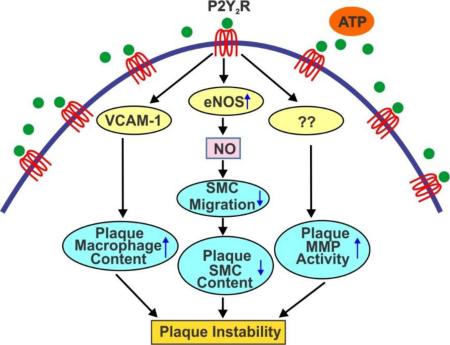
Abstract
Objective
Nucleotide P2Y2 receptor (P2Y2R) contributes to vascular inflammation by increasing vascular cell adhesion molecule (VCAM)-1 expression in endothelial cells (EC), and global P2Y2R deficiency prevents fatty streak formation in ApoE−/− mice. Since P2Y2R is ubiquitously expressed in vascular cells, we investigated the contribution of endothelial P2Y2R in the pathogenesis of atherosclerosis.
Approach and Results
EC-specific P2Y2R-deficient mice were generated by breeding VEcadherin5-Cre mice with the P2Y2R “floxed” mice. Endothelial P2Y2R deficiency reduced eNOS activity and significantly altered ATP-and UTP-induced vasorelaxation without affecting vasodilatory responses to acetylcholine. Telemetric blood pressure and echocardiography measurements indicated that EC-specific P2Y2R-deficient mice did not develop hypertension. We investigated the role of endothelial P2Y2R in the development of atherosclerotic lesions by crossing the EC-specific P2Y2R knockout mice onto an ApoE−/− background and evaluated lesion development after feeding a standard chow diet for 25 weeks. Histopathological examination demonstrated reduced atherosclerotic lesions in the aortic sinus and entire aorta, decreased macrophage infiltration and increased smooth muscle cell and collagen content leading to the formation of a subendothelial fibrous cap in EC-specific P2Y2R-deficient ApoE−/− mice. Expression and proteolytic activity of matrix metalloproteinase (MMP)-2 was significantly reduced in atherosclerotic lesions from EC-specific P2Y2R-deficient ApoE−/− mice. Furthermore, EC-specific P2Y2R deficiency inhibited NO production leading to significant increase in SMC migration out of aortic explants.
Conclusions
EC-specific P2Y2R-deficiency reduces atherosclerotic burden and promotes plaque stability in ApoE−/− mice through impaired macrophage infiltration acting together with reduced MMP-2 activity and increased SMC migration.
Keywords: Adenosine 5’-trisphosphate, Atherosclerosis, Endothelial cell, Receptor, Smooth muscle cell
Graphical Abstract
Introduction
Endothelial dysfunction and monocyte trans-endothelial migration have been recognized as integral components in the initiation and progression of atherosclerosis (1). P2Y2 receptor (P2Y2R) activation in vivo enhances neointimal hyperplasia in response to vascular injury (2).We recently reported that P2Y2R deficiency prevents fatty streak lesion formation in ApoE−/− mice (3). During atherosclerosis, inflammation-induced endothelial barrier dysfunction promotes lipoprotein and leukocyte transendothelial flux in the vascular wall (4). Extracellular nucleotides acting on P2Y2R regulate the expression of VCAM-1 (5), one of the earliest molecular events in the pathogenesis of atherosclerosis (6). These data suggest a prominent role for endothelial P2Y2R in the formation of atherosclerotic lesions. However, given that this nucleotide receptor is ubiquitously expressed in vascular and blood-derived cells (7-10), the relative contribution of endothelial P2Y2R to the pathogenesis of atherosclerosis is unclear. We hypothesized that endothelial cell P2Y2R contributes to atherosclerosis by conferring an endothelial inflammatory phenotype and promoting monocyte infiltration. In the present study, we have generated EC-specific P2Y2R-deficient mice on an ApoE−/− background, and characterized the functional contribution of endothelial P2Y2R to the development of atherosclerosis.
We determined that in ApoE−/− mice, loss of endothelial cell P2Y2R inhibited the progression of atherosclerosis and increased SMC and collagen content in atherosclerotic plaques resulting in the formation of a subendothelial fibrous cap. Mechanistically, we showed that endothelial P2Y2R deficiency decreased the proteolytic activity of matrix metalloproteinase MMP-2 and inhibited NO production leading to significant increase in SMC migration. Our findings suggest that targeted inhibition of this nucleotide receptor may increase plaque stability.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Generation of mice with endothelial-specific deletion of P2Y2 receptor
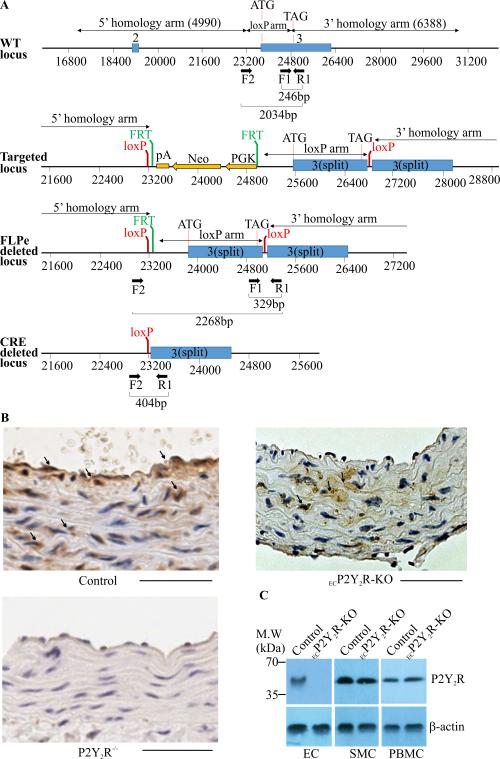
A conditional “floxed” allele of the murine P2Y2R genomic locus was generated by flanking the coding region of the P2Y2R gene with loxP sites (Fig. 1A). The P2Y2Rfl/fl mice were bred to cadherin 5-Cre (Cre) transgenic mice to generate ECP2Y2R-KO (P2Y2Rfl/fl Cre−/+) and control P2Y2Rfl/fl mice. All mice are on a C57BL/6 background. Immunostaining of the mouse aorta with antibodies against P2Y2R detected the presence of P2Y2R in both EC and SMC in control mice (Fig. 1A). As expected, P2Y2R was expressed in SMC but not in EC in the aorta of ECP2Y2R-KO mice (Fig. 1B) whereas, no P2Y2R-positive staining was detectable in the aorta of total body P2Y2R knockout mice (Fig. 1B). Western blot analysis of lysates from primary EC cultures further confirmed endothelial-specific knockout of the P2Y2R (Fig. 1C). In addition, endothelial deletion of P2Y2R did not result in P2Y2R deletion from peripheral blood mononuclear cells (Fig. 1C). The ECP2Y2R-KO mice and controls were born at the expected Mendelian ratios, and exhibited no obvious developmental defects. These data indicated that the P2Y2R is efficiently and specifically deleted in the endothelial compartment of ECP2Y2R-KO mice.
