Abstract
Apolipoprotein E (ApoE), which has been shown to influence serum lipid parameters, can bind to multiple types of lipids and plays an important role in the metabolism and homeostasis of lipids and lipoproteins. A previous study showed that ApoE concentration significantly affects serum lipid levels independently of ApoE polymorphism. The serum lipid levels were also closely correlated with dietary habits, and Shandong cuisine is famous for its high salt and oil contents, which widely differ among the different areas in China. Therefore, studying the effect of ApoE polymorphism on ApoE concentration and serum lipid levels in Shandong province is very important.
A total of 815 subjects including 285 men and 530 women were randomly selected and studied from Jinan, Shandong province. In order to evaluate the association of ApoE polymorphism and serum level on lipid profiles, the ApoE genotypes, as well as levels of fasting serum ApoE and other lipid parameters, were detected in all subjects.
The frequency of the ApoE E3 allele was highest (83.1%), while those of E2 and E4 were 9.4% and 7.5%, respectively, which are similar to those in other Asian populations. ApoE2 allele carriers showed significantly increased ApoE levels but lower levels of serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and Apolipoprotein B (ApoB).
We found that ApoE level is influenced by ApoE polymorphism in a gene-dependent manner. The ApoE polymorphism showed different influences on serum lipid parameters with increasing age and body mass index (BMI) in our Shandong Han population.
Keywords: ApoB, ApoE, LDL-C, polymorphism, serum lipid, TC
1. Introduction
The main functions of lipoproteins are to transport lipids in the blood and facilitate lipid transport through the cellular membrane into cells. Lipoproteins play an important role in the development of atherosclerotic cardiovascular disease in humans.[1–3] There are 5 main types of blood lipoproteins: high-density lipoprotein (HDL), low-density lipoprotein (LDL), intermediate-density lipoprotein, very low-density lipoprotein (VLDL), and chylomicrons. The concentrations and metabolism of these lipoproteins, which have different functions in lipids transport and homeostasis,[4,5] are modulated by apolipoproteins on the surface of lipid-rich particles.
The human apolipoprotein E (ApoE gene; OMIM 107741) located on chromosome 19q13.2, codes for a 34,200-kDa protein of 299 amino acids.[6] As a major determinant of lipoprotein metabolism, ApoE protein is synthesized principally in the liver, and has also been found in other tissues such as the brain, spleen, kidneys, gonads, adrenals, and macrophages. ApoE is a polymorphic glycoprotein attached to various plasma lipoproteins. ApoE participates in reverse cholesterol transport and functions as a ligand for receptor-mediated clearance of chylomicrons and VLDL remnants in lipid metabolism.[7]
Two single-nucleotide polymorphism variations in the ApoE gene, rs429358 and rs7412, lead to different protein isoforms that exert opposite effects on the metabolism of blood lipids.[8] Differentiated based on cysteine (Cys) and arginine (Arg) residue interchanges at positions 112 and 158 in the amino acid sequence, the allelic variants derived from the 2 single-nucleotide polymorphisms are commonly referred to as E2, E3, and E4. The E2 allele contains a Cys residue at both the 112 and 158 positions, the E3 allele has a Cys residue at position 112 and an Arg residue at position 158, and the e4 allele contains Arg residues at both positions in the receptor-binding region. The 3 variants give rise to 6 biallelic genotypes, 3 homozygous genotypes (E2/E2, E3/E3, and E4/E4), and 3 heterozygous genotypes (E2/E3, E2/E4, and E3/E4), which form 3 isoforms of ApoE, referred to as ApoE2, ApoE3, and ApoE4.[9] These amino acid substitutions cause the ApoE protein to have different functions. Among these 6 genotypes, E3/E3 is the wild-type and most prevalent in different populations, and the number of people with the E2 or E4 allele is low. Different ApoE isoforms have different receptor-binding abilities; ApoE4 shows the maximum-binding capacity, whereas the binding ability ApoE2 is defective.[10] Previous studies have shown that the different ApoE genotype results in an individual carrier's differential susceptibility to future coronary artery disease events and that ApoE4 is associated with increased coronary artery disease events compared to ApoE2 and ApoE3.[8,11]
Serum lipid levels are closely correlated with dietary habits,[12] which in Shandong are quite different from other areas of China. Shandong cuisine is famous for its high salt and soy sauce content, and sodium intakes were high among residents of Shandong province.[13] Shandong province is located on the northeast of China and there are more than 95,000,000 people in Shandong province which is nearly 1/14 of China according to the 6th national census of China in 2010.[14] There are 56 ethnic groups in China and Han is the largest group. Among this 95 million people, a very large group is Han nationality accounts for 99.32% of the proportion and the frequency of ApoE polymorphism differs in different ethnic groups.[2,14,15] But only a few of the study about ApoE polymorphism and ApoE concentration has been performed in Shandong Han population. Therefore, evaluating the effect of ApoE polymorphism on serum lipid levels in Shandong province is a complementary mechanism study of ApoE.
