFS based on race, with a restriction on the type of MTAs to EGFR inhibitors, the Asian dominant subgroup (HR = 0.56, 95% CI = 0.49–0.64) could live longer without their disease progressing than the Caucasian dominant subgroup (HR = 0.86, 95% CI = 0.76–0.97) from the addition of EGFR-TKIs. The pronounced survival benefit could be partly attributed to a higher occurrence of activating mutations found in Asian patients compared with Caucasian population[64] and at least somewhat suggest that the targeted subpopulation which most likely to benefit from EGFR-TKIs is not Caucasian NSCLC population. Identifying potential predictive markers to target MTA treatment to specific subpopulations should still be the key issue for future study.
Several limitations had to be mentioned in relation to this meta-analysis. First, the meta-analysis was not based on individual patient data. With the exception of 1 trial in which a stratification for biomarker analysis (human epidermal growth factor receptor-2 status) was reported, all the other trials were performed on unselected patient populations which meant that confounding factors such as demographic characteristics and specific biomarkers across the trials may not be incorporated. Possible survival benefits of combining targeted therapy with PG in different NSCLC patient groups with distinct histologic types, ages, mutation status of patients could not be discovered. Second, an accurate pooled analysis according to ethnicity was unable to perform since some trials such as SQUamous NSCLC treatment with the Inhibitor of EGF REceptor (SQUIRE)[47] enrolled 913 Caucasian patients and 180 others, while Mok et al's[44] study enrolled 145 Asian patients and 6 Caucasian patients. Subgroup analysis according to dominant ethnicity was conducted in order to explore the influence of ethnicity. Third, all trials included in this meta-analysis were performed in first-line treatment, which might lead to potential confounding effects from the bias of subsequent treatments. Finally, inevitable variations existing among the treatment plans, such as dosage regimen and cycle duration, could potentially affect the present results. Further studies are warranted to complete and follow-up the information.
In conclusion, PG chemotherapy plus MTA was superior to PG alone in terms of PFS and OR in patients with advanced NSCLC. More studies need to be done to draw a conclusion for OS. With respect to the toxicity profile, a higher frequency of grade 3 or higher toxic effects has been observed in the combination arm than PG arm. An overview of the advantage and disadvantage in terms of efficacy, and safety from the combination of MTAs to PG has been given by our study and could further inspire patients and doctors in choosing first-line NSCLC treatment therapies.
Supplementary Material
Footnotes
Abbreviations: MTA(s) = molecular targeted agent(s), PG = platinum–gemcitabine, NSCLC = non-small cell lung cancer, OS = overall survival, PFS = progression-free survival, ORR = objective response rate, AE(s) = adverse event(s), EGFR = epidermal growth factor receptor, RCT = randomized controlled trial, ECOG PS = Eastern Cooperative Oncology Group performance status, HR = hazard ratio, CI = confidence interval, RR = risk ratio.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol 2014;25:1475–84. [DOI] [PubMed] [Google Scholar]
- [3].Wakelee H, Belani CP. Optimizing first-line treatment options for patients with advanced NSCLC. Oncologist 2005;10(suppl. 3):1–0. [DOI] [PubMed] [Google Scholar]
- [4].National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Non-small Cell Lung Cancer, Version 4. 2016. [Google Scholar]
- [5].Scagliotti G, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non–small-cell lung cancer. J Clin Oncol 2002;20:4285–91. [DOI] [PubMed] [Google Scholar]
- [6].Le Chevalier T, Scagliotti G, Natale R, et al. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: a meta-analysis of survival outcomes. Lung Cancer 2005;47:69–80. [DOI] [PubMed] [Google Scholar]
- [7].Smit EF, van Meerbeeck JP, Lianes P, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non–small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group—EORTC 08975. J Clin Oncol 2003;21:3909–17. [DOI] [PubMed] [Google Scholar]
- [8].Grossi F, Aita M, Defferrari C, et al. Impact of third-generation drugs on the activity of first-line chemotherapy in advanced non-small cell lung cancer: a meta-analytical approach. Oncologist 2009;14:497–510. [DOI] [PubMed] [Google Scholar]
- [9].Stinchcombe TE, Socinski MA. Current treatments for advanced stage non–small cell lung cancer. Proc Am Thorac Soc 2009;6:233–41. [DOI] [PubMed] [Google Scholar]
- [10].Minguet J, Smith KH, Bramlage P. Targeted therapies for treatment of non-small cell lung cancer—recent advances and future perspectives. Int J Cancer 2016;138:2549–61. [DOI] [PubMed] [Google Scholar]
