Abstract
The aim of this study was to evaluate the efficacy of dexmedetomidine in combination with sufentanil or butorphanol for postoperative analgesia in patients undergoing laparoscopic resection of a gastrointestinal tumor.
This quasi-experimental trial was conducted in Nanchang, China, from January 2014 to December 2015. Eighty patients (age 27–70 years, American Society of Anesthesiologists physical status I–II) undergoing laparoscopic resection of a gastrointestinal tumor were randomized into 4 groups and offered intravenous patient-controlled analgesia for pain control after surgery. The patients received sufentanil 2.0 μg/kg in combination with dexmedetomidine 1.5 μg/kg (group S1) or 2.0 μg/kg (group S2), or butorphanol 0.15 mg/kg in combination with dexmedetomidine 1.5 0 μg/kg (group N1) or 2.0 μg/kg (group N2). Oxygen saturation, mean arterial pressure (MAP), heart rate, visual analog scale score, and Ramsay sedation score were recorded at enrollment (T0), at extubation (T1), and 4 (T2), 8 (T3), 12 (T4), 24 (T5), and 48 (T6) hours thereafter. Side effects and satisfaction scores were evaluated after surgery.
MAP increased in all groups at T1 but not significantly so when compared with T0. Heart rate decreased significantly in group S2 when compared with the other groups at T1–T5 (P < 0.05). MAP decreased significantly in group S2 when compared with group S1 at T4–T6 (P < 0.05). MAP increased significantly in group N1 when compared with group N2 at T4–T5 (P < 0.05). There was a statistically significant decrease in mean visual analog scale score in group S2 when compared with group S1 at T2 (P < 0.05) and group N2 at T1–T2 (P < 0.05). Two patients in group S1 had vomiting. There were no reports of drowsiness, respiratory depression, or other complications. The satisfaction score was higher in group S2 than in the other groups.
Dexmedetomidine in combination with sufentanil or butorphanol can be used safely and effectively for postoperative analgesia in patients undergoing laparoscopic resection of a gastrointestinal tumor. The combination of dexmedetomidine 2.0 μg/kg and sufentanil is particularly beneficial in these patients.
Keywords: butorphanol, dexmedetomidine, gastrointestinal tumor, postoperative analgesia, sufentanil
1. Introduction
Sufentanil and butorphanol are often used for postoperative analgesia. Sufentanil alone is more likely to cause side effects and respiratory depression than when combined with 1 or more adjunctive drugs in intravenous patient-controlled analgesia (PCA).[1,2] Further, it has been reported that lower doses of butorphanol may have ceiling effects.[3] Effective postoperative analgesia would not only improve patient satisfaction but also reduce the incidence of postoperative complications and shorten the duration of hospitalization.[4,5] According to the available protocols for postoperative pain management, the ideal method is a combination of drugs. Previous studies have reported that use of an α2-adrenoceptor agonist can decrease the risk of cardiovascular side effects postoperatively. Dexmedetomidine is a highly selective α2 adrenergic receptor agonist with sedative, analgesic, and antianxiety activity.[6,7] However, data on the effects of different concentrations of dexmedetomidine are inadequate. The primary aim of this study was to evaluate the efficacy of dexmedetomidine in combination with sufentanil or butorphanol for postoperative analgesia in patients undergoing laparoscopic resection of a gastrointestinal tumor.
