Abstract
To compare the performance of convex probe endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) with conventional endobronchial biopsy (EBB) or transbronchial lung biopsy (TBLB) in patients with mediastinal, and coexisting endobronchial or peripheral lesions.
Retrospective review of records of patients undergoing diagnostic EBUS-TBNA and conventional bronchoscopy in 2014.
A total of 74 patients had mediastinal, and coexisting endobronchial or peripheral lesions. The detection rate of EBUS-TBNA for mediastinal lesion >1 cm in short axis, EBB for visible exophytic type of endobronchial lesion, and TBLB for peripheral lesion with bronchus sign were 71%, 75%, and 86%, respectively. In contrast, the detection rate of EBUS-TBNA for mediastinal lesion ≤1 cm in short axis, EBB for mucosal hyperemia type of endobronchial lesion, and TBLB for peripheral lesion without bronchus sign were 25%, 63%, and 38%, and improved to 63%, 88%, and 62% respectively by adding EBB or TBLB to EBUS-TBNA, and EBUS-TBNA to EBB or TBLB. Postprocedure bleeding was significantly more common in patients undergoing EBB and TBLB 8 (40%) versus convex probe EBUS-TBNA 2 patients (2.7%, P = 0.0004).
EBUS-TBNA is a safer single diagnostic technique compared with EBB or TBLB in patients with mediastinal lesion of >1 cm in size, and coexisting exophytic type of endobronchial lesion, or peripheral lesion with bronchus sign. However, it requires combining with EBB or TBLB and vice versa to optimize yield when mediastinal lesion is ≤1 cm in size, and coexisting endobronchial and peripheral lesions lack exophytic nature, and bronchus sign, respectively.
Keywords: bronchoscopy, bronchus, cancer (lung), EBUS-TBNA, exophytic
1. Introduction
The planning of a bronchoscopic diagnostic procedure for a lesion in the lung begins from a computed tomography (CT). If the CT shows a peripheral mass beyond the visible segmental bronchi with a bronchus sign, the conventional bronchoscopic technique associated with the highest yield is transbronchial lung biopsy (TBLB).[1–6] If the CT raises suspicion of an exophytic endobronchial lesion, the bronchoscopic technique associated with the highest yield is endobronchial biopsy (EBB).[7–9] If the CT scan shows mediastinal lesion, the preferred modality is blind or convex probe endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA).[10–15]
The decision making is easy in patients with isolated mediastinal, peripheral, or endobronchial lesion. However, some patients present with concomitant “mediastinal lesion” amenable to convex probe EBUS-TBNA, “endobronchial lesion” amenable to bronchial biopsy, and/or “peripheral lesion” amenable to TBLB in the same patient.[16] Although combining various techniques such as bronchial washing (BW), brushing, and biopsy increases the diagnostic yield, whether all the sites should be sampled in these patients employing all above-stated techniques or one site with one technique is adequate remains an unanswered question.[17–21] It is conceivable that sampling one site with single technique would be more effective in terms of cost, labor, and time. Furthermore, any preferred technique would need to have good safety profile, and high diagnostic yield.
The diagnostic yield and safety profile of convex probe EBUS-TBNA for mediastinal lesion is reportedly excellent.[11,12] It is known that needle techniques provide a higher diagnostic yield than bronchoalveolar lavage (BAL), brush, or forceps biopsy due to their potential to bypass surface and sample viable tumor or lymph-nodes beneath the trachea and bronchi.[22,23] EBUS-TBNA bypasses the surface, and samples from beneath the trachea and bronchi; this obviates the biopsy of the airway or peripheral lesion thus preventing flooding of the airways with blood as is associated with bronchial or TBLB. Correspondingly, the performance of convex probe EBUS-TBNA has been compared with other bronchoscopic techniques, and its superiority established in diseases like sarcoidosis.[24–28] However, literature on such comparison among patients with lung cancer or other benign diseases is sparse.