In this study, the distribution of different genotypes of Shandong Han people was detected. It has been reported that serum ApoE concentration significantly modulates lipoprotein levels in an isoform-independent manner through its effects on clearance rate, lipolytic conversion, and VLDL production.[16] The association between ApoE polymorphism and serum lipid parameters may be confounded or masked by ApoE concentration. To evaluate the association of serum lipids with ApoE alleles, the serum levels of total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), and ApoA1/ApoB (A1/B) in a Shandong Han population were evaluated. The influences of sex, age, and body mass index (BMI) on lipid metabolism were also taken into account in this study to determine the association of ApoE polymorphism and lipid profiles, which may be partly attributed to genetic variations in ApoE.
2. Materials and methods
2.1. Sample and data collection
A total of 815 Han population from the Jinan Central Hospital Health examination center was randomly selected from Jinan, Shandong province. Subjects were general population, ranged in age from 20 to 60 years, BMI from 18.5 to 29.9, and did not recently take drugs that may affect blood lipid levels. There were 285 men and 530 women. The Ethics Committee of Jinan Central Hospital Affiliated to Shandong University approved the study, and samples were taken after obtaining informed consent from all subjects.
2.2. Laboratory analysis
Blood samples were collected and serum was separated within 4 hours and stored to measure serum lipid parameters. Subsequent analysis of serum TC, TG, LDL-C, HDL-C, ApoA1, and ApoB was performed using the Roche Cobas 8000 modular analyzer series (Basel, Switzerland) using a standard method. All analyses were performed in duplicate, and the examiners were blinded to the clinical and laboratory results.
2.3. ApoE genotyping
Blood used for genotyping was drawn into EDTA-containing tubes. Genomic DNA was prepared from peripheral blood leukocytes according to the standard procedure of the Magen HiPure Blood DNA Mini Kit (Guangzhou xinyan, Bioscience Co., Ltd., Guangdong, China) and stored at −20 °C. The genotypes of ApoE were detected using an ApoE Genotyping Detection Kit (gene chip assay) (ZhuHai Sinochips Bioscience Co., Ltd.) according to the manufacturer's instructions.
2.4. Statistical analysis
The results were reported as the means ± standard deviations or percentages. The allelic frequencies and genotype distributions were calculated using the gene-counting method. The χ2 test or Fisher exact test was used to evaluate the allelic and genotypic frequencies of ApoE. One-way analysis of variance and Student–Newman–Keuls post test were performed to determine the differences in lipid parameters among different genotypes and alleles. Differences were considered significant when P < 0.05. Statistical analysis was performed using SPSS version 22.0 (SPSS, Inc., Chicago, IL).
3. Results
3.1. Characteristics of subjects with different ApoE genotypes
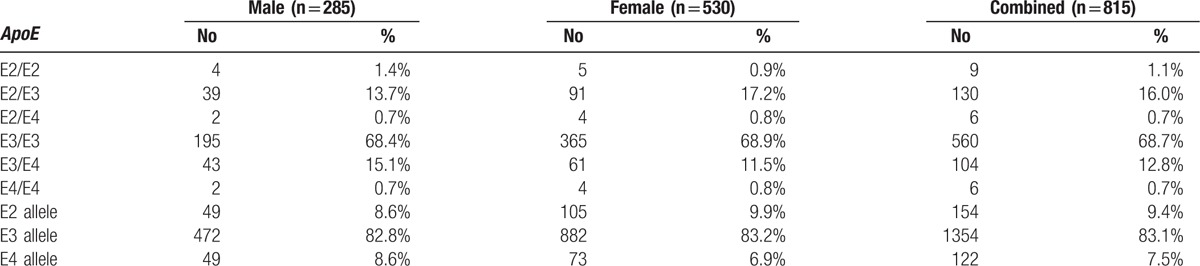
Here, 815 normal Han subjects were selected for the current study. The general characteristics and serum lipids of the Shandong Han population in this study are shown in Table 1. The allele and genotype frequencies are shown in Table 2. The frequency of the ApoE E3 allele was greatest (83.1%), while those of E2 and E4 were 9.4% and 7.5%, respectively, which is comparable to the values found in other studies performed in Asian populations.
