Abstract
Endoscopic submucosal dissection (ESD) has been widely accepted as a curative treatment for gastric neoplasm. Pyloric stenosis is a chronic complication that can be caused by ESD. The aim of this study is to clarify the risk factors and management for pyloric stenosis. From January 2004 to January 2014, a total of 126 patients who underwent ESD adjacent to pylorus were reviewed retrospectively. Pyloric mucosal defect was defined as when any resection margin of ESD was involved in the pyloric ring. Pyloric stenosis was defined as when a conventional endoscope could not be passed to the duodenum. Among the 126 patients, pyloric stenosis was identified in 9. In a univariate analysis, pyloric stenosis was more common in older patients (P < 0.05) and in lesions with resections over 75% of the pyloric ring circumference (P < 0.001). In a multivariate analysis, the factor that was associated with pyloric stenosis was the extent of the pyloric ring dissection (P < 0.001). Four of the 9 patients with pyloric stenosis had mild dyspepsia, and the others had gastric outlet obstruction symptoms. The 5 symptomatic patients underwent endoscopic balloon dilation (EBD), and the frequency of EBD was 1 to 8 times. The asymptomatic patients were treated conservatively. The incidence of pyloric stenosis was higher in lesions with resections over 75% of the pyloric ring circumference. Although EBD was an effective treatment for pyloric stenosis, conservative management was also helpful in patients who had mild symptoms.
Keywords: balloon dilation, endoscopic submucosal dissection, pylorus, stenosis
1. Introduction
Endoscopic resection has been widely used for the treatment of gastric adenoma or early gastric cancer that is limited to the mucosa without lymph node metastases.[1,2] It is divided into endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). ESD is the preferred technique, because it allows en bloc resection and complete excision of larger lesions.[3] ESD requires a higher degree of skill and more experience compared to that for EMR.[4] These procedures have various complications, such as bleeding, perforation, or pyloric stenosis. Although endoscopic balloon dilation (EBD) and metal stent insertion can be used to successfully treat post-ESD pyloric stenosis, it also carries the risk of severe complications, such as perforation or bleeding, and may require repetitive procedure due to restenosis.[5,6] Unfortunately, currently there is no established prevalence rate and treatment for post-ESD pyloric stenosis. Therefore, this study aimed to review the incidence rate and risk factors for post-ESD pyloric stenosis and to analyze treatment outcomes according to clinical symptoms.
2. Materials and methods
2.1. Study population
A total of 1381 patients with gastric cancer or gastric adenoma were treated with ESD at the Presbyterian Medical Center between January 2004 and January 2014. This retrospective study evaluated risk factors for pyloric stenosis and its prevalence in 126 patients, in whom the perimeter of the excised lesions involved the pyloric ring. The present study was carried out after obtaining ethical approval from the institutional review board (IRB) of the Presbyterian Medical Center (IRB no. 2013-06-21).
2.2. ESD indications and procedure
Epithelial neoplastic lesions are classified into 5 categories, some of which have subcategories in the Vienna classification.[7] ESD was performed for noninvasive low-grade dysplasia, noninvasive high-grade dysplasia, intramucosal carcinoma, gastric subepithelial lesions, and gastric lesions suspicious of neoplasm. The indication of ESD for gastric adenoma was a lesion that was ≥1 cm, regardless of its shape. In early gastric cancer, ESD was performed for differentiated-type cancer when the protruding lesion was ≤2 cm, when the excavated lesion was ≤1 cm, or when there was a lesions in which adenocarcinoma had arisen from an adenoma, regardless of size. For undifferentiated-type cancer, ESD was indicated for the lesions that were ≤1 cm when the patients wanted to undergo ESD rather than surgery, or in cases with a poor performance status due to severe cardiopulmonary disease. At our institution, a endoscope (GIF-Q240J or GIFQ260J; Olympus, Tokyo, Japan) equipped with a transparent cap (D-201, Olympus) was used for the ESD procedure, ESD is performed using an insulation-tipped knife (KD-610L or KD-611L, Olympus), a needle knife (KD-1L-1; Olympus), and a hook knife (KD-620LR; Olympus). In the first step, the normal mucosa that was >5 mm away from the edge of the lesion was marked circumferentially using a snare tip, and a mixture of normal saline and indigo carmine was injected into the submucosa slightly outside the marked region until the mucosa had been sufficiently lifted. Next, a 360° incision was made around the lesion using a needle knife. Finally, the lesion was directly dissected from the muscle layers along the submucosa using an insulation-tipped knife or hook knife.