- [11].Doebele RC, Spigel D, Tehfe M, et al. Phase 2, randomized, open-label study of ramucirumab in combination with first-line pemetrexed and platinum chemotherapy in patients with nonsquamous, advanced/metastatic non-small cell lung cancer. Cancer 2015;121:883–92. [DOI] [PubMed] [Google Scholar]
- [12].Yu H, Zhang J, Wu X, et al. A phase II randomized trial evaluating gefitinib intercalated with pemetrexed/platinum chemotherapy or pemetrexed/platinum chemotherapy alone in unselected patients with advanced non-squamous non-small cell lung cancer. Cancer Biol Therapy 2014;15:832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hirsch FR, Govindan R, Zvirbule Z, et al. Efficacy and safety results from a phase II, placebo-controlled study of onartuzumab plus first-line platinum-doublet chemotherapy for advanced squamous cell non–small-cell lung cancer. Clin Lung Cancer 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [14].Steuer CE, Ramalingam SS. Targeting EGFR in lung cancer: lessons learned and future perspectives. Mol Aspects Med 2015;45:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kloth M, Buettner R. Changing histopathological diagnostics by genome-based tumor classification. Genes 2014;5:444–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bonanno L, Favaretto A, Rugge M, et al. Role of genotyping in non-small cell lung cancer treatment. Drugs 2011;71:2231–46. [DOI] [PubMed] [Google Scholar]
- [17].Minguet J, Smith KH, Bramlage P. Targeted therapies for treatment of non-small cell lung cancer—recent advances and future perspectives. Int J Cancer 2016;138:2549–61. [DOI] [PubMed] [Google Scholar]
- [18].Salto-Tellez M, Tsao M-S, Shih J-Y, et al. Clinical and testing protocols for the analysis of epidermal growth factor receptor mutations in East Asian patients with non-small cell lung cancer: a combined clinical-molecular pathological approach. J Thorac Oncol 2011;6:1663–9. [DOI] [PubMed] [Google Scholar]
- [19].Xu C, Zhou Q, Wu Y. Can EGFR-TKIs be used in first line treatment for advanced non-small cell lung cancer based on selection according to clinical factors?—a literature-based meta-analysis. J Hematol Oncol 2012;5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xue C, Hu Z, Jiang W, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer 2012;77:371–5. [DOI] [PubMed] [Google Scholar]
- [21].Liang W, Wu X, Hong S, et al. Multi-targeted antiangiogenic tyrosine kinase inhibitors in advanced non-small cell lung cancer: meta-analyses of 20 randomized controlled trials and subgroup analyses. PLoS One 2014;9:e109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li X, Wang H, Lin W, et al. Efficacy of combining targeted therapy with pemetrexed or docetaxel as second-line treatment in patients with advanced non-small-cell lung cancer: a meta-analysis of 14 randomized controlled trials. Curr Med Res Opin 2014;30:2295–304. [DOI] [PubMed] [Google Scholar]
- [23].Sheng M, Zhao Y, Wang F, et al. Targeted drugs for unselected patients with advanced non-small-cell lung cancer: a network meta-analysis. J Thorac Dis 2016;8:98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Burotto M, Manasanch EE, Wilkerson J, et al. Gefitinib and erlotinib in metastatic non-small cell lung cancer: a meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist 2015;20:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non–small-cell lung cancer. J Clin Oncol 2008;26:3543–51. [DOI] [PubMed] [Google Scholar]
- [26].Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:64–70. [DOI] [PubMed] [Google Scholar]
- [27].Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med 2002;346:92–8. [DOI] [PubMed] [Google Scholar]
- [28].Li M, Li H, Cheng X, et al. Preclinical pharmacokinetic/pharmacodynamic models to predict schedule-dependent interaction between erlotinib and gemcitabine. Pharm Res 2013;30:1400–8. [DOI] [PubMed] [Google Scholar]
- [29].Higgins B, Kolinsky K, Smith M, et al. Antitumor activity of erlotinib (OSI-774, Tarceva) alone or in combination in human non-small cell lung cancer tumor xenograft models. Anticancer Drugs 2004;15:503–12. [DOI] [PubMed] [Google Scholar]
- [30].Samakoglu S, Deevi DS, Li H, et al. Preclinical rationale for combining an EGFR antibody with cisplatin/gemcitabine for the treatment of NSCLC. Cancer Genomics Proteomics 2012;9:77–92. [PubMed] [Google Scholar]
- [31].Steuer CE, Ramalingam SS. Targeting EGFR in lung cancer: lessons learned and future perspectives. Mol Aspects Med 2015;45:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barr Kumarakulasinghe N, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology 2015;20:370–8. [DOI] [PubMed] [Google Scholar]
- [33].Daga A, Ansari A, Patel S, et al. Current drugs and drug targets in non-small cell lung cancer: limitations and opportunities. Asian Pac J Cancer Prev 2015;16:4147–56. [DOI] [PubMed] [Google Scholar]