Table 1.
General characteristics and serum lipids of Shandong Han population.

Table 2.
Allele frequencies and prevalence of Apolipoprotein E (ApoE) genotypes in Shandong Han population.
3.2. Serum ApoE concentrations with different ApoE genotypes
To evaluate the association of serum lipids with ApoE alleles, the concentration of serum ApoE in our Shandong Han population was determined. We divided all subjects into 3 subgroups: E2 group (E2/E2 and E2/E3), E3 group (E2/E4 and E3/E3), and E4 group (E3/E4 and E4/E4). Table 3 shows that ApoE polymorphism influenced serum ApoE levels in a gene-dependent manner. The same pattern was observed in both the male and female groups. The concentration of serum ApoE in the E2 group was higher than those in the E3 and E4 groups (Fig. 1A).
Table 3.
Concentration of serum ApoE in Shandong Han population (mg/L).
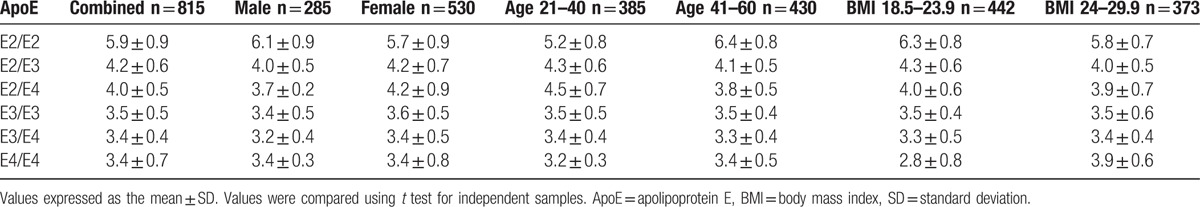
Figure 1.
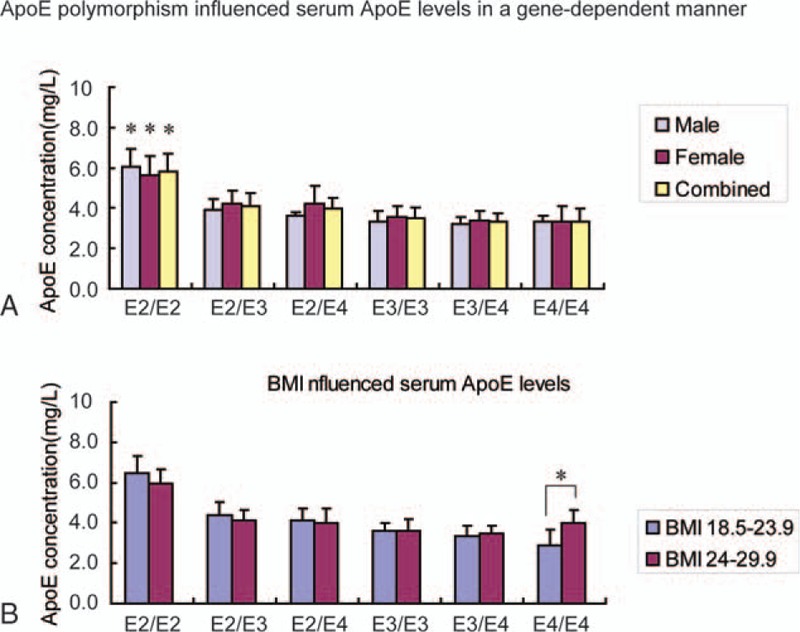
The influence of ApoE alleles on serum ApoE concentrations in Shandong Han population. (A) ApoE polymorphism influenced serum ApoE levels in a gene-dependent manner. (B) The concentration of serum ApoE was influenced by BMI. ApoE = apolipoprotein E, BMI = body mass index.