2.3. Pyloric stenosis risk factors and definition
We reviewed the clinical records, endoscopic finding, and histological reports of all patients. Sex, age, comorbidities, lesion position, endoscopic findings, pathological findings, lesion ulcers, submucosa invasion, pyloric ring lesions, and the ratio of the resected region to the pyloric ring circumference were considered the potential risk factors for pyloric stenosis. Location of the tumor was subdivided into the lesser curvature, greater curvature, anterior wall, and posterior wall. The macroscopic lesion types were classified with data collected from the endoscopic reports according to the early gastric cancer classification method that was proposed by the Japan Gastroenterological Endoscopy Society in 1962.[8] This method classifies gastric cancers into 3 types: elevated type (protruding and superficially elevated lesions), flat type (superficially flat lesions), and depressed type (superficially depressed and excavated lesions). Pyloric mucosal defect was defined when any resection margin of ESD was involved in pyloric ring. The circumferential ratio of the mucosal defect in the pyloric ring was evaluated by 1 physician via the endoscopic images, and was classified as <75% and ≥75%. The final histological diagnosis after the endoscopic resection was determined based on the histopathological diagnosis of the excised specimen including the degree of differentiation of the lesion, involvement of basal and lateral resection margin, and lymphovascular invasion. Pyloric ring stenosis was defined as when an endoscope (GIF-Q240J or GIF-Q260J; Olympus) could not pass through the pyloric ring, which prevented entry into the duodenum. Diameter of GIF-Q240J and GIF-Q260J endoscope are 10.2 and 9.2 mm, respectively.
2.4. Post-ESD follow-up
Each patient was treated with intravenous pantoprazole (Takeda GmbH, Oranienburg, Germany) 40 mg/d for 2 days, and then the treatment of most patients was changed to oral pantoprazole (40 mg/d) on day 3 if there were no complications. Chest and abdominal radiographs were taken immediately after the ESD and on the following day to determine if perforation was present. Follow-up endoscopy was performed again on the next day after the ESD to check for bleeding or the ulcer's condition, and patients were allowed to start a liquid diet on day 3 if there was no bleeding or perforation. Although the schedule of postdischarge follow-up endoscopy was individualized depending on the previous histological findings, endoscopy was performed 3 months postdischarge, and thereafter, every 6 or 12 months.
2.5. Statistical analysis
The SPSS version 20.0 (IBM Co, Chicago, IL) was used for the statistical analysis. Two-sample t test was used for continuous variables. Categorical variables were analyzed by Fisher exact test. Factors associated with pyloric stenosis were analyzed with logistic regression. A P values <0.05 were considered to be statistically significant.