We hypothesized that the convex probe EBUS-TBNA as the sole and 1st line bronchoscopic technique in patients with mediastinal and coexisting peripheral or bronchial lesion is equivalent to conventional TBLB and EBB, respectively, in terms of yield, and safer in terms of complications. This study compares the performance of convex probe EBUS-TBNA with TBLB or EBB done sequentially in the same sitting, in patients with mediastinal and coexistent peripheral, or endobronchial lesions.
2. Methods
Retrospective review of records of patients who underwent EBUS-TBNA in 2014 and had mediastinal, and coexisting endobronchial or peripheral lesions were done (Fig. 1). Data were collected on demographics, CT findings, bronchoscopic findings, endobronchial ultrasound findings, type of diagnostic technique employed, and pathological result. Approval from institutional review board (DSRB) was obtained.
Figure 1.
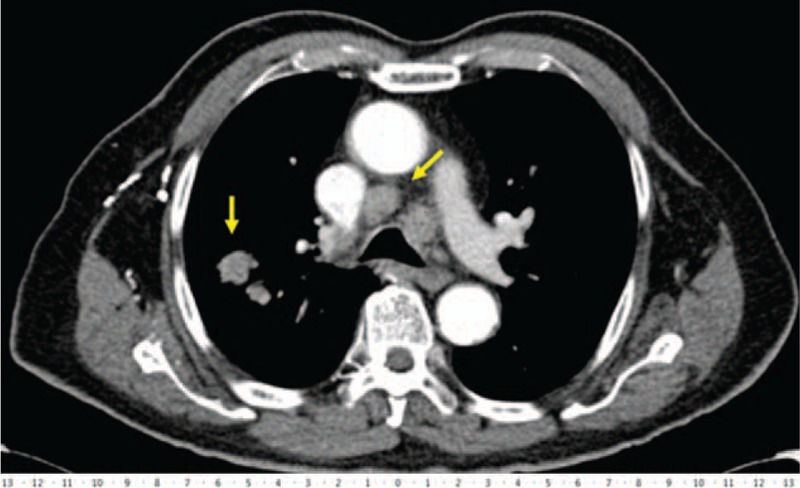
Representative case of mediastinal and coexisting peripheral parenchymal lesion in the same patient (scale bar in centimeters).
2.1. Definition of CT and bronchoscopic abnormalities
The size of mediastinal lesion was considered ≤1 cm when the diameter of the mediastinal lymph node or mass was ≤1 cm in short axis, and it was considered >1 cm when the diameter of the mediastinal lymph node or mass was >1 cm in short axis. Endobronchial exophytic lesion was defined as a lesion (tumorous growth) protruding into the lumen of the airways and visible on bronchoscopy. Endobronchial “mucosal hyperemia” was defined as an abnormal appearance of the mucosa in the form of erythema or neovascularization limited to the surface of the airway without any protrusion/elevation above the surface of the airways. Peripheral parenchymal lesion with bronchus sign was defined based on CT findings as a lesion ≥3 cm in largest diameter in the outer 3rd of the hemithorax with the finding of a bronchus leading directly to or contained within the peripheral lung mass. Peripheral parenchymal lesion without bronchus sign was defined as a lesion ≥3 cm in largest diameter in the outer 3rd of the hemithorax without any bronchus leading directly into the peripheral lung mass (Fig. 2).
Figure 2.