2. Methods
2.1. Study design and participants
Eighty patients (41 men, 39 women, aged 27–70 years) with American Society of Anesthesiologists physical status I to II and a body mass index < 28 kg/m2 undergoing laparoscopic resection of a gastrointestinal tumor were included. The study exclusion criteria included a history of cardiovascular disease, severe renal or hepatic insufficiency, bradycardia, atrioventricular block, chronic pain, and current use of analgesic medication. The patients were randomized into 4 groups of 20 patients each using a computer-generated randomization list to receive dexmedetomidine 1.5 μg/kgand sufentanil 2.0 μg/kg and ondansetron 0.4 mg/kg, diluted with 0.9% saline solution to 100 mL (group S1); dexmedetomidine 2.0 μg/kg and sufentanil 2.0 μg/kg and ondansetron 0.4 mg/kg, diluted with 0.9% saline solution to 100 mL (group S2); dexmedetomidine 1.5 μg/kg and butorphanol 0.15 mg/kg and ondansetron 0.4 mg/kg, diluted with 0.9% saline solution to 100 mL (group N1); and dexmedetomidine 2.0 μg/kg and butorphanol 0.15 mg/kg and ondansetron 0.4 mg/kg, diluted with 0.9% saline solution to 100 mL (group N2).
2.2. Anesthesia
The patients did not receive any medication before induction of anesthesia. At the start of anesthesia, peripheral venous access was established in the right upper extremity, and a 5-lead electrocardiogram, oxygen saturation (SpO2), and blood pressure were monitored continuously. Anesthesia was induced by an intravenous infusion of dexmedetomidine 1.0 μg/kg (15 minutes before the start of surgery), etomidate 0.3 mg/kg, sufentanil 0.4 μg/kg, and cisatracurium 0.2 mg/kg. When the trachea was intubated, ventilation was mechanically controlled to maintain a tidal volume of 7 to 10 mL/kg, a respiratory rate of 12 breath/min, and end-tidal carbon dioxide (PETCO2) at 35 to 45 mm Hg. Anesthesia was maintained by an intravenous infusion of propofol 4.0 to 8.0 mg/kg/min, remifentanil 4.0 to 8.0 μg/kg/min, and cisatracurium 0.1 mg/kg/min. Hemodynamic stability was maintained intraoperatively. All patients received an intravenous injection of flurbiprofen axetil 50 mg and ondansetron 4 mg 15 minutes before completion of surgery. Propofol, remifentanil, and cisatracurium were then discontinued. All patients were offered an electronic infusion pump for intravenous PCA after surgery.
2.3. Outcome measures
Oxygen saturation, mean arterial pressure (MAP), heart rate (HR), visual analog score (VAS), and Ramsay sedation score (RSS) were recorded at enrollment (T0), at extubation (T1), and at 4 (T2), 8 (T3), 12 (T4), 24 (T5), and 48 (T6) hours thereafter. Side effects and satisfaction scores were evaluated after surgery.
The VAS score was classified as no pain (score 0), mild pain (score 1–3), moderate pain (score 4–6), or severe pain (score 7–10). Sedation was assessed using the RSS (1, anxious; 2, cooperative and tranquil; 3, responding to command; 4, brisk response to stimulus; 5, sluggish response to stimulus; 6, no response to stimulus).
2.4. Ethics statement
The study protocol was approved by the local hospital ethics committee in Nanchang, China, and conducted from January 2014 to December 2015. Informed consent was obtained from all study participants.
2.5. Statistical analysis
Normally distributed data are expressed as the mean ± standard deviation. Between-group comparisons were performed using repeated-measures analysis of variance. Categorical variables were compared using the Chi-squared test, and intragroup comparisons were performed using the Wilcoxon rank-sum test. All P values < 0.05 were considered to be statistically significant. The statistical analysis was performed using Statistical Package for the Social Sciences version 17.0 software (SPSS Inc., Chicago, IL).
3. Results
The patient characteristics are summarized in Table 1. There was no difference in patient sex, age, or weight, or duration of surgery between the study groups (P > 0.05). MAP increased in all groups at T1 when compared with T0, but the difference was not statistically significant (Table 2). HR decreased significantly in group S2 at T1–T5 when compared with the other groups (P < 0.05). MAP decreased significantly in group S2 when compared with group S1 at T4–T6 (P < 0.05) and increased significantly in group N1 when compared with group N2 at T4–T5 (P < 0.05). A statistically significant decrease in VAS was seen in group S2 when compared with group S1 at T2 (P < 0.05) and with group N2 at T1–T2 (P < 0.05; Table 3). Two patients in group S1 developed vomiting. There were no reports of drowsiness, respiratory depression, or other complications in any of the groups (Table 4). The patient satisfaction rate was higher in group S2 (95%) than in the other groups (Table 5).