Figure 1.
Generation of ECP2Y2R-KO mice. A. The wild type mouse P2Y2R locus is indicated in the upper panel. The positions of the 5’, 3’ and loxP homology arms used to generating the targeting vector are indicated. Exons 2 and 3 of the gene are indicate by blue boxes. The positions of primers used for genotyping are also indicated. Schematics of the targeted locus, the FLPe deleted locus, in which the neo cassette was removed and the final cre deleted or knockout locus are also shown. Cre-mediated deletion results in excision of the entire coding region of the P2Y2R gene. B, Immunostaining of P2Y2R in cross sections of aortic vessels in control, ECP2Y2R-KO and P2Y2R−/− (total body P2Y2R knock out) mice. P2Y2R is expressed in both EC and SMC in aorta of control mice whereas in ECP2Y2R-KO mice expression was only detected in SMC. P2Y2R staining was absent from EC and SMC in the aorta of P2Y2R−/−mice. Scale bar represents 50μm. C, Western blot analysis of P2Y2R expression in primary cultured EC, SMC and PBMC from control and ECP2Y2R-KO mice. Data shown are representative of experiments performed from 4 mice for each genotype.
Endothelial P2Y2 receptor mediates nucleotide-induced vasodilation
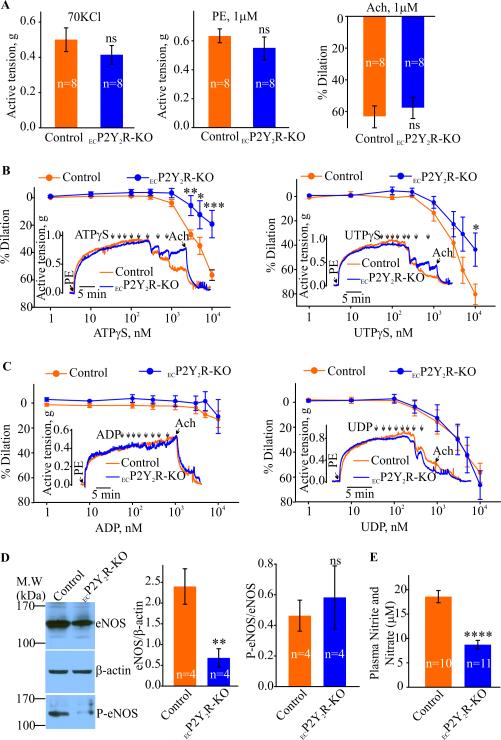
As extracellular nucleotides evoke endothelium-dependent relaxation in the mouse aorta (11) we used the ECP2Y2R-KO mice to clarify the role of the P2Y2R in this process. Aortic ring constrictions in response to KCl or phenylephrine (PE) were similar between control and ECP2Y2R-KO mice (Fig. 2A). Acetylcholine-induced dilations of PE preconstricted aortic arch rings were also not significantly different between the two groups (Fig. 3A). However, the vasodilatory responses evoked by ATPγS were significantly smaller in the ECP2Y2R-KO mice compared to controls (56.5 ± 3.8%, n=8 in controls vs 19.2 ± 10.0%, n=5 in ECP2Y2R-KO; Fig. 2B). UTPγS-induced dilations were also significantly impaired in the ECP2Y2R-KO aortic rings (80.4 ±8.4%, n=7 in controls vs. 43.6 ± 13.7%, n=5 in ECP2Y2R-KO; Fig. 2B). Aortic rings in control and ECP2Y2R-KO mice showed complete relaxation in response to the P2Y6 receptor agonist UDP (56.5 ± 7.7%, n=9 in controls vs 66.4 ± 11.5%, n=6 in ECP2Y2R-KO; Fig. 2C). Surprisingly, the P2Y1R agonist ADP, even at the highest concentration of 10 μM, dilated neither control nor ECP2Y2R-KO aortic rings (Fig. 2C). These data together with the normal vasodilatory responses to Ach demonstrate that although the P2Y2-deficiency mice have reduced eNOS activity, this is not sufficient to result in systemic arterial hypertension.
Figure 2.