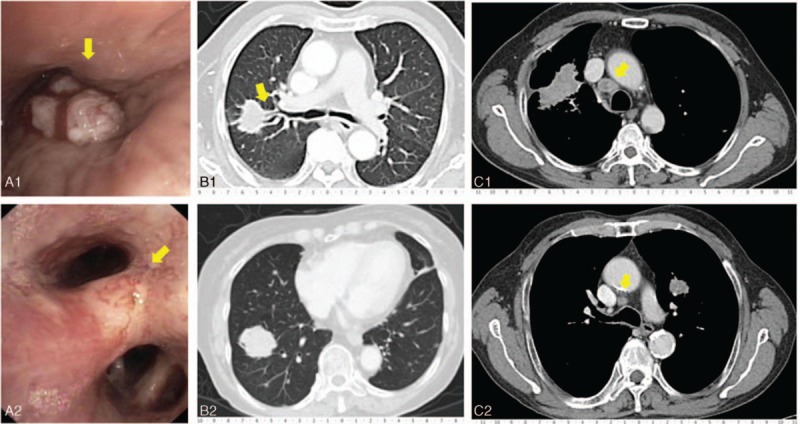
Representative case of visible endobronchial lesion amenable to EBB, peripheral lesion amenable to TBLB, and mediastinal lesion amenable to EBUS-TBNA. (A-1) Patient with exophytic endobronchial lesion in the right bronchus intermedius. (A-2) Patient with mucosal hyperemia type of endobronchial lesion in the right middle lobe. (B-1) Patient with a peripheral parenchymal lesion in the right upper lobe, showing bronchus of posterior segment of the right upper lobe leading into the mass (bronchus sign). (B-2) Patient with a peripheral parenchymal lesion in the right lower lobe without the bronchus sign. (C-1) Patient with a right para-tracheal lymph node of greater than 1 cm in diameter. (C-2) Patient with a right para-tracheal lymph node of lesser than 1 cm in diameter (scale bar in centimeters). EBB = endobronchial biopsy, EBUS-TBNA = endobronchial ultrasound guided transbronchial needle aspiration, TBLB = transbronchial lung biopsy.
2.2. Description of methodology of bronchoscopic techniques
All procedures were done by a single or 2 (including trainees) operators via the transoral route under local anesthesia (lignocaine) and moderate sedation (midazolam) in a unintubated state in the endoscopy center of a tertiary hospital.
2.3. Endobronchial biopsy
Tumors which were visibly protruding or polypoidal (exophytic) were biopsied using flexible biopsy forceps inserted through the working channel of the flexible bronchoscope. In case of tumors in difficult locations (e.g., lateral wall), the tip of the bronchoscope was flexed as far as possible so that opened forceps could be jammed against the surface of tumor. One or 2 biopsies were taken in each visible lesion. The biopsied material obtained by forceps was transferred to a bottle containing 10% formalin and sent for histopathological examination.[18] Postbiopsy bleeding was arrested by iced cold saline or adrenalin instillation.
2.4. Transbronchial lung biopsy
All transbronchial lung biopsies were done under fluoroscopy guidance. After the appropriate subsegmental bronchus was endoscopically selected, the forceps were advanced under fluoroscopic guidance and upon encountering resistance, the forceps were withdrawn 1 cm and opened and advanced again until resistance was met. Following this, the forceps were closed and withdrawn, while the bronchoscope was wedged in the biopsied segment/subsegment for 30 seconds. Two to 3 biopsies, or as permitted by the degree of the postbiopsy airway bleeding, were taken. Postprocedure chest roentgenograms were obtained within 2 or more hours.[29]
2.5. Endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA)
EBUS-TBNA was performed by 2 trained operators using a curvilinear scanning ultrasound bronchoscope (BF UC260F-OL8, Olympus Ltd., Tokyo, Japan) connected to an ultrasound unit (EU-C60 Olympus Ltd., Tokyo, Japan). For paratracheal lesions, the scope was positioned endotracheally. For peribronchial lesions the scope was positioned in the respective bronchi in order to visualize the lung lesion. TBNA was performed using a 22-gauge needle (NA-2015X-4022, Olympus Ltd., Tokyo, Japan). Two aspirates were performed with 15 revolutions (moving needle back and forth in the lesion) per aspirate. The core tissue was expelled onto piece of paper for histological examination and the needle was flushed with saline onto glass slides for cytological examination. The aspirate was smeared onto glass slides, air dried, fixed immediately with 95% alcohol. Histological cores were fixed with 10% neutral buffered formalin. Rapid on-site cytological examination was not available. A postprocedure chest X-ray was routinely performed to exclude any procedure-related complications.[13]
2.6. Data analysis
The diagnosis detection rate of various techniques was analyzed based on the radiological features of presence versus absence of bronchus sign, endobronchial features of mucosal hyperemia versus exophytic lesion, and size (diameter) of the mediastinal lesion in short axis of ≤1 cm versus >1 cm. We used software (SPSS, version 17; SPSS, Chicago, IL) for all statistical analyses. The results were compared using a Wilcoxon 2-sample test or Fisher exact test. P values were 2 sided and considered indicative of a significant difference if less than 0.05.