Table 1.
Patient characteristics in the treatment groups.
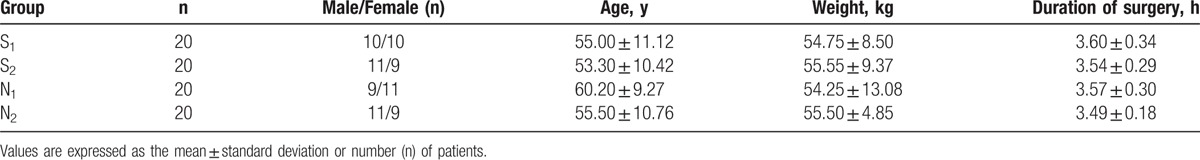
Table 2.
Comparison of hemodynamic changes in the treatment groups at the different time points.
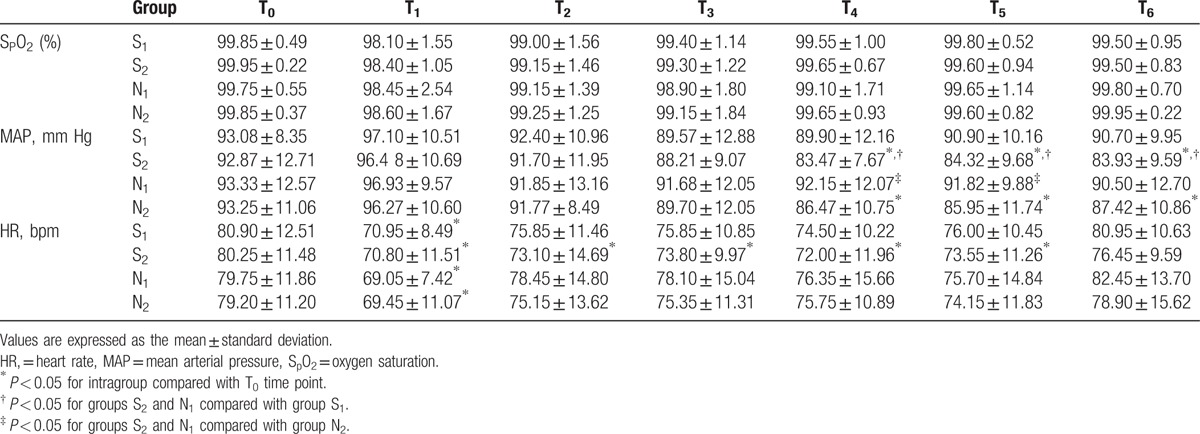
Table 3.
Comparison of visual analog score and Ramsay sedation score between the treatment groups at the different time points.
Table 4.
Side effects in the treatment groups.

Table 5.
Comparison of patient satisfaction levels between the treatment groups.

4. Discussion
Postoperative pain is a common patient complaint after surgery. Apfelbaum et al[8] reported that approximately 80% of their patients experienced pain after surgery and 86% had moderate to severe pain. Although postoperative pain is significantly less after laparoscopy than after open surgery,[9] the pain may still be considerable because of the transabdominal sutures and laparoscopic tacks used during the procedure.[2,10] Medication patches and percutaneous pump devices have been used to decrease postoperative pain with limited success, so improving postoperative analgesia is an area of continued interest in laparoscopic surgery.