Endothelial deletion of P2Y2R results in significant decrease in nucleotide- induced vasodilation and eNOS activity. ATPγS- or UTPγS-induced dilations are significantly decreased in the aortic rings from ECP2Y2R-KO mice. Phenylephrine (PE)- and 70 mM KCl-induced contractions and Ach-induced dilations in PE pre-constricted aortic rings from control and ECP2Y2R- KO mice are shown in A. Sample traces and concentration-response curves for ATPγS, UTPγS and ADP, UDP induced effects in PE pre-constricted aortic rings are shown in B and C, respectively. *p<0.05, **p<0.01 and ***p<0.001. D, Western blot analysis of total eNOS protein and phosphorylated eNOS (serine 1176) in aortic arch lysates from control and ECP2Y2R-KO. Densitometry analysis are shown in graphs (n= 7, **p < 0.01). E, Plasma nitrate and nitrite levels in control (n=10) and ECP2Y2R-KO mice (n= 11). Data are mean ± SEM; ****p<0,0001 by 2-tailed Student's t test.
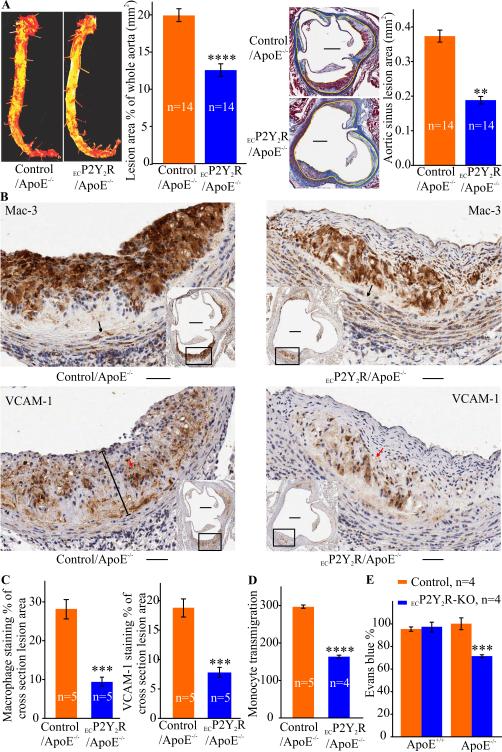
Figure 3.
Endothelial cell-specific deletion of P2Y2R reduces atherosclerosis, inflammation and endothelial barrier dysfunction in ApoE−/− mice. A, Analysis of atherosclerotic lesions in ECP2Y2R ApoE−/− and control ApoE−/− mice fed standard chow diet for 25 weeks. Left panel shows representative photomicrographs of Oil red O-stained aortas collected between the subclavian and iliac branches and quantitative computer- assisted image analysis of lipid deposition in the entire aorta. Data represent the percentage surface area of the aorta occupied by atherosclerotic lesions in control (n=14) and ECP2Y2R/ApoE−/− mice (n=14). ****p<0.0001. Right panel shows representative images of cross sections of aortic sinus from ECP2Y2R/ApoE−/− and control/ApoE−/− mice stained with Masson's trichrome. Scale bar represents 250 μm. The adjacent graphs shows the mean ±SEM lesion area measured in 5 sections for each of 14 animals. The lesion area in the aortic sinus was determined by computer-assisted image analysis. The yellow lines delineate the lesions. **p < 0.01. B, Representative images of immunohistological staining of atherosclerotic lesions in the aortic sinus stained with the anti-macrophage Mac-3 antibody or VCAM-1 as indicated. Scale bar represents 50 μm and the scale bar in inset represents 250 μm. Morphometric analysis of the stained areas are indicated in panel C. Data are the mean ±SEM of the percentage staining of the total plaque area in 5 consecutive sections in 5 mice for each genotype. ***p<0.001. D, Monocyte transmigration across endothelial cell monolayers from control and ECP2Y2R-KO mice. EC were grown on transwell membrane with monocytes from wild-type added to the top chamber and MCP-1 to the bottom. Transmigrated monocytes were counted and normalized to the non-treated condition (without MCP-1). ****p< 0.0001. E, Mice were injected with Evans blue dye and leakage of the dye into the aortas was quantified after extracting the dye and measuring absorbance at 620 nm. Values were normalized to the dry weight of the aorta. Data represent mean ±SEM; (n= 5). ***p < 0.001.
Endothelial P2Y2 receptor deletion attenuates eNOS activity in vivo but does not result in hypertension
Decreased endothelial nitric oxide synthase (eNOS) activity and nitric oxide (NO) production are critical contributors to endothelial dysfunction (12). Interestingly, extracellular nucleotides have also been implicated in activation of eNOS, leading to NO generation and vasodilation (13). To explore the mechanisms underlying the reduction of endothelium-dependent relaxation in response to nucleotides, we therefore examined the effect of ECP2Y2R deletion on eNOS expression and activity. Western blot analysis demonstrated a significant (**p<0.01) decrease in eNOS protein levels in the ECP2Y2RKO mouse aorta compared to control mice (Fig, 2D). The decreased eNOS protein expression correlated with a decrease in plasma nitrate levels in ECP2Y2R-KO mice (Fig. 2E, (**p<0.01). A tamoxifen-inducible endothelium-specific P2Y2-deficient mouse (Tie2-CreERT2 P2Y2Rfl/fl) has been previously reported to develop systemic hypertension (14). Blood pressure measurements were thus recorded in our ECP2Y2R-KO mice to determine if they also develop hypertension. Radio-telemetry monitoring of blood pressure and heart rate showed no difference between ECP2Y2R-KO and control mice (Table 1). Likewise, acute blood pressure measurements showed no difference between control and ECP2Y2R-KO mice (Table 2). Furthermore, transthoracic echocardiography revealed no significant difference in right ventricle diameter or thickness in 5 month-old ECP2Y2R-KO mice relative to control mice (Table 3), indicating that these mice did not develop pulmonary hypertension. Likewise, left ventricle thickness and diameter were similar in mice of both genotypes. Additional parameters of LV function (% fractional shortening, mitral and aortic velocities) are also similar between groups (data not shown).The blood pressure data coupled with the lack of hypertrophy in both ventricles indicates a lack of systemic hypertension in ECP2Y2R-KO mice. Together, these data demonstrate that endothelial P2Y2R-deficiency strongly reduces eNOS activity in vivo resulting in reduced NO formation which was not accompanied by arterial hypertension.