3. Results
Seventy four patients undergoing diagnostic bronchoscopic procedure for mediastinal and coexistent endobronchial or peripheral lesions in 2014 were studied (Table 1). All patients underwent convex probe EBUS-TBNA and BAL/BWs. Additionally, 19 underwent EBB and 20 underwent TBLB. Median (range) age was 63 (33–82) years and 51 (68.9%) were males. Final diagnosis was malignancy in 57, benign disease in 9, and unknown in 8 (Table 2).
Table 1.
General characteristics of the lesions and procedures performed in 74 patients.

Table 2.
Final diagnosis in 74 patients.

In the whole cohort, detection rate of EBUS-TBNA alone for the mediastinal lesion was similar to EBB via biopsy forceps alone for the endobronchial lesion, and TBLB via biopsy forceps alone for the peripheral lesion (≥3 cm in size). Detection rate of combined EBUS-TBNA plus EBB (89.4%) was superior to EBUS-TBNA alone (59.4%) in patients with mediastinal and coexisting endobronchial lesions (P = 0.01) (Fig. 3). Detection rate of combined EBUS-TBNA and EBB plus TBLB (79.4%) was superior to EBUS-TBNA alone (59.4%) in those who underwent combined procedures (P = 0.03) (Table 3).
Figure 3.
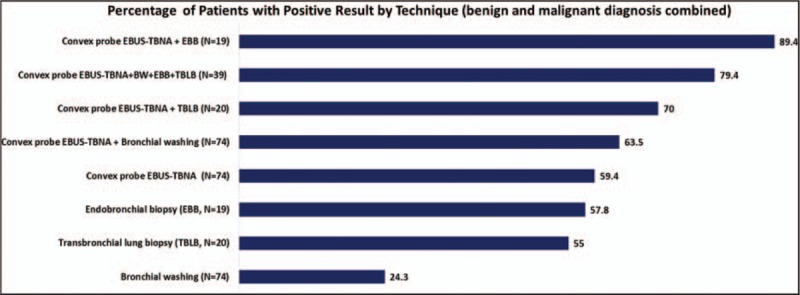
Analysis of diagnosis detection rate based on the bronchoscopic technique in the whole group.
Table 3.
Analysis of diagnosis detection rate based on the bronchoscopic technique.

In subgroup analysis based on the radiological features of presence versus absence of bronchus sign, endobronchial features of mucosal hyperemia versus exophytic lesion, and size of the mediastinal lesion of ≤1 versus >1 cm, the detection rate of EBUS-TBNA alone for mediastinal lesion >1 cm in short axis, EBB alone for visible exophytic type of endobronchial lesion, and TBLB alone for peripheral lesion with bronchus sign was 71%, 75%, and 86%, respectively. In contrast, the detection rate of EBUS-TBNA for mediastinal lesion ≤1 cm in short axis, EBB for mucosal hyperemia type of endobronchial lesion, and TBLB for peripheral lesion without bronchus sign were 25%, 63%, and 38%, respectively. The addition of EBB and TBLB to EBUS-TBNA for mediastinal lesion ≤1 cm in short axis, addition of EBUS-TBNA to EBB for mucosal hyperemia type of endobronchial lesion, and addition of EBUS-TBNA to TBLB for peripheral lesion without bronchus sign improved the detection rate exponentially to 63%, 88%, and 62%, respectively (Table 4).