The most common type of PCA involves use of an intravenous opioid because of its postoperative analgesic efficacy and prolonged duration of action.[11] However, this type of PCA has considerable side effects, including nausea, vomiting, motor block, urinary retention, and respiratory depression.[12,13] Use of sufentanil has been investigated in some laparoscopic surgery studies. Damen et al[14] reported that intraoperative sufentanil was comparable with remifentanil in patients undergoing laparoscopic cholecystectomy. Early pain was decreased in patients receiving sufentanil, but at the expense of opioid-related adverse effects. Butorphanol is a lipid-soluble narcotic agent with strong κ-receptor agonist and weak μ-receptor agonist/antagonist activity. The above-mentioned narcotic analgesics have been used frequently for postoperative analgesia.[15] In recent years, there have been attempts to reduce the frequency of side effects associated with postoperative pain management by use of a combination of two or more drugs.[16] Recent studies have focused on nonopioid receptors with additional analgesic effects. Previous studies have demonstrated that dexmedetomidine, an α2-adrenoceptor agonist, causes dose-dependent hypotension, bradycardia, and sedation. Dexmedetomidine decreases the HR and blood pressure by decreasing plasma levels of norepinephrine and epinephrine.[17] Saadawy et al[18] reported a decrease in HR and MAP in their dexmedetomidine group within 25 to 35 minutes of caudal administration. In our study, we also found a decrease in HR and MAP, particularly in the group that received dexmedetomidine 2.0 μg/kg. Further, MAP decreased significantly in the group that received dexmedetomidine 2.0 μg/kg and sufentanil 2.0 μg/kg when compared with the other groups. Addition of dexmedetomidine 2.0 μg/kg to sufentanil or butorphanol in this study was not associated with an increased incidence of side effects. Our results are consistent with those of studies in patients undergoing laparoscopic bariatric surgery. In one early study, patients undergoing laparoscopic colorectal surgery who received intraoperative dexmedetomidine reported lower pain scores during the first 12 postoperative hours, and no opioid-sparing effect was found.[19] Dexmedetomidine also showed significant anxiolytic efficacy and durable analgesic efficacy, with a decreased need for postoperative opioid analgesia. Our study found that satisfactory patient sedation contributed to the lessening of postoperative pain. Our results indicated that patients were generally satisfied with their intravenous PCA system because the adjuvant combination of dexmedetomidine and sufentanil or butorphanol therein achieved an acceptable level of sedation.
In conclusion, our results show that the 2 doses of dexmedetomidine as an adjuvant to sufentanil and butorphanol can be safely used for postoperative analgesia in patients undergoing laparoscopic resection of a gastrointestinal tumor. The most effective dose of dexmedetomidine that did not lead to any complications was 2.0 μg/kg combined with sufentanil 2.0 μg/kg.
Acknowledgment
We would like to express our gratitude to our coworkers and the patients who participated in this study. This study is supported by the National Natural Science Foundation of China (81660096).
Footnotes
Abbreviations: HR = heart rate, MAP = mean arterial pressure, PETCO2 = end-tidal carbon dioxide, RSS = Ramsay sedation score, SpO2 = oxygen saturation, VAS = visual analog scale.
X-K Z and Q-H C contributed equally to this work.
The authors have no conflicts of interest.