3. Results
3.1. General characteristics of the patients
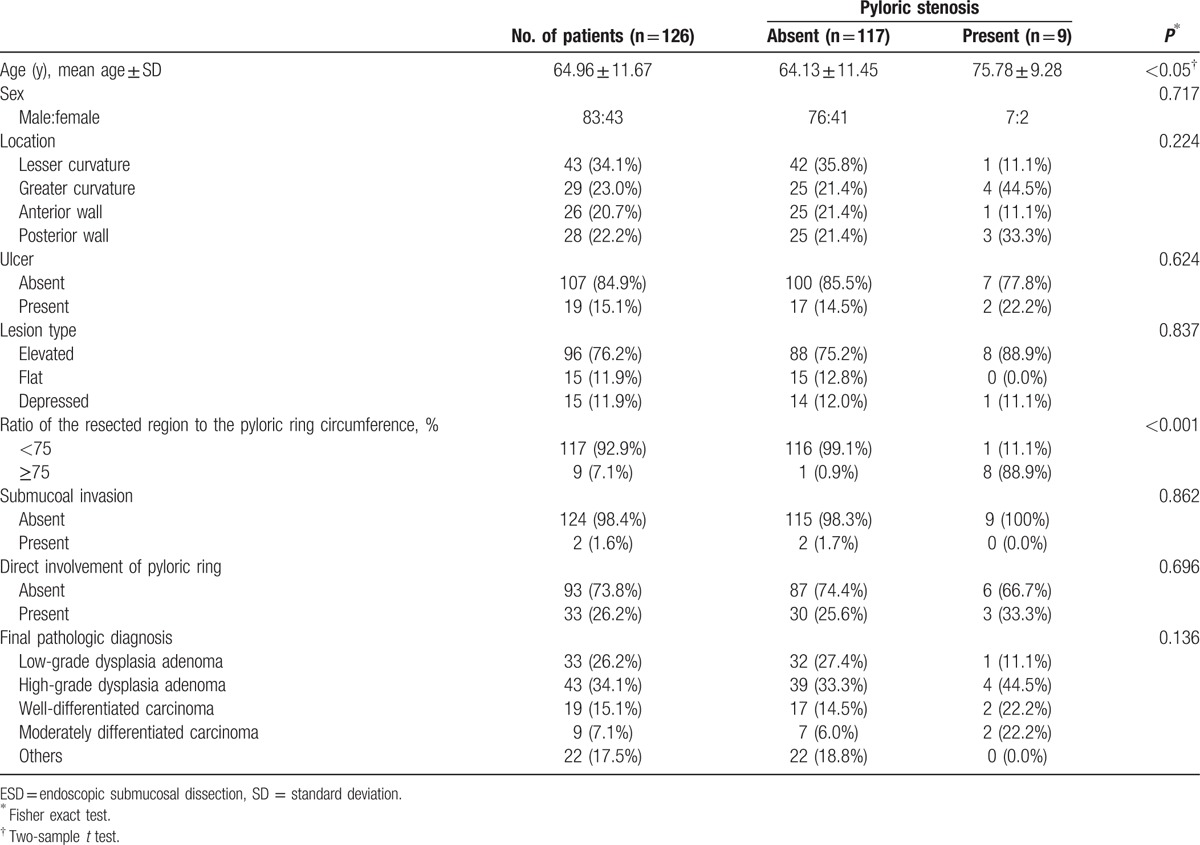
Among the 1381 patients who underwent gastric ESD, 126 patients involved the resected mucosal parts in the pyloric ring after ESD. The incidence rate of pyloric stenosis in gastric ESD was associated with 9 of the 1381 patients (0.6%). In these 126 cases, all lesions were located in the antrum; including 43 (34.1%) at the lesser curvature, 29 (23.0%) at the greater curvature, 28 (22.2%) in the anterior wall, and 26 (20.7%) in the posterior wall. Endoscopic findings revealed that there were 96 patients (76.2%) with the elevated type, 15 (11.9%) with the flat type, and 15 (11.9%) with the depressed type. A total of 107 patients (84.9%) did not have an ulcer in the lesion while 19 patients (15.1%) had an ulcer. The ratio of the resected lesion to the pyloric ring was <75% in 117 patients (92.9%) and ≥75% in 9 patients (7.1%). The submucosa was invaded in 2 patients (1.6%), while the lesions were limited to the mucosa in 124 patients (98.4%). Lesions were present within the pyloric ring in 33 patients (26.2%) and outside the pyloric ring in 93 patients (73.8%). The pathological findings after ESD were early gastric cancer in 28 patients (22.2%) and gastric adenoma in 76 patients (60.3%). The remaining 22 patients (17.5%) were diagnosed with gastritis in 15 patients, hyperplastic polyp in 4 patients, and lipoma in 3 patients. Early gastric cancer was well-differentiated type in 19 patients (15.1%) and moderately differentiated type in 9 patients (7.1%). Among the cases of gastric adenoma, 33 patients (26.2%) were diagnosed with low-grade dysplasia and 43 patients (34.1%) with high-grade dysplasia (Table 1).