Table 4.
Analysis of diagnosis detection rate (N [%]) based on characteristic features of the lesion and the procedures (EBUS-TBNA, BW, EBB, and TBLB) performed.

In subgroup analysis based on the etiology of the lesion, in patients with malignant lesion, the detection rate of EBUS-TBNA alone for the mediastinal lesion (72%), EBB alone for the endobronchial lesion (63%), and TBLB alone for the peripheral lesion (57%), were similar without any statistical difference. Detection rate of combined EBUS-TBNA plus EBB (100%) was superior to EBUS-TBNA alone (72%) in cancer patients with mediastinal and coexisting endobronchial lesions (P = 0.01). In patients with benign disease, there was no difference in detection rate between EBUS-TBNA alone and other techniques-single or in combination.
With regards to complications, postprocedure bleeding was significantly more common in patients who underwent EBB and TBLB 8 (40%) as compared to those undergoing convex probe EBUS-TBNA 2 (2.7%), P = 0.0004.
4. Discussion
We illustrated that EBUS-TBNA is adequate, and safer single technique of choice in patients with mediastinal lesion of short-axis diameter of >1 cm in size, and coexisting exophytic type of endobronchial lesion amenable to EBB, or peripheral lesion with bronchus sign amenable to TBLB. However, it requires combining with EBB or TBLB and vice versa to optimize yield when diameter of mediastinal lesion in short-axis is ≤1 cm in size, and coexisting endobronchial and peripheral lesions lacking exophytic nature, and bronchus sign, respectively.
Detection rate of BAL/BW in all patients combined was 24.3%. In patients with mediastinal lesion and coexistent peripheral lesion with bronchus sign, it was 43%. This is consistent with the existing literature where the diagnostic yield of BAL has been reported as 19.3 in all types of lesions, and 30% with a peripheral lung lesion.[30,31] The pooled sensitivity of BWs has been reported as 48% in patients with endobronchial lesions and 43% in patients with peripheral lesions.[19,30,31]
TBLB in patients with peripheral lesions of more than 3 cm in diameter regardless of the bronchus sign showed the detection rate of 50%. Among those with the bronchus sign, the detection rate of TBLB was 86%, whereas it was 38% in those without bronchus sign. The average yield of TBLB reported in the literature is 57% with range from 17% to 77% in patients with peripheral cancers depending of the size of the lesion and the presence or absence of bronchus sign.[17] In patients with ≥2 cm lesion, the yield is 63%, slightly higher than our cohort.[32] In patients with bronchus sign, our findings are consistent with the reported yield of 60% to 82% versus 0% to 44% for those without bronchus sign.[3–6]
Detection rate of bronchial biopsy using forceps in patients with endobronchial lesion regardless of whether the lesion was a “hyperemia type of mucosal abnormality” or an “exophytic lesion” showed the detection rate of 57.8% in the whole group. Among the “mucosal hyperemia” type of abnormality and the exophytic lesion, EBB was more diagnostic in exophytic lesions compared with “mucosal hyperemia” type of abnormality only (75% vs 62.5%). These findings are in keeping with the existing literature reporting the yield of EBB as 74% overall, and 90% in those with exophytic lesion.[31] The reason for relatively lower yield in our cohort of patients with exophytic lesions could be due to less number of biopsy attempts to minimize risk of bleeding. The incidence of bleeding in patients undergoing EBB or TBLB was 40% in our cohort.
