Abstract
Persistent exacerbation of a peptic ulcer may lead to a complicated peptic ulcer (perforation or/and bleeding). The management of complicated peptic ulcers has shifted from acid-reducing vagotomy, drainage, and gastrectomy to simple local suture or non-operative (endoscopic/angiographic) hemostasis. We were interested in the long-term effects of this trend change. In this study, complicated peptic ulcer patients who received acid-reducing vagotomy were compared with those who received simple suture/hemostasis to determine the risk of ischemic heart disease (IHD).
This retrospective cohort study analyzed 335,680 peptic ulcer patients recorded from 2000 to 2006 versus 335,680 age-, sex-, comorbidity-, and index-year matched comparisons. Patients with Helicobacter pylori (HP) infection were excluded. In order to identify the effect of vagus nerve severance, patients who received gastrectomy or antrectomy were also excluded. The incidence of IHD in both cohorts, and in the complicated peptic ulcer patients who received acid-reducing vagotomy versus those who received simple suture or hemostasis was evaluated.
The overall incidence of IHD was higher in patients with peptic ulcer than those without peptic ulcer (17.00 vs 12.06 per 1000 person-years), with an adjusted hazard ratio (aHR) of 1.46 based on multivariable Cox proportional hazards regression analysis controlling for age, sex, Charlson's comorbidity index, and death (competing risk). While comparing peptic ulcer patients with acid-reducing vagotomy to those with simple suture/hemostasis or those without surgical treatment, the aHR (0.58) was the lowest in the acid-reducing vagotomy group.
Patients with peptic ulcer have an elevated risk of IHD. However, complicated peptic ulcer patients who received acid-reducing vagotomy were associated with reduced risk of developing IHD.
Keywords: ischemic heart disease, peptic ulcer, truncal vagotomy
1. Introduction
The incidence of peptic ulcers has decreased over the past decades due to more understanding of the etiology and improvement in the pharmacological treatment.[1] There were about 500,000 cases of newly developed peptic ulcer disease in the United States each year, and the direct and indirect costs attributed to the disease are estimated at about $10 billion dollars annually.[2,3]
The major etiologies of peptic ulcer may include Helicobacter pylori (HP) infection[4] and increased acid production. Other risk factors include cigarette smoking,[5] alcohol consumption,[6] and the use of nonsteroidal anti-inflammatory drugs, which is frequently used for cardiovascular disease or arthropathy in the United States.[7]
Studies showed that HP infections were diagnosed in 90% to 100% of uncomplicated duodenal ulcer patients and in 60% to 100% of uncomplicated gastric ulcer patients.[8] Eradication of HP is important in peptic ulcer management.[9] The advances in acid-inhibiting pharmacological agent (especially proton pump inhibitors, PPIs) have improved management outcome of peptic ulcer.[9,10]
Persistent exacerbation of a peptic ulcer may lead to a complicated peptic ulcer (perforation or/and bleeding), which is an emergency that needs surgery or immediate management. However, the reported HP infection rate in patients with complicated peptic ulcer is about 65% to 70% in perforated peptic ulcer,[11] and 57% to 73% in bleeding peptic ulcer.[12–14] The prevalence might raise consideration of the role of HP infection in complicated peptic ulcer patients.[15]
The traditional management for complicated peptic ulcers was acid-reducing vagotomy with/without drainage, such as truncal vagotomy with pyloroplasty (TVP) or highly selective vagotomy (HSV), as well as gastrectomy or antrectomy to remove acid-producing cells.[16] However, management of complicated peptic ulcer has now shifted to a more simple surgical procedure or various non-operative (endoscopic/angiographic) hemostasis procedures, followed by postoperative or postprocedural PPIs treatment and HP eradication.[10,17,18] Therefore, the role of acid-reducing vagotomy and acid-producing cells removal has become less important in the current management for complicated peptic ulcer.[19]
On the other hand, ischemic heart disease (IHD) is one of the major causes of death and disability in the developed countries and remains responsible for about one-third or more of all deaths in individuals over age 35.[20,21] Cardiovascular diseases are expected to become the main cause of death globally due to increasing prevalence in Eastern Europe and developing countries,[22] which gives emphasis to the need for a broad understanding of the risk factors for IHD.
We were interested in potential associations between surgical procedures and development of IHD in this changing trend for management of complicated peptic ulcers. Studies have shown that HP infection in peptic ulcer patients was associated with development of IHD.[23,24] Therefore, in this retrospective population study, excluding ulcer patients with HP infection, we assessed the risk of IHD in complicated peptic ulcer patients who underwent simple suture/hemostasis (SSH) procedure versus those who received acid-reducing vagotomy.