Table 1.
Risk factors of pyloric stenosis after ESD.
3.2. Characteristics of the patients with pyloric stenosis
Pyloric stenosis occurred in 9 (7.1%) of the 126 patients. The mean age of the patients with pyloric stenosis was 75.78 ± 9.28 years, which was higher than that of the patients without pyloric stenosis (64.13 ± 11.45 years). There was no significant difference in the proportion of men and women in the 2 groups. The rate of post-ESD pyloric stenosis was significantly higher when the resected mucosal region was ≥75% of the pyloric ring (Table 1). In a multivariate analysis, risk factor related to pyloric stenosis was the resected area over 75% of pyloric ring circumference.
3.3. Pyloric stenosis treatment outcomes
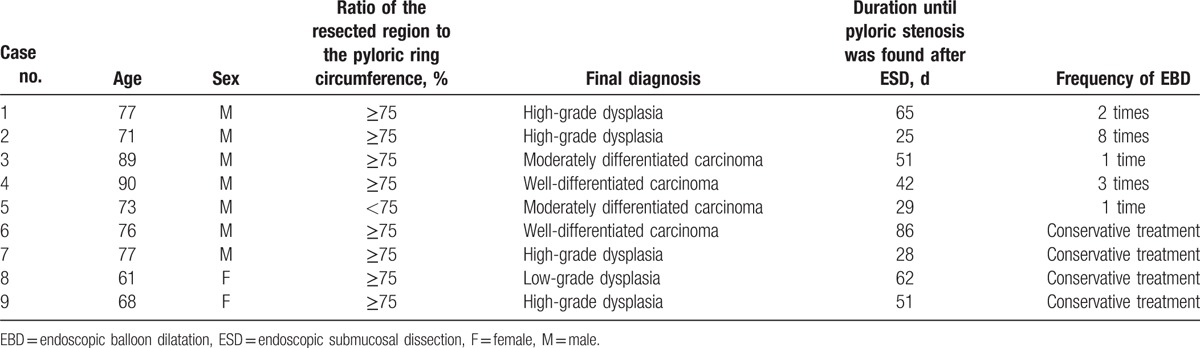
Among the 9 patients with pyloric stenosis, 5 developed new symptoms, including nausea, vomiting, and abdominal discomfort, while the remaining 4 patients only had mild dyspepsia. The 5 patients with severe symptoms underwent EBD using a controlled radial expansion balloon (CRE balloon; Boston Scientific, Natick, MA). After EBD, the patients reported temporary abdominal discomfort, but there were no severe complications, such as bleeding or perforation. The median number of EBD procedures in each patient was 3. The number of procedures ranged from 1 in 2 patients to 8 in 1 patient who experienced recurrent symptoms. Among the 5 patients who were treated with EBD, follow-up endoscopy was performed for 2 patients, due to which there was substantial improvement in the pyloric stenosis that allowed the endoscope to pass through the pyloric ring. The remaining 3 patients exhibited reduction in nausea and vomiting without follow-up endoscopy, and had no specific complaints even after eating solid foods, which indicated that their pyloric stenosis had improved. The 4 patients with less severe symptoms did not undergo EBD and were advised to eat small amounts of food frequently, and were prescribed proton pump inhibitor and prokinetic drugs, while their progress was monitored. Follow-up endoscopy confirmed that the pyloric stenosis had improved in all 4 patients (Table 2).
Table 2.
Clinical characteristics of patients who developed pyloric stenosis after ESD.