The highest concentration of serum ApoE was observed for the E2/E2 allele compared to E2/E3, E2/E4, E3/E3, E3/E4, and E4/E4 in both the male and female groups. ApoE concentration in the lower BMI group was slightly higher than that in the higher BMI group with the E2/E2 allele, while ApoE concentration in the lower BMI group was lower than that in the higher BMI group with the E4/E4 allele (Fig. 1B).
3.3. Influence of serum ApoE concentration and polymorphisms on serum lipids parameters
Table 4 shows that the concentration of different serum components was not always the same for variant alleles in both the male and female groups. We found that when ApoE was higher, TC and LDL-C concentrations for the E2/E2 allele were lower than in other groups (Fig. 2A, B). These results indicate that together with ApoE level, ApoE polymorphism may influence serum TC and LDL-C levels in a gene-dependent manner. For serum TG, the concentration in men was higher than that in women with the E2/E2, E2/E3, and E3/E3 alleles (Fig. 2D). Serum HDL-C and ApoA1 levels in women were higher than that in men except for E2/E4 allele (Fig. 2E, F).
Table 4.
Influences of ApoE genotypes and alleles on serum lipid concentrations in Shandong Han population.
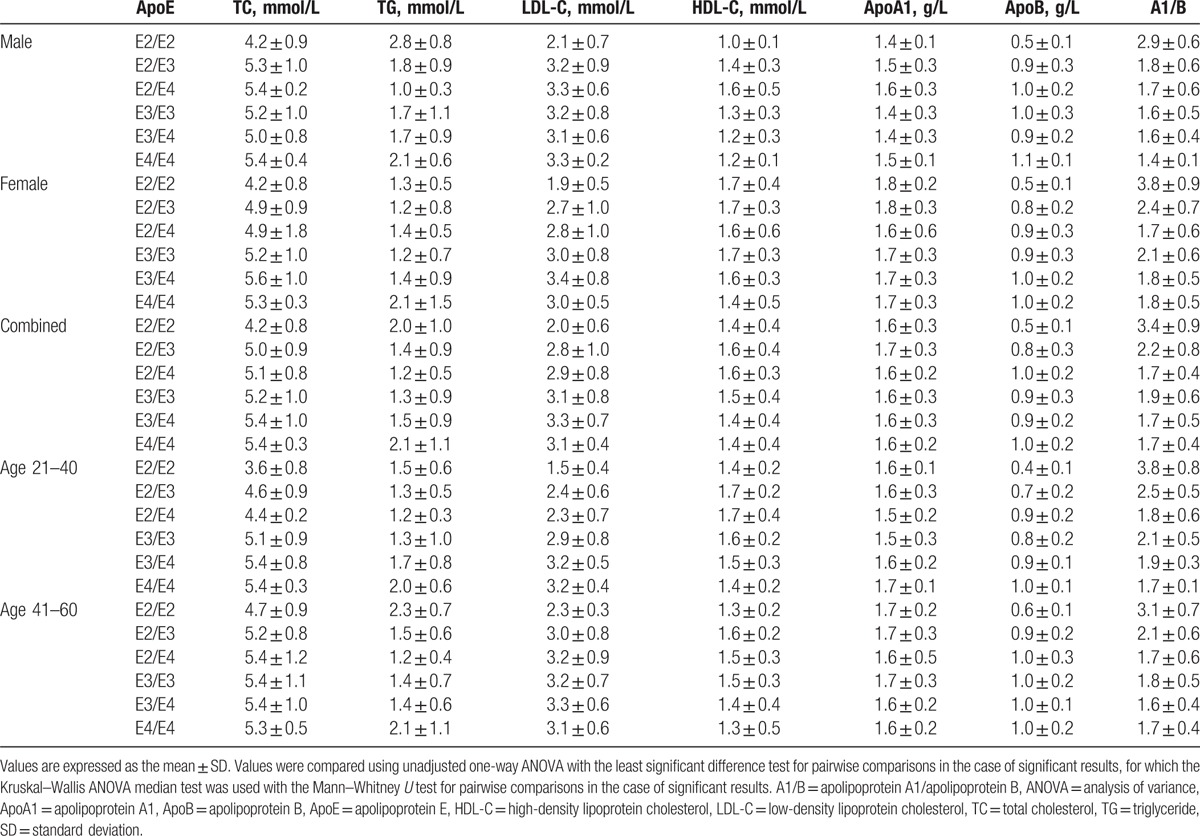
Figure 2.