2. Method
2.1. Data source
We obtained an inpatient file, a part of the National Health Insurance Research Databases (NHIRD), from Taiwan National Health Research Institutes (TNHRI). Taiwan Bureau of National Health Insurance (TBNHI) established a National Health Insurance program in 1995. This program has a coverage ratio over 99% of the population in Taiwan. TNHRI was commissioned to maintain NHIRD for researches conducted by TBNHI. The inpatient database contained all inpatient claims from the start of 1996 to the end of 2011. To avoid a violation of the Personal Information Protection Act, the identification was re-coded before being sent to researchers. Disease was defined based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). We performed this study with the approval of the Ethics Review Committee at Chinese Medical University and Hospital. Though written informed consent was not provided by the participants for the use of their clinical records in this study, all patient information was anonymized and de-identified prior to the analysis.
The diagnoses of disease in NHIRD were judged and determined by a related specialists and physicians committee. Therefore, the data regarding IHD, truncal vagotomy and pyloroplasty, highly selective vagotomy, simple suture/hemostasis procedure, and peptic ulcer diagnoses were highly reliable.
2.2. Study subject
We collected 535,969 patients who were admitted with newly diagnosed peptic ulcers from 2000 to 2006 (ICD-9-CM 531–534). The date of peptic ulcer diagnosis was defined as the index date. Complicated peptic ulcer was defined as peptic ulcer induced perforation and/or bleeding. Patients who received ulcer-associated procedure before the index date were excluded from this study. In order to assess the effect of vagus nerve severance, we excluded patients that received gastrectomy or antrectomy. Those patients who underwent selective vagotomy were also excluded due to rarity.
Peptic ulcer patients with previous malignancy (ICD-9-CM 140–208), IHD (ICD-9-CM 410–414), age <20 y/o, or follow-up duration <1 year were all excluded. To minimize the effect of HP infection, we also excluded ulcer patients with HP infection (ICD-9-CM 041.86) during admission. Based on the management of ulcers and complicated peptic ulcers, we grouped the patients into 4 subgroups by the management received: 1. with TVP; 2. with SSH; 3. with HSV, and 4. without documented surgery or SSH procedures.
Furthermore, we selected 1 control group of people without peptic ulcers, ulcer-associated procedure, malignancy, and IHD history. This control group was matched with peptic ulcer patients according to age, sex, comorbidity including Charlson Comorbidity Index (CCI) score,[25] hyperlipidemia, diabetes and hypertension, and index-year at a ratio 1:1. The details of selecting subjects were presented in Fig. 1.
Figure 1.
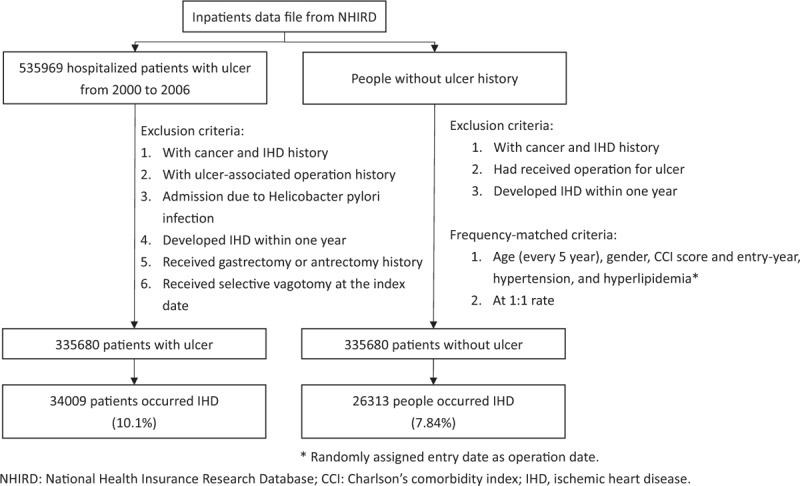
Flow chart for study subjects.
2.3. Surgery for ulcer, comorbidity, and outcome
Surgical treatments for complicated peptic ulcers included TVP (ICD-9 operation code 44.01 and 44.2), SSH (ICD-9 operation code 44.4), gastrectomy (ICD-9 operation code 43.5–43.9), and HSV (ICD-9 operation code and 44.02) in our study. Because this study assessed the association between peptic ulcer and IHD, we excluded peptic ulcer, malignancy, and myocardial infarction from Charlson Comorbidity Index. All study subjects with IHD were followed up from the index date until the date of IHD admission (ICD-9-CM 410–414). Subjects without IHD were followed up until the date they withdrew from the program or the end of 2011, whichever came first.
2.4. Statistical analysis
All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA) and the significant level was set at P < 0.05. The incidence of IHD (per 1000 person-years) was calculated in both cohorts. Cox proportional hazard regression was used to assess the risk of IHD in all subjects in crude and adjusted model, adjusted for age, sex, and comorbidity. We also evaluated the association between ulcer and IHD, stratified by sex, age group (20–44, 45–64, and 65+ years-old), CCI score (0, 1, 2, and 3+), hyperlipidemia, and hypertension. The interaction test between sex and ulcer, age and ulcer, CCI score and ulcer, hyperlipidemia, and hypertension and ulcer were assessed using crude Cox proportional hazard regression. The association between IHD and ulcer-associated procedure was also assessed. Kaplan–Meier analysis was used to plot the cumulative incidence of IHD and log-rank test was used to test the differences among ulcer-associated treatments.