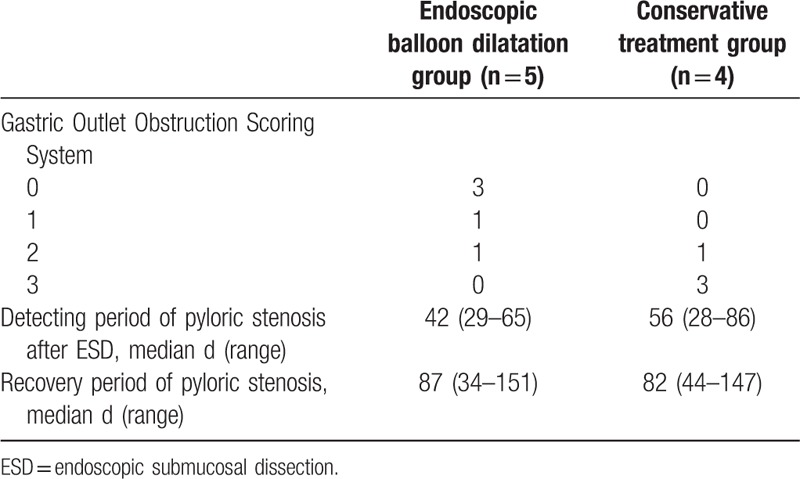
The Gastric Outlet Obstruction Scoring System (GOOSS) was used to evaluate the symptoms of the 9 patients with pyloric stenosis. The GOOSS presented by Adler[9] (0 = no oral intake, 1 = liquids only, 2 = soft solids, 3 = low-residue or full diet). This system assigns a point score based on the level of oral intake (Table 3). The GOOSS scores for the patients who had undergone EBD procedure were 0 points in 3 patients, 1 point in 1 patient, and 2 points in 1 patient. In contrast, the scores for the patients who had received conservative treatment were 2 points in 1 patient and 3 points in 3 patients. The median detection time for post-ESD pyloric stenosis was 42 days (range, 29–65 days) for the patients who had undergone EBD procedure and 56 days (range, 28–86 days) for the patients who had received conservative treatment. The median time from the diagnosis of pyloric stenosis to symptom alleviation was 87 days (range, 34–151 days) and 82 days (range, 44–147 days) in the EBD group and the conservative treatment group, respectively (Table 4).
Table 3.
Gastric Outlet Obstruction Scoring System.

Table 4.
Comparison of clinical symptoms and outcomes associated with endoscopic balloon dilatation and conservative treatment.
4. Discussion
Endoscopic treatment for early gastric cancer via ESD has been widely used,[2,10] and the incidence of the related complications has increased. Bleeding and perforation are the most common complications.[4,11] while pyloric stenosis, emphysematous gastritis, and pneumonia are also reported, although these complications rare. Perforation and bleeding can be controlled successfully, because they are mostly detected early during ESD, whereas pyloric stenosis occasionally occurs later after the lapse of considerable time.
Post-ESD stenosis mostly occurs at the esophagus, gastroesophageal junction, or pylorus. In particular, as the pylorus has a thick muscle layer and its lumen suddenly narrows (compared to the wider lumen in the antrum), gastric outlet obstruction is likely to occur due to fibrosis that develops during the recovery from ulcers after ESD.[12] Tsunada et al[13] have reported a post-ESD stenosis rate of 0.9% (5/532) and all of their cases occurred in the antrum, and Coda et al[14] have reported a post-ESD stenosis rate of 0.8% (15/1819) with 8 cases in the antrum and 7 in the cardia. Various studies have reported on the risk factors of post-ESD stenosis. Coda et al[14] reported through univariate analysis that circumferential extent of mucosa defect of 3/4 and longitudinal extent of >5 cm in length were risk factors of pyloric stenosis. Kakushima et al[15] reported through multivariate analysis that wide resection of >75% of the circumference is associated with post-ESD pyloric stenosis. In this study, ratio of the resected region to the pyloric ring circumference ≥75% was found to be statistically significant risk factor.
Post-ESD stenosis has been treated using EBD, stent insertion, and steroid injection at the ulcer region. Since EBD is a relatively simple and easy-to-use procedure, it has been used as the first choice for the treatment of stenosis. However, it can also induce severe complications like perforation and bleeding, and the repetitive procedures that are necessitated by restenosis can cause economical and psychological issues. Iizuka et al[16] performed an average of 2.8 EBD procedures in 6 patients with stenosis, and perforation was only observed in 1 case, which was successfully resolved with conservative treatment. In our study, the frequency of EBD was 1 to 8 times (average, 3 times) in 5 patients with pyloric stenosis and any serious complications were not observed.