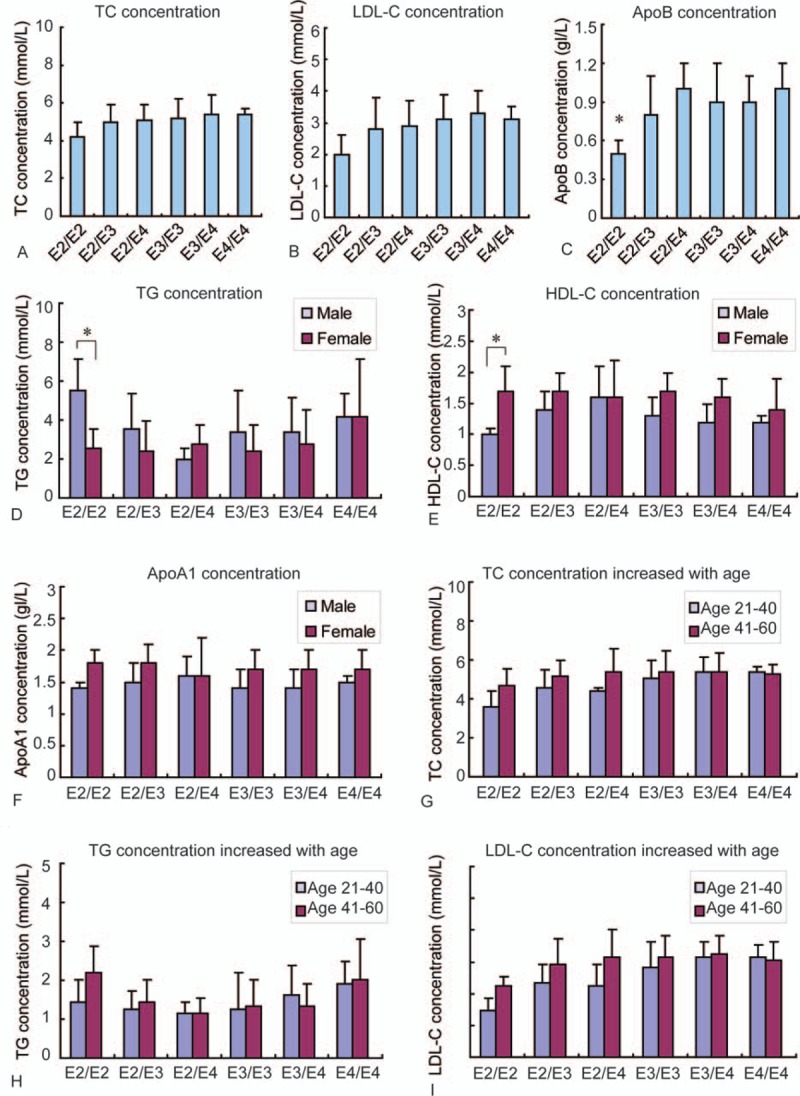
ApoE concentration and polymorphism influence serum lipid levels in Shandong Han population. (A) The concentration of serum TC in the 6 different ApoE alleles. (B) The concentration of serum LDL-C in the 6 different ApoE alleles. (C) The concentration of serum ApoB in the 6 different ApoE alleles. (D) The concentration of serum TG in the 6 different ApoE alleles in both male and female. (E) The concentration of serum HDL-C in the 6 different ApoE alleles in both male and female. (F) The concentration of serum ApoA1 in the 6 different ApoE alleles in both male and female. (G) The changes of serum TC concentration with different age in the 6 different ApoE alleles. (H) The changes of serum TG concentration with different age in the 6 different ApoE alleles. (I) The changes of serum LDL-C concentration with different age in the 6 different ApoE alleles. (∗P < 0.05). ApoA1 = apolipoprotein A1, ApoB = apolipoprotein B, ApoE = apolipoprotein E, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, TC = total cholesterol, TG = triglyceride.