The detection rate of convex probe EBUS-TBNA regardless of the size of the lesion was 59.4% in the whole group. This diagnostic yield is less than what is reported in the literature. However, the detection rate was 71% in those with the mediastinal lesion of greater than 1 cm in size and 25% in those with the mediastinal lesion of less than 1 cm in size. The diagnostic yield of convex probe EBUS-TBNA has been reported as 86% to 94% in the literature.[11,12,33] The reasons for lower yield in our cohort could be the inclusion of patients with mediastinal lesions of ≤1 cm in diameter, and reduced number of passes in each lesion. We performed 2 passes per station or lesion due to suboptimal patient cooperation as we perform the procedures without endotracheal tube and monitored anesthesia care. In the absence of a protected airway and monitored anesthetic care, patients’ cooperation was suboptimal. Care was taken during the procedures to avoid over sedation which may result in respiratory suppression and airway compromise. We postulate that diagnostic yield from EBUS-TBNA would be expected to be higher in centers where EBUS-TBNA is performed via the endotracheal route and where dedicated anaesthetic care is available.
Combining the bronchoscopic techniques has been shown to increase the yield. The overall yield by all, washing, brushing, and endobronchial biopsy was found to be 80% in patients with suspected lung cancer in 1 study and authors concluded that for maximal yield, bronchoscopists should perform all 3 techniques.[18] The benefit of combination was seen in our cohort too. The addition of EBUS-TBNA improved the detection rate of BAL/BW from 24.3% to 63.5% in the whole group. In case of TBLB, addition of EBUS-TBNA increased the overall yield from 50% to 70% in the whole group. In the case of EBB, the addition of EBUS-TBNA improved the detection rate from 57.8% to 89.4%. By combining techniques, the detection rate in the group that had lower yield such as “mucosal hyperemia” type of endobronchial abnormality, “peripheral lesion lacking bronchus sign,” and “small size mediastinal lesion” improved from 63% to 88%, 38% to 78%, and 25% to 63%, respectively, similar to their counterparts with exophytic lesion, bronchus sign, and large size mediastinal lesion. These findings imply that in patients with mediastinal, and coexisting parenchymal or endobronchial lesions, the decision regarding the single best bronchoscopic technique of choice should be based on the size of the mediastinal lesion, presence or absence of exophytic type of endobronchial lesion, and presence or absence of bronchus sign in case of peripheral lesion (Fig. 4).
Figure 4.
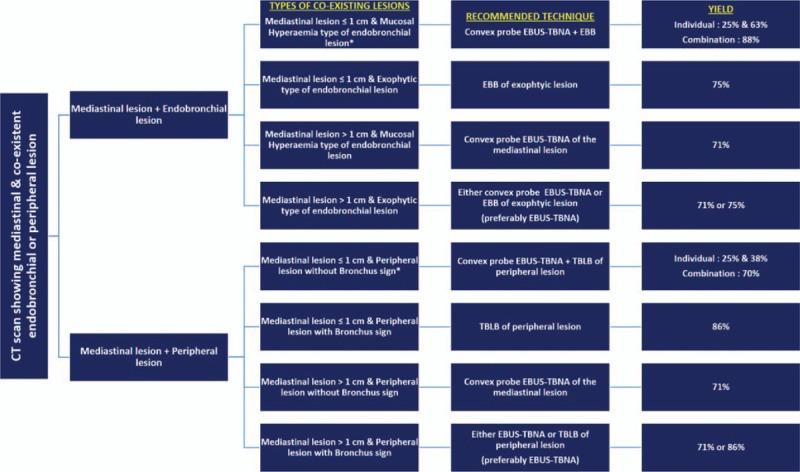
Proposed algorithm for the decision making based on the type of lesions in patients with mediastinal, and coexisting endobronchial or peripheral lesions.
Our study has limitations. First, it is a single center retrospective study with small numbers. Second, using a single bronchoscopic technique in patients with lesions at multiple sites may carry the risk of missing a double pathology. Third, it is not applicable to centers that do not practice EBUS-TBNA.