Novel biodegradable esophageal stent successfully improved the condition of 2 patients with benign esophageal stenosis after ESD and may be useful in treating post-ESD pyloric stenosis.[17] Several studies reported that esophageal strictures can be successfully treated with endoscopic intralesional steroid injections.[18,19] Mori et al[20] have also experienced that a local injection of triamcinolone acetonide effectively prevented the gastric deformation that occurs during post-ESD ulcer treatment.
In previous reports, all cases of post-ESD pyloric stenosis were treated with EBD, and none of the patients received conservative treatment. In the present study, post-ESD pyloric stenosis occurred in 9 patients, including 5 cases with severe gastric outlet obstruction symptoms that were treated with EBD and 4 cases with less severe symptoms that alleviated with only dietary and medicinal treatments. Therefore, it is desirable to identify the patients who will benefit from conservative treatment prior to EBD, and individualized treatment program according to the degree of symptoms and stenosis, should be given for safer and noninvasive treatments.
The invasion of peptic ulcers ranges from the mucosal erosion to the muscularis propria. In contrast, since the iatrogenic ulcers that are caused by ESD occur immediately after the procedure, they feature relatively less fibrosis and fewer inflammatory findings. In addition, they are limited to the submucosa, while the muscle layer in the vicinity of the ulcer retains its contractility. Furthermore, postexcision rapid shrinkage of the mucosa around the ulcer reduces the ulcer's size, forms granulation tissue, and induces mucosal repopulation, which typically results in recovery within 8 weeks.[21] Therefore, EBD can be considered for patients with severe post-ESD pyloric stenosis symptoms. In contrast, natural convalescence from pyloric stenosis may be possible with conservative care when they are associated with mild symptom and detected in follow-up endoscopy. As the treatment indications for ESD have expanded, it is predicted that the incidence of stenosis-related complications will gradually increase, necessitating a systematic study of its features.
Treatment plans should be established via early follow-up endoscopy, which can predict pyloric stenosis in the high-risk group, thereby facilitating the prevention of symptoms that are caused by pyloric stenosis and improving the patient's quality of life.
The present study has several limitations. First, since the study was a retrospective study based on clinical records and endoscopic images, cases could have been omitted, or bias could have been present during the review of results. Second, the procedure was conducted at a single center and by 1 endoscopic expert for most cases and therefore, it is difficult to generalize the results. Third, magnifying endoscopy with narrow-band imaging (NBI) is a promising endoscopic technique that may improve the accuracy of diagnosis of gastric lesions.[22] Unfortunately, we could not use magnifying endoscopy with NBI to evaluate the gastric lesions due to a limitation of our hospital equipment. Lastly, as only 9 patients had pyloric stenosis, the result interpretation is limited. Moreover, in the statistical analysis, chi-squared test was conducted through Fisher exact test due to low expected frequencies.
Although Coda et al[14] mentioned that longitudinal extent of >5 cm was also relevant as a risk factor for pyloric stenosis, this study did not consider longitudinal extent as a risk factor because it is unlikely that a longitudinal extent would directly affect pyloric stenosis, although it would affect structural transformation in the healing process. In conclusions, the pyloric ring circumference ≥75% was the only risk factor of pyloric stenosis. In the future, the study of the risk factors affecting post-ESD stenosis needs to be performed with numerous cases through a multicenter trial, and additional studies on the characteristics of stenosis group that can be recovered by conservative treatments should also be conducted.