3. Result
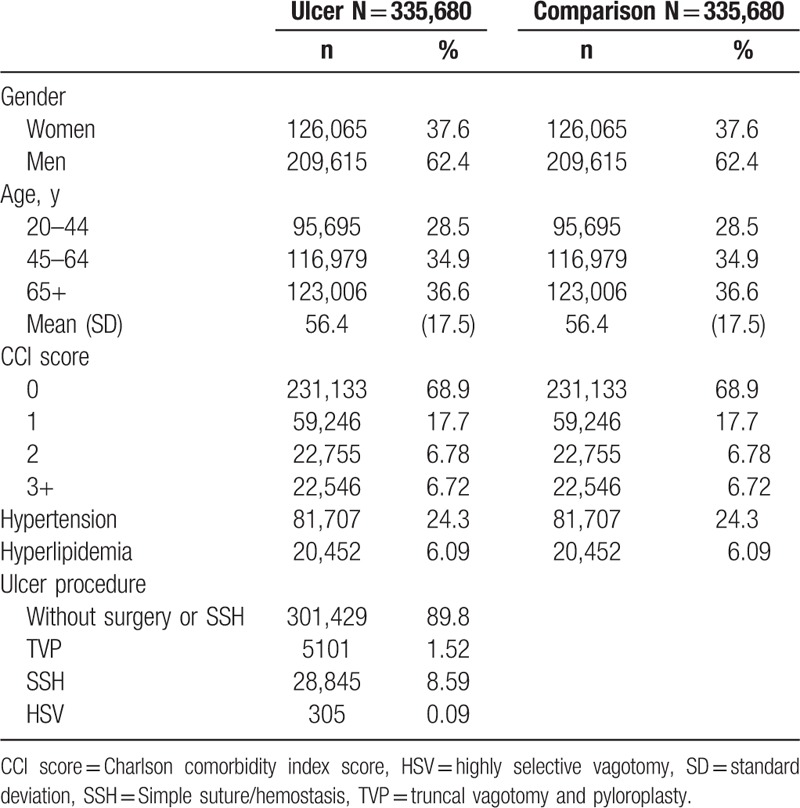
There were selected 335,680 peptic ulcer patients and 335,680 matched comparisons in this retrospective cohort study. In the peptic ulcer patients cohort, there were more men than women (62.4% vs 37.6%), and the mean age was 56.4 y/o (standard deviation = 17.5) (Table 1). There were 13.5% ulcer patients with higher CCI score (≥2), 24.3% with hypertension, and 6.09% with hyperlipidemia. The majority of peptic ulcer patients did not receive ulcer-associated surgical procedures (89.8%); whereas there were 8.59% patients received SSH, 1.52% patients received TVP, and 0.09% patients received HSV.
Table 1.
Demographics in study subjects.
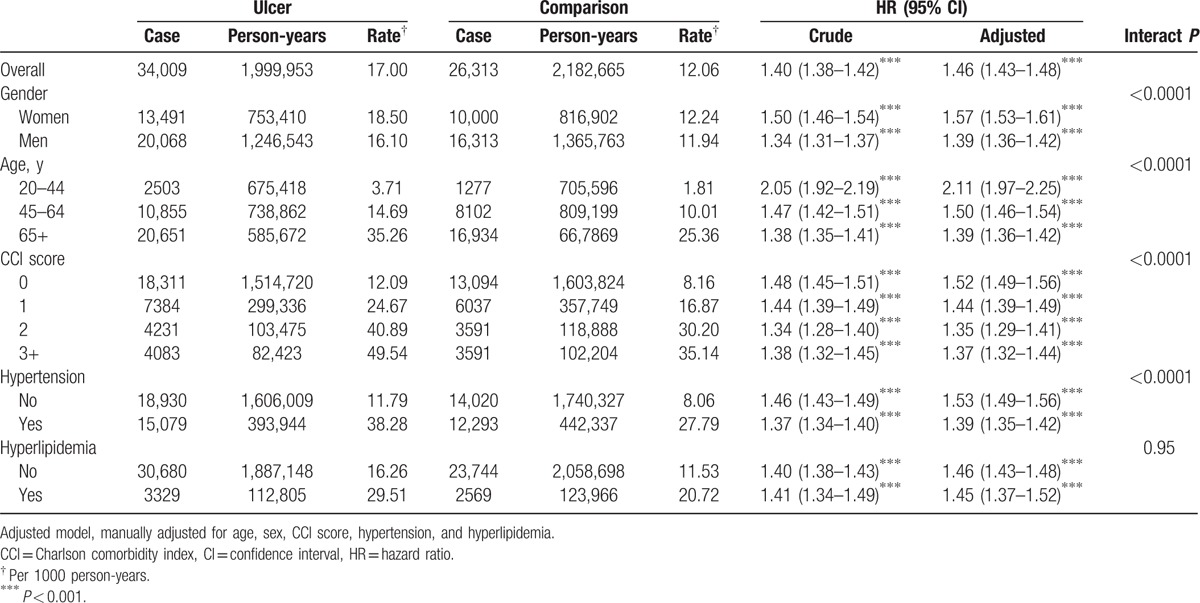
During a mean 6.2-years follow-up, the incidence of IHD were 17.00 and 12.06 per 1000 person-years in ulcer and comparison cohort, respectively (Table 2). The IHD risk in peptic ulcer patients compared with comparisons was 1.40 and 1.46 in crude and multivariable model (95% CI = 1.38–1.42 and 1.43–1.48). In multivariable model, the IHD risk in woman was higher than in man (hazard ratio [HR] = 1.57 vs 1.39, 95% CI = 1.53–1.61 vs 1.36–1.42, Table 2). Age-specific risk decreased with aging from 2.11, 1.50 to 1.39 at age 20 to 44, 45 to 64, and 65+ years-old (95% CI = 1.97–2.25, 1.46–1.54, and 1.36–1.42). The CCI-specific risk at score 0 was the highest (HR = 1.52, 95% CI = 1.49–1.56). When stratified with hypertension, the risk of IHD in patients without hypertension was higher