In conclusion, endobronchial lesions that are exophytic in nature, peripheral parenchymal masses with the bronchus sign, and mediastinal lesion that are larger than 1 cm in diameter in short axis have an equally better detection rate (>70%) with EBB, TBLB, and convex probe EBUS-TBNA, respectively, as compared to ≤60% in endobronchial lesion with just a “mucosal hyperemia” type of abnormality, peripheral mass without the bronchus sign, and mediastinal lesions smaller than 1 cm in size. Hence, in patients with large mediastinal lesion, and either coexistent exophytic endobronchial lesion in the airways, or peripheral mass with the bronchus sign, any one of the 3 above-stated techniques may be adequate. However, since EBUS-TBNA is least associated with the complication of bleeding, it may be the most preferable single diagnostic procedure due to its yield, safety, and ability to provide nodal staging. On the other hand, in patients with small (≤1 cm) mediastinal lesion, and coexisting “nonexophytic” type of endobronchial lesion, or peripheral lesion without the bronchus sign, combining EBUS-TBNA with EBB or TBLB may be preferable to optimize the detection rate.
Acknowledgments
The authors thank Ms Ivy Yu Ling Ling for her valuable contribution in editing the figures and administrative work.
Footnotes
Abbreviations: BAL = bronchoalveolar lavage, BW = bronchial washing, CT = computed tomography, EBB = endobronchial biopsy, EBUS-TBNA = endobronchial ultrasound guided transbronchial needle aspiration, TBLB = transbronchial lung biopsy.
The authors have no conflicts of interest to disclose.
References
- [1].Singh SP. The positive bronchus sign. Radiology 1998;209:251–2. [DOI] [PubMed] [Google Scholar]
- [2].Naidich DP, Sussman R, Kutcher WL, et al. Solitary pulmonary nodules. CT-Bronchoscopic correlation. Chest 1988;93:595–8. [DOI] [PubMed] [Google Scholar]
- [3].Gaeta M, Barone M, Russi EG, et al. Carcinomatous solitary pulmonary nodule: evaluation of tumor bronchi relationship with thin-section CT. Radiology 1993;187:535–9. [DOI] [PubMed] [Google Scholar]
- [4].Bilaceroglu S, Kumcuoglu Z, Alper H, et al. CT-bronchus sign guided bronchoscopic multiple diagnostic procedures in carcinomatous pulmonary nodules and masses. Respiration 1998;65:49–55. [DOI] [PubMed] [Google Scholar]
- [5].Gaeta M, Pandolfo I, Volta S, et al. Bronchus sign on CT in peripheral carcinoma of the lung. Value in predicting results of transbronchial biopsy. Am J Roengenol 1991;157:1181–5. [DOI] [PubMed] [Google Scholar]
- [6].Gaeta M, Russi EG, La Spada F, et al. Small bronchogenic carcinomas presenting as solitary pulmonary nodules. Biopsy approach guided by CT-positive bronchus sign. Chest 1992;102:1167–70. [DOI] [PubMed] [Google Scholar]
- [7].Wilson RW, Frazier AA. Pathological-radiological correlations: pathological and radiological correlation of endobronchial neoplasms: part II, malignant tumours. Ann Diagn Pathol 1998;2:31–4. [DOI] [PubMed] [Google Scholar]
- [8].Simoff MJ. Endobronchial management of advanced lung cancer. Cancer Control 2001;8:337–43. [DOI] [PubMed] [Google Scholar]
- [9].Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123:115S–28S. [DOI] [PubMed] [Google Scholar]
- [10].Nakajima T, Yasufuku K. The techniques of endobronchial ultrasound-guided transbronchial needle aspiration. Innovations 2011;6:57–64. [DOI] [PubMed] [Google Scholar]
- [11].Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389–96. [DOI] [PubMed] [Google Scholar]
- [12].Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156–64. [DOI] [PubMed] [Google Scholar]