Footnotes
Abbreviations: EBD = endoscopic balloon dilation, EMR = endoscopic mucosal resection, ESD = endoscopic submucosal dissection, GOOSS = Gastric Outlet Obstruction Scoring System, IRB = institutional review board, NBI = narrow-band imaging.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219–25. [DOI] [PubMed] [Google Scholar]
- [2].Gunji Y, Suzuki T, Hori S, et al. Prognostic significance of the number of metastatic lymph nodes in early gastric cancer. Dig Surg 2003;20:148–53. [DOI] [PubMed] [Google Scholar]
- [3].Gotoda T. Endoscopic resection for premalignant and malignant lesion of the gastrointestinal tract from the esophagus to the colon. Gastrointest Endosc Clin N Am 2008;18:435–50. [DOI] [PubMed] [Google Scholar]
- [4].Fujishiro M. Kaminishi M, Takubo K, Mafune K. Endoscopic resection for early gastric cancer. The Diversity of Gastric Carcinoma: Pathogenesis, Diagnosis, and Therapy. Springer-Verlag:Tokyo; 2005. 243–52. [Google Scholar]
- [5].Kim HJ, Park JJ, Kang CD, et al. Effect of the temporary placement of stent in benign pyloric stenosis. Gastroint Endosc 2004;59:153A. [Google Scholar]
- [6].Cheng YS, Li MH, Chen WX, et al. Follow-up evaluation for benign stricture of upper gastrointestinal tract with stent insertion. World J Gastroenterol 2003;9:2609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Akahoshi K, Chijiwa Y, Hamada S, et al. Pretreatment staging of endoscopically early gastric cancer with a 15 MHz ultrasound catheter probe. Gastrointest Endosc 1998;48:470–6. [DOI] [PubMed] [Google Scholar]
- [9].Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol 2002;97:72–8. [DOI] [PubMed] [Google Scholar]
- [10].Eguchi T, Gotoda T, Oda I, et al. Is endoscopic one-piece mucosal resection essential for early gastric cancer? Dig Endosc 2003;15:113–6. [Google Scholar]
- [11].Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005;17:54–8. [Google Scholar]
- [12].Lee WW, Park JJ, Oh CR, et al. A case of endoscopic temporary stent insertion to treat a pyloric stenosis caused by endoscopic submucosal dissection for early gastric cancer. Korean J Gastrointest Endosc 2008;37:429–32. [Google Scholar]
- [13].Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008;67:979–83. [DOI] [PubMed] [Google Scholar]
- [14].Coda S, Oda I, Gotoda T, et al. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009;41:421–6. [DOI] [PubMed] [Google Scholar]
- [15].Kakushima N, Tanaka M, Sawai H, et al. Gastric obstruction after endoscopic submucosal dissection. United European Gastroenterol J 2013;1:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Iizuka H, Kakizaki S, Sohara N, et al. Stricture after endoscopic submucosal dissection for early gastric cancers and adenomas. Dig Endosc 2010;22:282–8. [DOI] [PubMed] [Google Scholar]
- [17].Saito Y, Tanaka T, Andoh A, et al. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig Dis Sci 2008;53:330–3. [DOI] [PubMed] [Google Scholar]
- [18].Lee M, Kubik CM, Polhamus CD, et al. Preliminary experience with endoscopic intralesional steroid injection therapy for refractory upper gastrointestinal strictures. Gastrointest Endosc 1995;41:598–601. [DOI] [PubMed] [Google Scholar]
- [19].Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011;74:1389–93. [DOI] [PubMed] [Google Scholar]
- [20].Mori H, Rafiq K, Kobara H, et al. Local steroid injection into the artificial ulcer created by endoscopic submucosal dissection for gastric cancer: prevention of gastric deformity. Endoscopy 2012;44:641–8. [DOI] [PubMed] [Google Scholar]
- [21].Kakushima N, Yahagi N, Fujishiro M, et al. The healing process of gastric artificial ulcers after endoscopic submucosal dissection. Dig Endosc 2004;16:327–31. [Google Scholar]
- [22].Yao K, Takaki Y, Matsui T, et al. Clinical application of magnification endoscopy and narrow-band imaging in the upper gastrointestinal tract: new imaging techniques for detecting and characterizing gastrointestinal neoplasia. Gastrointest Endosc Clin N Am 2008;18:415–33. [DOI] [PubMed] [Google Scholar]