than those with hypertension (HR = 1.53 vs 1.39, 95% CI = 1.49–1.56 vs 1.35–1.42, Table 2). No matter patients were with or without hyperlipidemia, there was similar higher IHD effect in peptic ulcer patients compared with comparisons.
Table 2.
Risk for ischemic heart disease in ulcer patients compared to comparisons.
The association between IHD and different types of ulcer surgery was presented in Table 3. When compared with comparisons, either peptic ulcer patients without surgical procedure/SSH or complicated peptic ulcer patients with SSH showed higher IHD risk (HR = 1.48 and 1.30, 95% CI = 1.46–1.51 and 1.25–1.35), while patient with TVP had a lower IHD risk (HR = 0.76, 95% CI = 0.66–0.87).
Table 3.
Risk for ischemic heart disease in comparison cohort and ulcer patients categorized by treatment method in multivariable Cox proportional hazard regression after adjusted for age, sex, CCI score, hypertension, and hyperlipidemia.
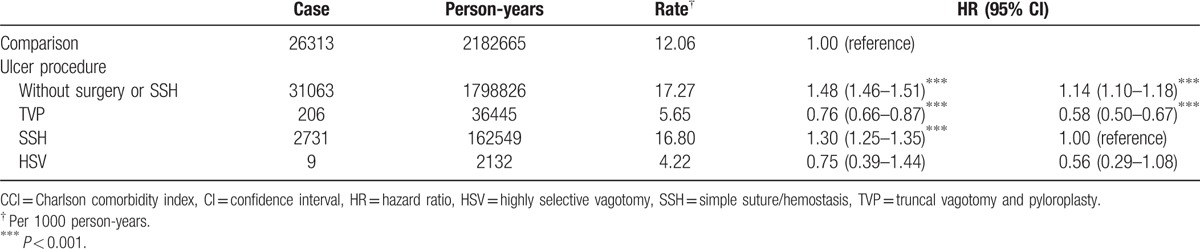
When compared with complicated peptic ulcer patients that received SSH or acid reducing vagotomy, patients underwent TVP had a lower IHD risk (HR = 0.58, 95% CI = 0.50–0.67, Table 3).
The cumulative incidence of IHD, after a cumulative 11 years of follow up, was highest in peptic ulcer patients without surgery or SSH (15.82%), followed by complicated peptic ulcer patients with SSH (15.48%), the comparison cohort (11.80%), patients who underwent TVP treatment (6.14%), and patients who underwent HSV (3.74%) (Fig. 2).
Figure 2.