Table 1.
Radiotelemetry blood pressure measurements
| Control (n=5) | ECP2Y2R-KO (n=5) | |
|---|---|---|
| Baseline SPB (mmHg) | ||
| Night time | 144.92±12.51 | 143.06±11.50 |
| Daytime | 93.28±13.06 | 88.40±13.53 |
| Heart rate (beats/min) | ||
| Night time | 579.04±74.70 | 585.10±56.67 |
| Daytime | 402.68±57.38 | 374.03±47.14 |
Table 2.
Acute blood pressure measurements
| Control (n=12) | ECP2Y2R-KO (n=12) | |
|---|---|---|
| Baseline SPB (mmHg) | 82.84±4.05 | 82.43±4.50 |
| Heart rate (beat/min) | 524.01±33.94 | 520.11±95.13 |
Table 3.
Echocardiography data in conscious mice
| Mice | Weight (g) | RVW (mm) | RVD (mm) | LVD (mm) | LVW (mm) |
|---|---|---|---|---|---|
| Control | 30.25±2.63 | 0.36±0.09 | 1.84±0.21 | 4.07±0.23 | 0.64±0.09 |
| ECP2Y2R-KO | 30.58±3.09 | 0.36±0.09 | 1.72±0.23 | 4.15±0.45 | 0.63±0.08 |
P<0.05 versus RVW, Right ventricle wall thickness; RVD, Right ventricle chamber diameter; LVD, Left ventricle chamber diameter; LVW, Left ventricle posterior wall thickness.
Loss of endothelial P2Y2 receptor reduces atherosclerotic lesions in ApoE−/− mice
Endothelium-dependent vasodilation is involved in atherosclerotic lesion formation (1). To examine the contribution of endothelial cell P2Y2R to atherosclerosis, we generated atherosclerosis-susceptible mice with endothelial-specific deletion of P2Y2R. To do this, we crossed both the P2Y2Rfl/fl mice and the Cad5Cre mice onto an ApoE−/− background to generate P2Y2Rfl/fl ApoE−/− Cad5Cre+/− (referred to as ECP2Y2R/ApoE−/−) experimental mice and P2Y2Rfl/fl ApoE−/− Cad5Cre−/− controls (referred as to control/ApoE−/−). The mice were maintained on a standard chow diet for 25 weeks and analyzed for lipoprotein and atherosclerotic lesion characteristics. The two groups of mice did not differ significantly in body weight, total plasma cholesterol, or fasting lipoprotein profile (Table 3). En face aortic preparations stained with oil-red-O indicate a significant (*p<0.05) reduction in total plaque area in ECP2Y2R/ApoE−/− mice as compared to control/ApoE−/− (Fig. 3A). Cross sections of aortic sinus specimens stained with Mason's trichrome showed a 40.6% decrease in the mean lesion area in the aortic root of ECP2Y2R/ApoE−/− mice compared to control/ApoE−/− mice (0.320 ±0.019 μm2 , n=5 vs. 0.193 ± 0.009 μm2, n=6 respectively; (**p<0.01); Fig. 3A). These results demonstrate that endothelial deletion of P2Y2R reduces atherosclerotic plaque burden.
P2Y2 receptor deficiency attenuates inflammation and endothelial barrier dysfunction
We next determined the effect of endothelial cell P2Y2R deficiency on the cellular composition of atherosclerotic lesions. Immuno-staining with the anti-macrophage antibody Mac-3 revealed that macrophage plaque content in the aortic root lesions of ECP2Y2R/ApoE−/− mice was reduced by more than 66% as compared with control mice (***p<0.001) Fig. 3B-C). To determine the mechanisms underlying the reduced macrophage plaque content in ECP2Y2R/ApoE−/− mice, we examined the effect of endothelial P2Y2R deletion on the transmigration of peripheral blood monocytes across endothelial monolayers. As shown in Figure 3D, monocyte chemoattractant protein (MCP)-1 increased monocyte transmigration across endothelial monolayers from control P2Y2R fl/fl mice whereas loss of endothelial P2Y2R (ECP2Y2R-KO mice) significantly impaired monocyte migration. Consistent with reduced plaque macrophage content, the percentage of lesion area positive for VCAM-1 staining was significantly reduced in the aortic root lesions of ECP2Y2R ApoE−/− mice(***p<0.001, Fig. 3B-C). These results suggest that absence of endothelial P2Y2R attenuates endothelial barrier dysfunction under pathological conditions. To examine the effect of endothelial P2Y2R deletion on vascular integrity in vivo, mice were injected with Evans blue dye, and vascular permeability within the aorta was assessed by measuring leakage of the dye. As shown in Figure 3E, there was no significant (***p<0.001) difference in the amount of dye recovered between control and ECP2Y2R-KO mice on a wild type background. In contrast, on an ApoE−/−background, loss of endothelial P2Y2R significantly reduced the amount of dye deposited in the aorta compared to control/ApoE−/− mice (Fig. 3E, (***p<0.001). These results indicate that absence of endothelial P2Y2R attenuates endothelial barrier dysfunction under pathological conditions.