References
- [1].Wang Y, Tang H, Guo Q, et al. Effects of intravenous patient-controlled sufentanil analgesia and music therapy on pain and hemodynamics after surgery for lung cancer: a randomized parallel study. J Altern Complement Med 2015;21:667–72. [DOI] [PubMed] [Google Scholar]
- [2].Sjovall S, Kokki M, Kokki H. Laparoscopic surgery: a narrative review of pharmacotherapy in pain management. Drugs 2015;75:1867–89. [DOI] [PubMed] [Google Scholar]
- [3].Bharti N, Chari P. Epidural butorphanol-bupivacaine analgesia for postoperative pain relief after abdominal hysterectomy. J Clin Anesth 2009;21:19–22. [DOI] [PubMed] [Google Scholar]
- [4].Blumenthal S, Borgeat A, Nadig M, et al. Postoperative analgesia after anterior correction of thoracic scoliosis: a prospective randomized study comparing continuous double epidural catheter technique with intravenous morphine. Spine 2006;31:1646–51. [DOI] [PubMed] [Google Scholar]
- [5].Yukawa Y, Kato F, Ito K, et al. A prospective randomized study of preemptive analgesia for postoperative pain in the patients undergoing posterior lumbar interbody fusion: continuous subcutaneous morphine, continuous epidural morphine, and diclofenac sodium. Spine 2005;30:2357–61. [DOI] [PubMed] [Google Scholar]
- [6].Al-Zaben KR, Qudaisat IY, Abu-Halaweh SA, et al. Comparison of caudal bupivacaine alone with bupivacaine plus two doses of dexmedetomidine for postoperative analgesia in pediatric patients undergoing infra-umbilical surgery: a randomized controlled double-blinded study. Paediatr Anaesth 2015;25:883–90. [DOI] [PubMed] [Google Scholar]
- [7].Nie Y, Liu Y, Luo Q, et al. Effect of dexmedetomidine combined with sufentanil for post-caesarean section intravenous analgesia: a randomised, placebo-controlled study. Eur J Anaesthesiol 2014;31:197–203. [DOI] [PubMed] [Google Scholar]
- [8].Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 2003;97:534–40. [DOI] [PubMed] [Google Scholar]
- [9].Newcomb W, Lincourt A, Hope W, et al. Prospective, double-blinded, randomized, placebo-controlled comparison of local anesthetic and nonsteroidal anti-inflammatory drugs for postoperative pain management after laparoscopic surgery. Am Surg 2007;73:618–24. [PubMed] [Google Scholar]
- [10].Fields AC, Gonzalez DO, Chin EH, et al. Laparoscopic-assisted transversus abdominis plane block for postoperative pain control in laparoscopic ventral hernia repair: a randomized controlled trial. J Am Coll Surg 2015;221:462–9. [DOI] [PubMed] [Google Scholar]
- [11].Grass JA. Patient-controlled analgesia. Anesth Analg 2005;101(5 Suppl):S44–61. [DOI] [PubMed] [Google Scholar]
- [12].Sultan P, Gutierrez MC, Carvalho B. Neuraxial morphine and respiratory depression: finding the right balance. Drugs 2011;71:1807–19. [DOI] [PubMed] [Google Scholar]
- [13].Bromage PR, Camporesi EM, Durant PA, et al. Nonrespiratory side effects of epidural morphine. Anesth Analg 1982;61:490–5. [PubMed] [Google Scholar]
- [14].Damen SL, Nieuwenhuijs VB, Joosten W, et al. The effects of remifentanil and sufentanil on the quality of recovery after day case laparoscopic cholecystectomy: a randomized blinded trial. J Laparoendosc Adv Surg Tech A 2004;14:87–92. [DOI] [PubMed] [Google Scholar]
- [15].Jose DE, Ganapathi P, Anish Sharma NG, et al. Postoperative pain relief with epidural buprenorphine versus epidural butorphanol in laparoscopic hysterectomies: a comparative study. Anesth Essays Res 2016;10:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murali Krishna T, Panda NB, Batra YK, et al. Combination of low doses of intrathecal ketamine and midazolam with bupivacaine improves postoperative analgesia in orthopaedic surgery. Eur J Anaesthesiol 2008;25:299–306. [DOI] [PubMed] [Google Scholar]
- [17].Talke P, Chen R, Thomas B, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg 2000;90:834–9. [DOI] [PubMed] [Google Scholar]
- [18].Saadawy I, Boker A, Elshahawy MA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand 2009;53:251–6. [DOI] [PubMed] [Google Scholar]
- [19].Cheung CW, Qiu Q, Ying AC, et al. The effects of intra-operative dexmedetomidine on postoperative pain, side-effects and recovery in colorectal surgery. Anaesthesia 2014;69:1214–21. [DOI] [PubMed] [Google Scholar]