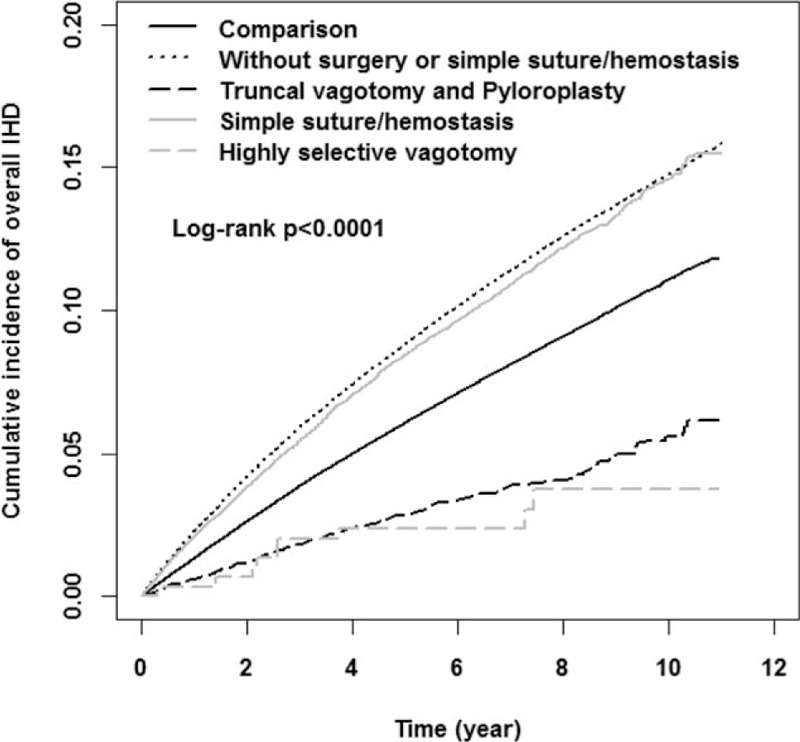
Cumulative incidence for ischemic heart disease among comparison cohort and different treatment ulcer cohorts in Kaplan–Meier analysis.
4. Discussion
After excluding patients with HP infection, we found that patients with peptic ulcer were associated with an overall higher risk of IHD when compared with comparisons (HR = 1.46, Table 2). The results were similar when stratified by sex, age, CCI score, hyperlipidemia, and hypertension. Our results showed association between peptic ulcers and the risk of developing IHD, indicating that there were not only local effects but also systemic impacts in patients with peptic ulcer disease.
The treatment of peptic ulcer disease has drastically changed over the past 50 years. The progress from surgical to medical management demonstrates the diminished role for surgery. Currently, the management of complicated peptic ulcers (perforation or/and bleeding) may include initial laparoscopic or open simple suture closure in patients with perforation or hemorrhage, as well as nonoperative (endoscopic/angiographic) hemostasis procedures for bleeding peptic ulcers.[11] In addition, the use of PPIs was efficacious in the early postoperative period for facilitating ulcer healing.[26] This has led to the diminished role of acid-reducing vagotomy in the management of complicated peptic ulcers.
In our series, comparing the risk for the development of ischemic heart disease in ulcer patients to the control group, the age-specific risk decreased with aging from 2.11, 1.50 to 1.39 at age 20 to 44, 45 to 64, and 65+ years-old (Table 2). This result showed that young peptic ulcer patients had a higher risk of developing ischemic heart disease than older patients, indicating that there might be more stress and higher level of systemic inflammation in young peptic ulcer patients.
Furthermore, we found that peptic ulcer patients without surgical procedure or SSH had a higher incidence of IHD (HR = 1.48, Table 3) than comparison group, while patients underwent SSH also had a higher incidence of IHD (HR = 1.30, Table 3). In comparing surgery/procedure type, patients who underwent TVP had a lower incidence of IHD than in SSH group (HR = 0.58, Table 3). In addition, there was reduced risk of IHD in patients who received HSV (HR = 0.56, Table 3). Our results show that acid-reducing vagotomy may have a protective effect against long-term IHD development. Since there was remaining integral sub-diaphragm vagus nerve in patients underwent SSH or those without surgical procedure, it seems that there might be a beneficial role for vagus nerve severance (i.e., truncal vagotomy) in the management of complicated peptic ulcer patients.
Studies showed that vagal hyperactivity was involved in the development of gastric stress ulceration,[27] and increased vagal parasympathetic tone is related to peptic ulcer diseases in humans.[28,29] Application of electrical stimulation to the vagus nerve had been reported to cause excessive secretion of acid and development of gastric ulcers,[27,30] indicating a potential association between increased vagal tone and peptic ulcer. Moreover, increased vagal tone was reported to account for the observed immune paralysis in traumatic brain injury patients.[31] Therefore, complicated peptic ulcer patients could be a result of persistent vagal hyperactivity and systemic inflammation.[32]
There are balances between systemic inflammatory and anti-inflammatory activities that control the progression of atherosclerosis.[33] Atherosclerosis is an important factor in the progress of cardiovascular diseases,[33] ischemic stroke,[34] and cancer.[35] Study showed that there were linkages between infections, atherosclerosis, and IHD, which could be due either to direct effect in plaques or to distant signaling by inflammatory mediators.[36] However, persistent systemic inflammation was associated with adverse outcomes. Previous studies had shown that persistent chronic inflammatory processes played a role in causing endothelial dysfunction and accelerating atherosclerosis that increased the risk of ischemic stroke,[34] and the mechanism is similar in other chronic diseases (e.g., rheumatoid arthritis,[37] psoriasis,[38] and periodontitis[39]).