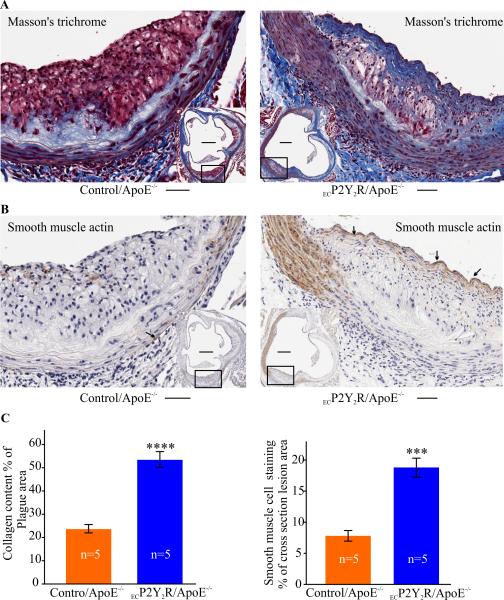
Endothelial P2Y2 receptor deficiency promotes fibrous cap formation
The decrease in macrophage content observed in ECP2Y2R ApoE−/− mice suggested that there may be an overall increase in SMC or collagen content of the lesions. Total SMC content in ECP2Y2R ApoE−/− lesions measured by the SMC marker α-SM-actin represented 18% of the total lesion area and was 2.2 fold higher that that seen in control/ApoE−/− mice (***p<0.001, Fig. 4B-C). Cap-like structures were observed in ECP2Y2R/ApoE−/− atherosclerotic lesions but not in control/ApoE−/− lesions (Fig. 4A). The increase in collagen content observed in ECP2Y2R/ApoE−/− mouse plaques correlated with a dramatic increase in the cross-section area stained with Masson's Trichrome (53.4%± 3.6% as compared to 23.6 ± 2.0% in controls; ***p<0.001; Fig. 4A-C). Our results strongly suggest that deletion of endothelial P2Y2R shifts plaque phenotype from an inflammatory status to a more stable plaque characterized by an abundance of SMC and fibrous connective tissue.
Figure 4.
Endothelial cell-specific deletion of P2Y2R promotes fibrous cap formation. A, Masson's trichrome showing collagen fibers (blue) and immunohistochemical staining for αSM-actin (B) in atherosclerotic lesions of ApoE−/− and ECP2Y2R/ApoE−/− mice. The areas occupied by α-SM actin staining or collagen were expressed as percentage of total cross-section lesion area. Scale bar represents 50 μm and the scale bar in inset represents 250 μm. (C). A continuous fibrous cap can be observed in the lesions of ECP2Y2R/ApoE−/− mice but was notably absent in lesions from ApoE−/− mice. Data represent mean ±SEM; ***p<0.001, ****p<0.0001. (n=5 mice for each genotype).
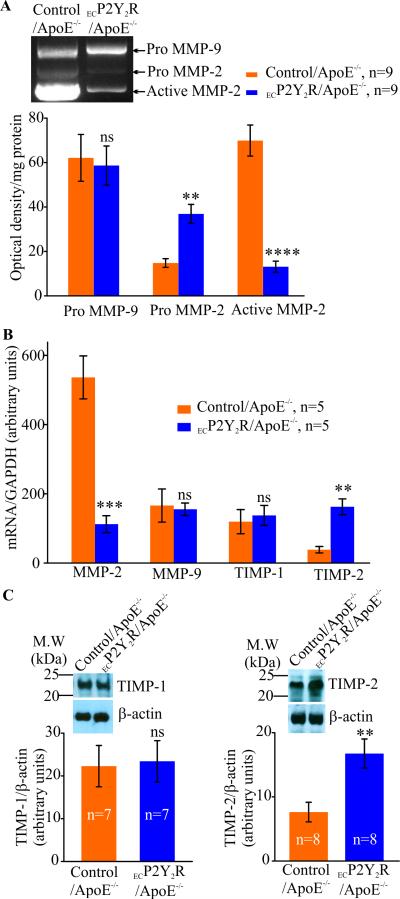
Endothelial deletion of P2Y2R decreases MMP-2 activity in atherosclerotic lesions
MMPs are thought to enhance migration and proliferation of SMC during early stages of the atherosclerotic process while in advanced plaques, inflammation-derived proteolytic activity may weaken the plaque. As atherosclerotic lesions in ECP2Y2R/ApoE−/− mice exhibited increased SMC and collagen content compared to lesions in ApoE−/− mice, we examined whether endothelial P2Y2R deficiency alter MMP proteolytic activity in atherosclerotic lesions of 25 week old mice. Gelatin zymography analysis of protein extracts from the aortic arch revealed a significant (*p < 0.05) decrease in MMP-2 activity in ECP2Y2R/ApoE−/− mice compared to control/ApoE−/− (Fig. 5A). This reduction in MMP-2 activity was paralleled by a decrease in MMP-2 mRNA expression as demonstrated by qPCR (***p < 0.001; Fig. 5B). Pro-MMP-9 expression in the lesions was similar between control/ApoE−/− and ECP2Y2R/ApoE mice. However, MMP-9 proteolytic activity was not detected in the lesions. Protein and mRNA levels of tissue inhibitor of metalloproteinase (TIMP)-2 were significantly (**p < 0.01) higher in the aortic arch of ECP2Y2R/ApoE−/− mice whereas TIMP-1 expression levels were similar between the two genotypes (Fig. 5B-C). These data indicate that endothelial P2Y2R deficiency inhibits MMP-2 activity in the atherosclerotic lesions in ApoE−/− mice.
Figure 5.
Endothelial deletion of P2Y2R increases MMP-2 activity in atherosclerotic lesions. A, Reduced gelatinolytic activity in 25 week-old ECP2Y2R/ApoE−/− mice. Representative zymogram demonstrating a significantly reduced MMP-2 activity (****p<0.0001) in the ECP2Y2R/ApoE−/− aortic arch compared to control/ApoE−/−. Data represent mean ±SEM; (n=9 mice per group); ****p <0.0001. There was no significant differences in pro-MMP-9 activity between control/ApoE−/− and ECP2Y2R/ApoE−/− mice. This decrease in MMP-2 activity was correlated with decreased MMP-2 mRNA levels (**p<0.01, n=5) as shown by qPCR (B).C, Western blot analysis shows a significant increase in TIMP-2 protein levels in ECP2Y2R/ApoE−/− mice compared to control/ApoE−/− (n=5, **p< 0.01) whereas TIMP-1 protein levels were unchanged.