- [13].Verma A, Jeon K, Koh W-J, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of central lung parenchymal lesions. Yonsei Med J 2013;54:672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tournoy KG, Rintoul RC, van Meerbeeck JP, et al. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer 2009;63:45–9. [DOI] [PubMed] [Google Scholar]
- [15].Nakajima T, Yasufuku K, Fujiwara T, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrapulmonary lesions. J Thorac Oncol 2008;3:985–8. [DOI] [PubMed] [Google Scholar]
- [16].Verma A, Lim AY, Tai DYH, et al. Timeliness of diagnosing lung cancer: number of procedures and time needed to establish diagnosis: being right the first time. Medicine 2015;94:e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mazzone P, Jain P, Arroliga AC, et al. Bronchoscopic and needle biopsy techniques for diagnosis and staging of lung cancer. Clin Chest Med 2002;23:137–58. [DOI] [PubMed] [Google Scholar]
- [18].Fuladi AB, Munje RP, Tayade BO. Value of washings, brushings, and biopsy at fibreoptic bronchoscopy in the diagnosis of lung cancer. JIACM 2004;5:137–42. [Google Scholar]
- [19].Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123(1 Suppl):115S–28S. [DOI] [PubMed] [Google Scholar]
- [20].Gasparini S, Ferrety M, Such E, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses: experience with 1027 consecutive cases. Chest 1995;108:131–7. [DOI] [PubMed] [Google Scholar]
- [21].El-Bayoumi E, Silvestri GA. Bronchoscopy for the diagnosis and staging of lung cancer. Semin Respir Crit Care Med 2008;29:261–70. [DOI] [PubMed] [Google Scholar]
- [22].Dasgupta A, Jain P, Minai O, et al. Utility of transbronchial needle aspiration in the diagnosis of endobronchial lesions. Chest 1999;115:1237–41. [DOI] [PubMed] [Google Scholar]
- [23].Caglayan B, Akturk U, Fidan A, et al. Transbronchial needle aspiration in the diagnosis of endobronchial malignant lesions. Chest 2005;128:704–8. [DOI] [PubMed] [Google Scholar]
- [24].Navani N, Booth HL, Kocjan G, et al. Combination of endobronchial ultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology 2011;16:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Plit M, Pearson R, Havryk A, et al. Diagnostic utility of endobronchial ultrasound-guided transbronchial needle aspiration compared with transbronchial and endobronchial biopsy for suspected sarcoidosis. Intern Med J 2012;42:434–8. [DOI] [PubMed] [Google Scholar]
- [26].Hong G, Lee KJ, Jeon K, et al. Usefulness of endobronchial ultrasound-guided transbronchial needle aspiration for diagnosis of sarcoidosis. Yonsei Med J 2013;54:1416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dziedzic DA, Peryt A, Orlowski T. The role of EBUS-TBNA and standard bronchoscopic modalities in the diagnosis of sarcoidosis. Clin Respir J 2015;doi: 10.1111/crj.12304 (published ahead of print). [DOI] [PubMed] [Google Scholar]
- [28].Goyal A, Gupta D, Agarwal R, et al. Value of different bronchoscopic sampling techniques in diagnosis of sarcoidosis: a prospective study of 151 patients. J Bronchol Interv Pulmonol 2014;21:220–6. [DOI] [PubMed] [Google Scholar]
- [29].Anders GT, Johnson JE, Bush BA, et al. Transbronchial biopsy without fluoroscopy. A seven-year perspective. Chest 1988;94:557–60. [DOI] [PubMed] [Google Scholar]
- [30].Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Herth FJF. Bronchoscopic techniques in diagnosis and staging of lung cancer. Breathe 2011;7:325–37. [Google Scholar]
- [32].Rivera MP, Mehta AC. Initial diagnosis of lung cancer. ACCP evidence-based clinical practice guidelines. 2nd edition. Chest 2007;132:131S–48S. [DOI] [PubMed] [Google Scholar]
- [33].Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