The mechanism may include immune cells- (macrophages, mast cells, T-cells, etc.) directed early atherosclerotic lesions. Activation of T cells may produce Th1 cytokines (e.g., interferon-gamma), which activate macrophages and vascular cells, leading to inflammation, while endogenous and microbial molecules may activate macrophages through toll-like receptors and lead to release of inflammatory cytokines, oxygen and nitrogen radicals, chemokines, and other inflammatory molecules, resulted in inflammation and tissue damage.[40,41] The effector molecules then accelerated progression of the lesions, enhanced by the effects of cytokines, including activation of inflammation, and thus resulted in acute coronary syndromes.[33] In addition, the use of statins showed reduced inflammation and subsequent benefit in coronary artery disease patients,[42–44] which highlighted the importance between systemic inflammation and development of IHD.
The decreased risk of IHD in TVP patients in our series shows that there is possible close association between the vagus nerve and development of IHD. This might be attributed to the role of the vagus nerve in the inflammation mechanism and immune system. It was reported that the vagus nerve was associated with metabolic homeostasis, and there was close connection between immune and nervous systems which interact to regulate inflammation.[45,46] In animal studies, Pavlov and Tracey[47] reported that efferent vagus nerve-mediated cholinergic signaling regulates immune function and proinflammatory responses via the inflammatory reflex, while vagotomy significantly exacerbates tumor necrosis factor responses to inflammatory stimuli, which is known as cholinergic anti-inflammatory pathway.[48,49] However, in contrast, another animal study showed that subdiaphragmatic vagotomy induces inhibition of NF-kappa B activation and attenuated inflammatory process by increasing protective mediators and anti-inflammatory cytokines in animal model.[50]
In a long-term human case-control study to evaluate the risk of rheumatoid arthritis after surgical vagotomy, Carlens et al[51] found that vagotomy has no specific effect on the risk of developing rheumatoid arthritis in humans, and the notable effects of vagotomy on acute inflammation in rodents are not mirrored in rheumatoid arthritis in humans. They proposed that the difference might be attributable to observation time scale for immune-regulatory mechanisms,[51] that is, minutes or hours in an animal study versus years to induce rheumatoid arthritis in human. In the current study, there is a protective effect for TVP patients in the development of IHD after long-term follow up. Our results are also different from the immune-regulatory mechanism in animal models. Therefore, our results suggest that in addition to timescale, other factors needed to be considered as well, such as the differences between species, and the experimental rodents versus diseased ulcer patients.
A recent study reported that vagotomy and drainage is superior to local over-sewing in bleeding peptic ulcer patients who required emergent surgery,[52] indicating the importance of acid-reducing procedure in managing complicated bleeding peptic ulcers. In the current series, we have found that peptic ulcer patients had an elevated risk of IHD, and complicated peptic ulcer patients who underwent TVP had a lower incidence of developing IHD compared with patients who had SSH.
To the best of our knowledge, there was no similar study focusing on the long-term impact of SSH in complicated peptic ulcer patients. Our results show that there might be a potential beneficial role for vagus nerve severance, which could lead to reappraisal of current surgical treatments in complicated peptic ulcers. However, further studies are warranted for definitive recommendations.
4.1. Limitation of the study
This study has the strengths of including a large study population, longitudinal design, reliable diagnosis, and high follow-up rate. However, certain limitations also exist. First, several risk factors such as complete lifestyle information with respect to drinking, smoking, blood cholesterol levels, obesity, metabolic syndrome, physical activity, diet style, socioeconomic status, and genetic factors were not available for IHD risk adjustment. To reduce this bias, we tried to control for diseases derived from bad lifestyle, such as: smoking-associated diseases; including chronic obstructive pulmonary disease, stroke, and lung cancer; alcohol-associated diseases, including alcoholic cirrhosis, and chronic hepatitis; metabolic syndrome-associated diseases, including hyperlipidemia, and diabetes. We chose CCI comorbidity score because the diseases mentioned above are considered in the CCI score. Second, in spite of the meticulous design of our study to control for confounding factors, bias resulting from the retrospective nature of the study could have influenced our results. Third, there was a lack of information on the status of hyperacidity, which is a potential risk factor of peptic ulcer. Therefore, we might have overestimated the incidence of IHD in all study subjects. However, the results still showed a higher risk of IHD in peptic ulcer patients than normal controls. Fourth, all data used were anonymous. Therefore, relevant clinical variables, such as pathology findings, imaging results, and serum laboratory data were unavailable in our study. Nonetheless, the data regarding IHD, TVP, highly selective vagotomy, simple closure surgery, and the diagnosis of peptic ulcers were highly reliable.
5. Conclusion
In this long-term cohort study, peptic ulcer patients were found to have an elevated risk of IHD. However, complicated peptic ulcer patients who received acid-reducing truncal vagotomy were associated with a reduced risk of subsequent IHD.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, CCI = Charlson's comorbidity index, CI = confidence interval, HP = Helicobacter pylori, HR = hazard ratio, HSV = highly selective vagotomy, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, IHD = ischemic heart disease, NHRID = National Health Research Institutes Database, SSH = simple suture/hemostasis, TBNHI = Taiwan Bureau of National Health Insurance, TNHRI = Taiwan National Health Research Institutes, TVP = truncal vagotomy and pyloroplasty.