ApoE level and ApoE polymorphism influenced not only the levels of serum lipids described above, but also the concentrations of ApoB. We found that the concentration of ApoB for subjects with the E2/E2 allele was the lowest and there was a significant difference between those with the E2/E2 allele and those with the other four alleles, including E2/E4, E3/E3, E3/E4, and E4/E4 (P < 0.05) (Fig. 2C); this pattern was observed in both men and women. The value of ApoA1/ApoB in the E2/E2 allele was the highest and was similar to the concentration of serum ApoE.
We also found that the concentrations of TC, TG, and LDL-C in the E2 group increased with increasing age, particularly for the E2/E2 allele, but this difference was not observed in the E4 group (Table 4). TC and LDL-C concentrations also increased with increasing age in the E3 group (Table 4). These results indicate that age influences serum TC, TG, and LDL-C levels in a gene-dependent manner.
4. Discussion
ApoE is a plasma glycoprotein associated with the transport of cholesterol and other lipids involved in growth and maintenance during development.[17] As the largest group, the Han ethnic group accounts for approximately 99.3% of the population in Shandong province. In this study, the distribution of different ApoE genotypes in a Shandong Han population was studied. The frequency of the ApoE E3 allele was the highest (83.1%), while those of E2 and E4 were 9.4% and 7.5%, respectively, which are comparable to values found in other studies of Asian populations.[18]
Our results showed that ApoE polymorphism significantly influenced serum ApoE levels in a gene-dependent manner in both male and female groups. The highest concentration of serum ApoE was found in the E2 group, while the lowest was found in the E4 group; these results agree with those previously reported in other populations and are consistent with those of a previous study by Liberopoulos.[14]
ApoE binds to multiple types of lipids and it has been implicated in cholesterol and TG homeostasis. An important part of our study was determining the influence of ApoE genotypes and alleles on serum lipid concentrations in a Shandong Han population. Previous studies have indicated that ApoE level is important for determining serum lipid levels. Although no significant differences in serum lipid concentrations were observed for the various genotypes, some general trends were observed. Individuals with the E2 allele showed lower levels of serum cholesterol, whereas individuals with the E4 allele showed higher levels compared to individuals who are homozygous for the E3 allele.[8,19]
Our results that ApoE2 carriers have lower levels of serum TC, LDL-C, and APO-B than ApoE3 carriers and greater levels in ApoE4 carriers in contrast to ApoE levels in our Shandong Han population were consistent with a previous study performed in Beijing and Shanghai.[18] Women showed a higher ApoA1 level than men with the E2/E2, E2/E3, E3/E3, E3/E4, and E4/E4 alleles except for the E2/E4 allele. We also found that some serum lipid levels were influenced by age in the E2 group, which is consist with a previous study.[20,21] The concentrations of TC, TG, and LDL-C in the E2 group increased with increasing age. Unlike in the E2 and E3 groups, age was not associated with serum lipid levels in the E4 group in our study.
In this study, the combined influence of ApoE polymorphism, age, and BMI on serum lipids was evaluated. The associations between ApoE polymorphism, ApoE level, age, BMI, and serum lipid profiles may be partially attributed to genetic variations in ApoE.
Footnotes
Abbreviations: ApoA1 = apolipoprotein A1, ApoB = apolipoprotein B, ApoE = apolipoprotein E, Arg = arginine, BMI = body mass index, Cys = cysteine, HDL = high-density lipoprotein, HDL-C = high-density lipoprotein cholesterol, IDL = intermediate-density lipoprotein, LDL = low-density lipoprotein, LDL-C = low-density lipoprotein cholesterol, TC = total cholesterol, TG = triglyceride, VLDL = very low-density lipoprotein.