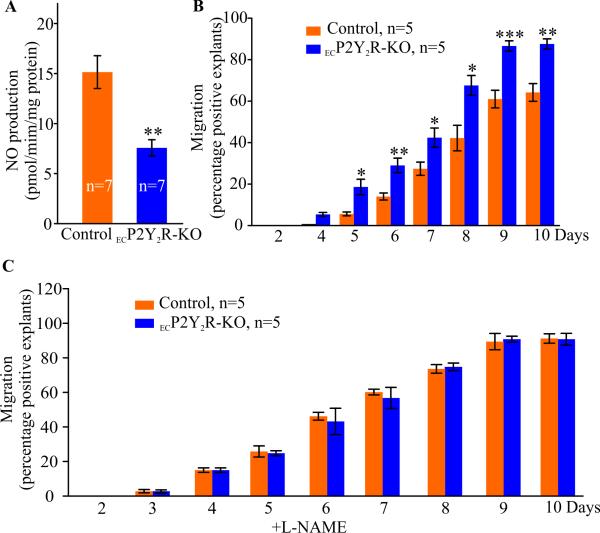
Endothelial deletion of P2Y2R increases SMC migration ex vivo
To explore the mechanisms underlying the increased accumulation of SMC in ECP2Y2R ApoE−/− lesions, we examined the effect endothelial P2Y2R deletion on migration of SMC out of aortic explants. NO derived from endothelial cells has been shown to inhibit SMC migration and proliferation (15-16). We, therefore, examined if SMC migration is altered in ECP2Y2R-KO mice that exhibit reduced eNOS levels. We first established that aortic explants from ECP2Y2R-KO mice released significantly less NO in the culture medium than explanted aortas from control mice (Fig. 6A, **p < 0.01, n=7). About 90% of aortic explants from ECP2Y2R-KO mice exhibit migrating SMC at day 10 compared to only 65% in control/ApoE−/− (**p < 0.01), Fig. 6B). At day 7, an average of 15± 4.3 (p<0.05, n=7) migrated cells per explant (p<0.05, n=7) was observed in ECP2Y2R/ApoE−/− mice compared to 6± 2.2 migrated cells per explant (n=15) in control mice. Immunofluorescence staining indicated that the migrated cells expressed the SMC marker desmin and were negative for the endothelial cell marker CD31 (not shown). Notably, addition of the nitric oxide synthase inhibitor L-NAME (10 mmol/L) to the culture media equally enhanced SMC migration out of the explants from the control mice such that there was no difference in the migration of P2Y2R and control cells (Fig. 6C). These data indicate that loss of endothelial P2Y2R enhances SMC migration through decreased NO release thereby contributing to SMC accumulation in ECP2Y2R-KO lesions.
Figure 6.
Endothelial deletion of P2Y2R decreases NO production but increases SMC migration from aortic explants. A, NO release from the aortic explants from control and ECP2Y2R-KO mice (n=7 for each genotype) was measured by determining the production of nitrite in the cultured medium after 4 days. Data represent mean ±SEM, **p < 0.01. B, Cell migration was expressed as percent of explants exhibiting at least one SMC outside the explant. SMC migration was significantly increased in explants from ECP2Y2R/ApoE−/− mice as compared to control/ApoE−/− mice (n=10 mice per group. *p < 0.05, **p < 0.01,***p < 0.001 by 2-tailed Student's t test. C, SMC migration out of aortic explants in presence of the eNOS inhibitor L-NAME (10 mmol/L) was similar in control and ECP2Y2R-KO mice.
Discussion
To examine the contribution of endothelial P2Y2 receptor (P2Y2R) to the pathogenesis of atherosclerosis, we generated mice harboring an EC-specific P2Y2R deletion mice on an ApoE−/− background. We demonstrated that on the ApoE−/− background EC-specific P2Y2R deficiency attenuates endothelial barrier dysfunction, decreases both inflammation and the burden of atherosclerosis. Most notably, EC-specific P2Y2R deficiency increases SMC and collagen plaque content leading to the formation of sub-endothelial fibrous cap. Mechanistically, endothelial P2Y2R-deficiency decreases VCAM-1 expression resulting in decreased recruitment of macrophages into the atherosclerotic plaques. Conversely, endothelial P2Y2R deficiency also decreases eNOS activity resulting in reduced NO production and increased SMC migration. Futhermore, we demonstrated that EC-specific P2Y2R deficiency reduced MMP-2 proteolytic activity in atherosclerotic lesions in ApoE−/− mice. Thus, our data suggest that therapies directed against EC P2Y2R might decrease plaque burden and increase plaque stability.
Extracellular nucleotides are maintained at very low levels due to rapid hydrolysis by ectonucleotidases (17). Under physiological conditions ATP released from the endothelium may exert a potent regulation on vascular tone (18). Aortic rings from P2Y2 receptor knockout mice (P2Y2R−/−) exhibit impaired vasorelaxation in response to nucleotides (19). In these mice, it was reported that ATP-induced dilation is mediated by both P2Y1 and P2Y2 receptors, while UTP-dependent vasodilation is attributed mostly to the P2Y6R. As these P2Y2R−/− mice lack the P2Y2R in both endothelial and smooth muscle cells, in the present study we sought to clarify the role of endothelial P2Y2R in endothelium-dependent relaxation. Using mice with EC-specific deletion of P2Y2R, we demonstrated that the vasodilatory effects of ATP and UTP are mainly mediated by endothelial P2Y2R. In addition, we established that P2Y1R is not involved in ATP-induced relaxation since ADP even at high concentrations could not elicit relaxation of aortic rings in either control or ECP2Y2R-KO mice. Furthermore, our results showed that Ach-induced dilation of PE pre-constricted aortic rings were similar between control and ECP2Y2R-KO mice, indicating that loss of endothelial P2Y2R does not result in generalized endothelial dysfunction. Although nucleotide-mediated vasomotor responses may vary among different vascular beds (20), our findings reveal that endothelial P2Y2R is the main receptor that mediates ATP -and UTP-dependent vasodilation in the aorta.