S-CW and C-WF have contributed equally to this study.
Author contributions: S-CW and C-WF conceived and designed the study and wrote the initial draft of the manuscript; WT-LC participated in the study design and conception; C-HM performed the data analysis and interpretation and was involved in writing the initial draft of manuscript; S-CW performed data analysis and interpretation, as well as manuscript drafting and revision.
Disclosure: This study has used the National Health Insurance Research Database established by the National Health Research Institutes with the authorization of the Bureau of National Health Insurance, Ministry of Health and Welfare of Taiwan. The interpretations and conclusions contained herein do not represent the opinion of the aforementioned agencies and institutions.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039 -005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan
Guarantor of the article: Shih-Chi Wu, MD.
The authors declare no conflicts of interest related to this study.
References
- [1].Yuan Y, Padol IT, Hunt RH. Peptic ulcer disease today. Nat Clin Pract Gastroenterol Hepatol 2006;3:80e9. [DOI] [PubMed] [Google Scholar]
- [2].Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician 2007;76:1005–12. [PubMed] [Google Scholar]
- [3].University of Michigan Health System. Peptic ulcer disease. Available at: http://www.cme.med.umich.edu/pdf/guideline/PUD05.pdf Accessed May 4, 2007. [Google Scholar]
- [4].Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;323:1311–5. [DOI] [PubMed] [Google Scholar]
- [5].Parasher G, Eastwood GL. Smoking and peptic ulcer in the Helicobacter pylori era. Eur J Gastroenterol Hepatol 2000;12:843–53. [DOI] [PubMed] [Google Scholar]
- [6].Zhang L, Ren JW, Wong CCM, et al. Effects of cigarette smoke and its active components on ulcer formation and healing in the gastrointestinal mucosa. Curr Med Chem 2012;19:63–9. [DOI] [PubMed] [Google Scholar]
- [7].Sostres C, Gargallo CJ, Arroyo MT, et al. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol 2010;24:121–32. [DOI] [PubMed] [Google Scholar]
- [8].Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther 1995;9(Suppl 2):59–69. [PubMed] [Google Scholar]
- [9].Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009;374:1449–61. [DOI] [PubMed] [Google Scholar]
- [10].Vakil N, Fennerty MB. Direct comparative trials of the efficacy of proton pump inhibitors in the management of gastro-oesophageal reflux disease and peptic ulcer disease. Aliment Pharmacol Ther 2003;18:559–68. [DOI] [PubMed] [Google Scholar]
- [11].Gisbert JP1, Pajares JM. Helicobacter pylori infection and perforated peptic ulcer prevalence of the infection and role of antimicrobial treatment. Helicobacter 2003;8:159–67. [DOI] [PubMed] [Google Scholar]
- [12].Lee JM, Breslin NP, Fallon C, et al. Rapid urease tests lack sensitivity in Helicobacter pylori diagnosis when peptic ulcer disease presents with bleeding. Am J Gastroenterol 2000;95:1166–70. [DOI] [PubMed] [Google Scholar]
- [13].Wu CY, Poon SK, Chen GH, et al. Interaction between Helicobacter pylori and non-steroidal anti-inflammatory drugs in peptic ulcer bleeding. Scand J Gastroenterol 1999;34:234–7. [DOI] [PubMed] [Google Scholar]
- [14].Aalykke C, Lauritsen JM, Hallas J, et al. Helicobacter pylori and risk of ulcer bleeding among users of nonsteroidal anti-inflammatory drugs: a case-control study. Gastroenterology 1999;116:1305–9. [DOI] [PubMed] [Google Scholar]
- [15].Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med 1994;331:717–27. [DOI] [PubMed] [Google Scholar]
- [16].Nyhus LM, Baker RJ, Fischer JE. Mastery of Surgery. 3rd ed.Boston:Little, Brown; 1997. [Google Scholar]
- [17].Wong CS, Chia CF, Lee HC, et al. Eradication of Helicobacter pylori for prevention of ulcer recurrence after simple closure of perforated peptic ulcer: a meta-analysis of randomized controlled trials. J Surg Res 2013;182:219–26. [DOI] [PubMed] [Google Scholar]
- [18].Søreide K, Thorsen K, Harrison EM, et al. Perforated peptic ulcer. Lancet 2015;386:1288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014;207:120–6. [DOI] [PubMed] [Google Scholar]
- [20].Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010;121:948–54. [DOI] [PubMed] [Google Scholar]
- [21].Nichols M, Townsend N, Scarborough P, et al. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 2014;35:2950–9. [DOI] [PubMed] [Google Scholar]
- [22].Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997;349:1436–42. [DOI] [PubMed] [Google Scholar]