SYH and YHX contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Scanu AM. The role of lipoprotein(a) in the pathogenesis of atherosclerotic cardiovascular disease and its utility as predictor of coronary heart disease events. Curr Cardiol Rep 2001;3:385–90. [DOI] [PubMed] [Google Scholar]
- [2].Afroze D, Yousuf A, Tramboo NA, et al. ApoE gene polymorphism and its relationship with coronary artery disease in ethnic Kashmiri population. Clin Exp Med 2015;1–6. [DOI] [PubMed] [Google Scholar]
- [3].El-Lebedy D, Raslan HM, Mohammed AM. Apolipoprotein E gene polymorphism and risk of type 2 diabetes and cardiovascular disease. Cardiovasc Diabetol 2015;15:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mahley RW, Innerarity TL. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta 1983;737:197–222. [DOI] [PubMed] [Google Scholar]
- [5].Dolphin PJ. Lipoprotein metabolism and the role of apolipoproteins as metabolic programmers. Can J Biochem Cell Biol 1985;63:850–69. [DOI] [PubMed] [Google Scholar]
- [6].Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988;240:622–30. [DOI] [PubMed] [Google Scholar]
- [7].Greenow K, Pearce NJ, Ramji DP. The key role of apolipoprotein E in atherosclerosis. J Mol Med 2005;83:329–42. [DOI] [PubMed] [Google Scholar]
- [8].Clark D, Skrobot OA, Adebiyi I, et al. Apolipoprotein-E gene variants associated with cardiovascular risk factors in antipsychotic recipients. Eur Psychiatry 2009;24:456–63. [DOI] [PubMed] [Google Scholar]
- [9].Suarez BK, Schonfeld G. Characterization of apolipoprotein E (ApoE) apoprotein levels in the various ApoE phenotypes. J Clin Endocrinol Metab 1981;53:435–8. [DOI] [PubMed] [Google Scholar]
- [10].Mamotte CD, Sturm M, Foo JI, et al. Comparison of the LDL-receptor binding of VLDL and LDL from apoE4 and apoE3 homozygotes. Am J Physiol 1999;276:553–7. [DOI] [PubMed] [Google Scholar]
- [11].Karahan Z, Uğurlu M, Uçaman B, et al. Relation between Apolipoprotein E gene polymorphism and severity of coronary artery disease in acute myocardial infarction. Cardiol Res Pract 2015;2015: 363458. doi: 10.1155/2015/363458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yan HQ, Yuan Y, Zhang P, et al. Association of the ApoE gene polymorphism and dietary factors with cerebral infarction and circulating lipid concentrations. Genet Mol Res 2015;14:665–70. [DOI] [PubMed] [Google Scholar]
- [13].Lu Z, Zhang X, Li J, et al. [Dietary sodium intakes and resources among residents in Shandong province]. Zhonghua Yu Fang Yi Xue Za Zhi 2014;48:7–11. [PubMed] [Google Scholar]
- [14].Sumita M. Communiqué of the National Bureau of Statistics of People's Republic of China on Major Figures of the 2010 Population Census(No.2). China Popul Today 2011;6:24–5. [Google Scholar]
- [15].Hu P, Qin YH, Lei FY, et al. Variable frequencies of apolipoprotein E genotypes and its effect on serum lipids in the Guangxi Zhuang and Han children. Int J Mol Sci 2010;12:5604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Villeneuve S, Brisson D, Gaudet D. Influence of abdominal obesity on the lipid-lipoprotein profile in apoprotein E2/4 carriers: the effect of an apparent duality. J Lipids 2015;48:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, et al. Data on plasma levels of apolipoprotein E, correlations with lipids and lipoproteins stratified byAPOEgenotype, and risk of ischemic heart disease. Data Brief 2016;6:923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xia Y, Sass C, Shen X, et al. Associations of apolipoprotein E concentration and polymorphism with lipids and apolipoprotein levels in Chinese from Beijing and Shanghai. Clin Chem Lab Med 2000;38:655–9. [DOI] [PubMed] [Google Scholar]
- [19].Mendes-Lana A, Pena GG, Freitas SN, et al. Apolipoprotein E polymorphism in Brazilian dyslipidemic individuals: Ouro Preto study. Braz J Med Biol Res 2007;40:49–56. [DOI] [PubMed] [Google Scholar]
- [20].Ward H, Mitrou PN, Bowman R, et al. APOE genotype, lipids, and coronary heart disease risk: a prospective population study. Arch Intern Med 2009;169:1424–9. [DOI] [PubMed] [Google Scholar]
- [21].Li L, Hua J, Jianping H, et al. Association between the lipid levels and single nucleotide polymorphisms of ABCA1, APOE and HMGCR genes in subjects with spontaneous preterm delivery. Plos One 2015;10: e0135785. doi: 10.1371/journal.pone.0135785. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