Impaired endothelium-dependent vasodilation is associated with the development of essential hypertension in humans and mice (20-21). P2Y2R−/− mice were found to develop salt-resistant hypertension (22), and P2Y2R activation decreases blood pressure in mice (23). Furthermore, a tamoxifen-induced endothelium-specific P2Y2-deficient mouse (Tie2-CreERT2 P2Y2Rfl/fl) was reported to develop spontaneous hypertension (14). Interestingly, our ECP2Y2R-KO did not develop hypertension as assessed by either acute or chronic measurements of blood pressure and the lack of hypertrophy in either ventricle. We demonstrated that endothelial deletion of P2Y2R using cadherin 5-Cre transgenic mice did not result in P2Y2R deletion from peripheral blood mononuclear cells. As Tie2-Cre–mediated deletion of P2Y2R may also lead to excision from P2Y2R bone marrow derived cells, together these data suggest that it may be the loss of P2Y2R from bone marrow derived cells that accounts for the development of hypertension in P2Y2R−/− mice. Heterologous bone marrow transplants into global P2Y2R−/− mice will be required to resolve this issue.
Although our data suggest that loss of endothelial P2Y2R does not result in systemic hypertension, functional impairment of the vascular endothelium is found in most forms of cardiovascular diseases including atherosclerosis (1). Endothelial dysfunction is associated with increased leukocyte transmigration and increasd permeability of endothelium (1, 24). Significant amounts of extracellular ATP may locally accumulate in the vasculature during traumatic and pathological events (7) and P2Y2R activation has been shown to induce a transient increase in venular permeability to albumin (25), suggesting that the P2Y2R may play a role in regulating vascular permeability. Accordingly, we found that EC-specific P2Y2R deletion attenuated endothelial barrier dysfunction that occurs in ApoE−/− mice as evidenced by decreased Evans blue dye accumulation observed in ECP2Y2R/ApoE−/− mice compared to control/ApoE−/− mice. In addition, monocyte transmigration across endothelial monolayers isolated from ECP2Y2R-KO mice was significantly decreased compared to endothelial cells isolated from control mice. These data strongly imply that the decreased monocyte content of atherosclerotic lesions in ECP2Y2R/ApoE−/− mice is likely to be due at least in part to impaired monocyte transmigration. We have also shown that loss of P2Y2R attenuates VCAM-1 expression in endothelial cells (3) suggesting that decreased binding of the monocytes likely also contributes to the decreased monocytes seen in atherosclerotic plaques of ECP2Y2R/ApoE−/− mice. Significantly less VCAM-1-positive areas were also observed in atherosclerotic lesions in ECP2Y2R/ApoE−/− mice. We recently reported that global P2Y2R deficiency prevents fatty streak lesion formation in ApoE−/− mice (3). Altogether, these data indicate a major effect of endothelial P2Y2R deletion may be to reduce vascular inflammation.
The formation of fibrous caps in atherosclerotic lesions of ECP2Y2R/ApoE−/− mice was a major finding of the present study. SMC accumulation could be the result of both increased matrix metalloproteinase activity and migration of vascular SMC. However, gelatin zymography showed significantly lower activity of MMP-2 in the lesions of 25 week-old ECP2Y2R/ApoE−/− mice and no MMP-9 activity was detected at this time point. The decrease in MMP-2 activity was paralleled by a significant increase in TIMP-2 protein and mRNA levels in the plaques. Although these data do not rule out a possible transient increase in MMP activity at an earlier stage of plaque development, they suggest that in mature plaques decreased MMP2 activity in ECP2Y2R/ApoE−/− mice could have a beneficial effect by increasing plaque stability. Despite the decreased MMP activity, we observed a significant increase in SMC migration of aortic segments from ECP2Y2R/ApoE−/− mice (Figure 6B) suggesting that the increased SMC content of the fibrous plaque may be due to increased SMC migration. We postulate that this occurs as a consequence of decreased endothelial NO. In support of this, studies have shown that transfection of eNOS gene into SMC can effectively inhibit their migration in response to chemotactic agents (26). Futhermore, the difference in SMC migration between explants derived from the control and ECP2Y2R/ApoE−/− mice was abrogated in the presence of a specific eNOS inhibitor (L-NAME, Figure 6C). Together these findings suggest that EC-specific deletion of P2Y2R may be contributing to plaque stability by not only increasing its SMC content but also by downregulating MMP-2 activity.
In conclusion, this study shows that decreased VCAM-1 expression leading to impaired macrophage infiltration acting together with MMP-2 activity and increased SMC migration resulting from diminished eNOS activity could explain the beneficial effects on plaque stabilization observed in ECP2Y2R ApoE−/− mice. Thus, targeted inhibition of P2Y2R-mediated signals in endothelial cells may be a viable approach to decrease plaque burden and improve plaque stability.
Supplementary Material
Table 4.
Body weight and plasma lipid profile of 25-week old ECP2Y2R/ApoE−/− and control/ApoE−/−
| Control/ApoE−/− (n=12) | ECP2Y2R/ApoE−/− (n=12) | |
|---|---|---|
| Body weight (g) | 34.4± 4 | 33.7± 5 |
| Total cholesterol (mg/dl) | 489± 28 | 481± 22 |
| Triglycerides (mg/dl) | 68± 7 | 67± 6 |
Highlights.
Endothelial cell-specific deletion of P2Y2 receptor reduces eNOS activity and reveals a major role for P2Y2 receptor in nucleotide-mediated vasodilation.
Endothelial cell-specific deletion of P2Y2 receptor attenuates vascular permeability in ApoE-null mice.