- [34].Khanal N, Ganti AK. Emerging targeted therapies in non-small cell lung cancer. Expert Rev Anticancer Ther 2016;16:177–87. [DOI] [PubMed] [Google Scholar]
- [35].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [36].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [38].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Butts CA, Bodkin D, Middleman EL, et al. Randomized phase II study of gemcitabine plus cisplatin or carboplatin [corrected], with or without cetuximab, as first-line therapy for patients with advanced or metastatic non small-cell lung cancer. J Clin Oncol 2007;25:5777–84. [DOI] [PubMed] [Google Scholar]
- [41].Gatzemeier U, Groth G, Butts C, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 2004;15:19–27. [DOI] [PubMed] [Google Scholar]
- [42].Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 2007;25:1545–52. [DOI] [PubMed] [Google Scholar]
- [43].Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol 2004;22:777–84. [DOI] [PubMed] [Google Scholar]
- [44].Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009;27:5080–7. [DOI] [PubMed] [Google Scholar]
- [45].Paz-Ares LG, Biesma B, Heigener D, et al. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol 2012;30:3084–92. [DOI] [PubMed] [Google Scholar]
- [46].Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin–gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 2010;21:1804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763–74. [DOI] [PubMed] [Google Scholar]
- [48].Wang Y, Wang L, Liu Y, et al. Randomize trial of cisplatin plus gemcitabine with either sorafenib or placebo as first-line therapy for non-small cell lung cancer. Chin J Lung Cancer 2011;14:239–44. [Chinese]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777–86. [DOI] [PubMed] [Google Scholar]
- [50].Zhang L, Fu Q, Hu G. Sorafenib plus chemotherapy for first-line treatment of non-small cell lung cancer. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong 2014;43:190–4. [Google Scholar]
- [51].Dy GK, Mandrekar SJ, Nelson GD, et al. A randomized phase II study of gemcitabine and carboplatin with or without cediranib as first-line therapy in advanced non-small-cell lung cancer North Central Cancer Treatment Group Study N0528. J Thorac Oncol 2013;8:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013;19:1389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Janjigian YY, Azzoli CG, Krug LM, et al. Phase I/II trial of cetuximab and erlotinib in patients with lung adenocarcinoma and acquired resistance to erlotinib. Clin Cancer Res 2011;17:2521–7. [DOI] [PubMed] [Google Scholar]
- [54].Ma W, Xu M, Liu Y, et al. Safety profile of combined therapy inhibiting EFGR and VEGF pathways in patients with advanced non-small-cell lung cancer: a meta-analysis of 15 phase II/III randomized trials. Int J Cancer 2015;137:409–19. [DOI] [PubMed] [Google Scholar]
- [55].Hong S, Tan M, Wang S, et al. Efficacy and safety of angiogenesis inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2015;141:909–21. [DOI] [PubMed] [Google Scholar]
- [56].OuYang P-Y, Su Z, Mao Y-P, et al. Combination of EGFR-TKIs and chemotherapy as first-line therapy for advanced NSCLC: a meta-analysis. PLoS One 2013;8:e79000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gao G, Ren S, Li A, et al. A meta-analysis of comparing EGFR-TKI with chemotherapy as the second-line treatment of NSCLC patients with wild-type EGFR. Paper presented at: ASCO Annual Meeting Proceedings; 2013. [Google Scholar]
- [58].Xiao B-K, Yang J-Y, Dong J-X, et al. Meta-analysis of seven randomized control trials to assess the efficacy and toxicity of combining EGFR-TKI with chemotherapy for patients with advanced NSCLC who failed first-line treatment. Asian Pac J Cancer Prev 2014;16:2915–21. [DOI] [PubMed] [Google Scholar]
- [59].Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008;7:3129–40. [DOI] [PubMed] [Google Scholar]
- [60].Wang W-L, Tang Z-H, Xie T-T, et al. Efficacy and safety of sorafenib for advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. Asian Pac J Cancer Prev 2013;15:5691–6. [DOI] [PubMed] [Google Scholar]
- [61].Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527–37. [DOI] [PubMed] [Google Scholar]
- [62].Xu JL, Jin B, Ren ZH, et al. Chemotherapy plus erlotinib versus chemotherapy alone for treating advanced non-small cell lung cancer: a meta-analysis. PLoS One 2015;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jiang H. Overview of gefitinib in non-small cell lung cancer: an Asian perspective. Jpn J Clin Oncol 2009;39:137–50. [DOI] [PubMed] [Google Scholar]
- [64].Yang C-H. EGFR tyrosine kinase inhibitors for the treatment of NSCLC in East Asia: present and future. Lung Cancer 2008;60:S23–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