- [23].Vafaeimanesh J, Hejazi SF, Damanpak V, et al. “Association of Helicobacter pylori infection with coronary artery disease: Is Helicobacter pylori a risk factor?”. ScientificWorldJournal 2014;2014:6.Article ID 516354. doi:10.1155/2014/516354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Khodaii Z, Vakili H, Ghaderian SM, et al. Association of Helicobacter pylori infection with acute myocardial infarction. Coron Artery Dis 2011;22:6–11. [DOI] [PubMed] [Google Scholar]
- [25].Romano PS, Roos LL, Jollis JG, et al. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075–9. [DOI] [PubMed] [Google Scholar]
- [26].Feliciano DV. Do perforated duodenal ulcers need an acid-decreasing procedure now that omeprazole is available? Surg Clin North Am 1992;72:369–80. [DOI] [PubMed] [Google Scholar]
- [27].Cho CH, Qui BS, Bruce IC. Vagal hyperactivity in stress induced gastric ulceration in rats. J Gastroenterol Hepatol 1996;11:125–8. [DOI] [PubMed] [Google Scholar]
- [28].Nada T, Nomura M, Iga A, et al. Autonomic nervous function in patients with peptic ulcer studied by spectral analysis of heart rate variability. J Med 2001;32:333–47. [PubMed] [Google Scholar]
- [29].Yukinaka M, Nomura M, Saijyo T, et al. Evaluation of autonomic nervous function in patients with essential hypertension complicated with peptic ulcer. J Gastroenterol Hepatol 2000;15:40–4. [DOI] [PubMed] [Google Scholar]
- [30].Cho CH, Ogle CW, Dai S. Acute gastric ulcer formation in response to electrical vagal stimulation in rats. Eur J Pharmacol 1976;35:215e9. [DOI] [PubMed] [Google Scholar]
- [31].Kox M, Pompe JC, Pickkers P, et al. Increased vagal tone accounts for the observed immune paralysis in patients with traumatic brain injury. Neurology 2008;70:480–5. [DOI] [PubMed] [Google Scholar]
- [32].Huang KW, Luo JC, Leu HB, et al. Chronic obstructive pulmonary disease: an independent risk factor for peptic ulcer bleeding: a nationwide population-based study. Aliment Pharmacol Ther 2012;35:796–802. [DOI] [PubMed] [Google Scholar]
- [33].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- [34].Tseng CH, Chen JH, Muo CH, et al. Increased risk of ischemic stroke among patients with chronic osteomyelitis: a population-based cohort study in Taiwan. Eur J Neurol 2015;22:633–9. [DOI] [PubMed] [Google Scholar]
- [35].Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16:217–26. 229. [PubMed] [Google Scholar]
- [36].Kalayoglu MV, Libby P, Byrne GI. Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease. JAMA 2002;288:2724–31. [DOI] [PubMed] [Google Scholar]
- [37].de Groot L, Posthumus MD, Kallenberg CG, et al. Risk factors and early detection of atherosclerosis in rheumatoid arthritis. Eur J Clin Invest 2010;40:835–42. [DOI] [PubMed] [Google Scholar]
- [38].Patel RV, Shelling ML, Prodanovich S, et al. Psoriasis and vascular disease risk factors and outcomes: a systematic review of the literature. J Gen Intern Med 2011;26:1036–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tonetti MS. Periodontitis and risk for atherosclerosis: an update on intervention trials. J Clin Periodontol 2009;36(Suppl 10):15–9. [DOI] [PubMed] [Google Scholar]
- [40].Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002;20:197–216. [DOI] [PubMed] [Google Scholar]
- [41].Robertson AKL, Rudling M, Zhou X, et al. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest 2003;112:1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kwak B, Mulhaupt F, Myit S, et al. Statins as a newly recognized type of immunomodulator. Nat Med 2000;6:1399–402. [DOI] [PubMed] [Google Scholar]
- [43].Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352:20–8. [DOI] [PubMed] [Google Scholar]
- [44].Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005;352:29–38. [DOI] [PubMed] [Google Scholar]
- [45].Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458–62. [DOI] [PubMed] [Google Scholar]
- [46].Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013;369:840–51. [DOI] [PubMed] [Google Scholar]
- [47].Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat Rev Endocrinol 2012;8:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tracey KJ. The inflammatory reflex. Nature 2002;420:853–9. [DOI] [PubMed] [Google Scholar]
- [49].Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009;9:418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Linard C, Marquette C, Clarenc D, et al. Acute ileal inflammatory cytokine response induced by irradiation is modulated by subdiaphragmatic vagotomy. J Neuroimmunol 2005;168:83–95. [DOI] [PubMed] [Google Scholar]
- [51].Carlens C, Brandt L, Klareskog L, et al. The inflammatory reflex and risk for rheumatoid arthritis: a case-control study of human vagotomy. Ann Rheum Dis 2007;66:414–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schroder VT, Pappas TN, Vaslef SN, et al. Vagotomy/drainage is superior to local oversew in patients who require emergency surgery for bleeding peptic ulcers. Ann Surg 2014;259:1111–8. [DOI] [PubMed] [Google Scholar]