Endothelial cell-specific deletion of P2Y2 receptor decreases metalloproteinase activity and smooth muscle migration in atherosclerotic lesions.
Endothelial cell-specific deletion of P2Y2 receptor promotes fibrous cap formation in ApoE-null mice.
Acknowledgments
Funding Sources
This work was supported by grants from the National Institutes of Health: Cheikh I. Seye (1R01HL112883) ; Alexander G. Obukhov and Xingjuan Chen (R01HL115140).
Nonstandard Abbreviations
- ATP
Adenosine 5-triphosphate
- ADP
Adenosine diphosphate
- ApoE−/−
Applipoprotein E null
- EC
Endothelial cells
- L-NAME
Nω-Nitro-L-arginine methyl ester hydrochloride
- PBMC
Peripheral blood mononuclear cells
- MMP
Matrix metalloproteinases
- SMC
Smooth muscle cells
- SMA
Smooth muscle actin
- TIMP
Tissue inhibitor of matrix metalloproteinase
Footnotes
Disclosures: None
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, Gonzalez FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- 3.Qian Shaomin, Hoggatt April, Jones-Hall Yava L., Ware Carl F., Herring Paul, Seye Cheikh I. Deletion of P2Y2 Receptor Reveals a Role for Lymphotoxin-α in Fatty Streak Formation. Vasc Pharmacol. 2016;85:11–20. doi: 10.1016/j.vph.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornerup K, Nordestgaard BG, Jensen TK, et al. Transendothelial exchange of low-density lipoprotein is unaffected by the presence of severe atherosclerosis. Cardiovascular Research. 2004;64:337–345. doi: 10.1016/j.cardiores.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Seye CI, Yu N, Jain R, Kong Q, Tess M, Newton J, Erb L, Gonzalez F, Weisman G. The P2Y2 Nucleotide Receptor Mediates UTP-induced Vascular Cell Adhesion Molecule-1 Expression in Coronary Artery Endothelial Cells. J Biol Chem. 2003;278:24960–24965. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- 6.Cybulsky MI, Liyama K, Li H, Zhu S, Chen M, Liyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107(10):1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem. J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malam-Souley R, Seye C, Gadeau AP, Loirand G, Pillois X, Campan M, Pacaud P, Desgranges C. Nucleotide receptor P2u partially mediates ATP-induced cell cycle progression of aortic smooth muscle cells. J. Cell. Physiol. 1995;166:57–65. doi: 10.1002/(SICI)1097-4652(199601)166:1<57::AID-JCP7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Erlinge D, You J, Wahlestedt C, Edvinsson L. Characterisation of an ATP receptor mediating mitogenesis in vascular smooth muscle cells. Eur J Pharmacol. 1995;289:135–49. doi: 10.1016/0922-4106(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 10.Jin J, Dasari VR, Sistare FD, Kunapuli SP. Distribution of P2Y receptor subtypes on haematopoietic cells. Br J Pharmacol. 1998;123:789–94. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guns PJ, Van Assche T, Fransen P, Robaye B, Boeynaems JM, Bult H. Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice. Br J Pharmacol. 2006;147:569–74. doi: 10.1038/sj.bjp.0706642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;4(113):1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 13.da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation. 2009;119:871–879. doi: 10.1161/CIRCULATIONAHA.108.764571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Iring A, Strilic B, Albarrán Juárez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S. P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J Clin Invest. 2015;125:3077–86. doi: 10.1172/JCI81067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22:S1–S14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996;78:225–230. doi: 10.1161/01.res.78.2.225. [DOI] [PubMed] [Google Scholar]
- 17.Robson Simon C., Sévigny Jean, Zimmermann Herbert. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ralevic V, Burnstock G. Involvement of purinergic signaling in cardiovascular diseases. Drug News Perspect. 2003;16:133–140. doi: 10.1358/dnp.2003.16.3.876886. [DOI] [PubMed] [Google Scholar]
- 19.Guns PJ, Van Assche T, Fransen P, Robaye B, Boeynaems JM, Bult H. Endothelium-dependent relaxation evoked byATP and UTP in the aorta of P2Y2-deficient mice. Br J Pharmacol. 2006;147:569–74. doi: 10.1038/sj.bjp.0706642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panza JA, García CE, Kilcoyne CM, Quyyumi AA, Cannon RO., 3rd Impaired endothelium-dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–8. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 21.Savoia C, Sada L, Zezza L, Pucci L, Lauri FM, Befani A, Alonzo A, Volpe M. Vascular inflammation and endothelial dysfunction in experimental hypertension. Int J Hypertens. 2011;2011:281240. doi: 10.4061/2011/281240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+and water reabsorption. FASEB J. 2007a;21:3717–3726. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- 23.Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V. P2Y(2) receptor activation decreases blood pressure and increases renal Na(+) excretion. Am J Physiol Regul Integr Comp Physiol. 2011;301:R510–R518. doi: 10.1152/ajpregu.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun C, Wu MH, Yuan SH. Nonmuscle Myosin Light-Chain Kinase Deficiency Attenuates Atherosclerosis in Apolipoprotein E–Deficient Mice via Reduced Endothelial Barrier Dysfunction and Monocyte Migration. Circulation. 2011;124:48–57. doi: 10.1161/CIRCULATIONAHA.110.988915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey J, Erb L, Huxley V, Weisman GA, Garrad R, Wang J. P2Y2 Receptor Dependent Modulation of Microvascular Barrier Function. FASEB Journal. 2012:26. Abstract 855.4. [Google Scholar]
- 26.Gurjar MV, Sharma RV, Bhalla RC. eNOS gene transfer inhibits smooth muscle cell migration and MMP-2 and MMP-9 activity. Arterioscler Thromb Vasc Biol. 1999;19(12):2871–7. doi: 10.1161/01.atv.19.12.2871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.