Abstract
The length of telomeric DNA is often considered a cellular biomarker of aging and general health status. Several telomere length measuring assays have been developed, of which the most common is the Telomere Restriction Fragment (TRF) analysis, which typically involves the use of radioactively labeled oligonucleotide probes. While highly effective, this method potentially poses substantial health concerns and generates radioactive waste. Digoxigenin (DIG) alternatives to radioactive probes have been developed and used successfully in a number of assays. Here we optimize the DIG protocol to measure telomere length in the model plant Arabidopsis thaliana and present evidence that this approach can be used successfully to efficiently and accurately measure telomere length in plants. Specifically, hybridization temperature of 42 °C instead of the typical 55 °C appears to generate stronger signals. In addition, DIG incorporation at 5′-end instead of 3′-end of the labeled oligonucleotide greatly enhances signal. We conclude that non-radioactive TRF assays can be as efficient as radioactive methods in detecting and measuring telomere length in plants, making this assay suitable for medical and research laboratories unable to utilize radioactivity due to hazardous waste disposal and safety concerns.
Keywords: Nonradioactive telomere restriction fragment analysis, Southern blot, DNA, Aging, Plant
1 Introduction
Telomeres are evolutionarily conserved protein-DNA complexes found at the ends of linear eukaryotic chromosomes. Telomeres are involved in genome maintenance and regulation of cellular lifespan. Telomeric DNA in most plants contains hundreds of TTTAGGG repeats [1]. While the overall length of telomeric DNA is species-specific, considerable variations in average telomere length exist within natural populations of different organisms. The most widely-used method to detect telomere length variations is Telomere Restriction Fragment (TRF) analysis, where genomic DNA is digested with a frequently-cutting restriction enzyme to free up terminal telomeric repeats from the rest of genomic DNA. The digested DNA is then run on a gel, transferred to a membrane and hybridized to a 32P-labeled oligonucleotide probe of telomeric sequence [2]. Although this method is highly effective, the use of radioactivity raises a number of health and environmental concerns which can be prohibitive for many research laboratories without access to radioactive waste disposal services. Here we present a modified protocol to measure telomere length in plants using a DIG-labeled telomeric oligonucleotide.
2 Materials and methods
Genomic DNA for TRF analysis was isolated using the standard CTAB protocol [3], treated with RNase A and diluted to 1000 ng/ul. Up to 50 μg DNA of each sample was digested by Tru1I (ThermoFisher Scientific, USA), which cuts genomic DNA frequently (TTAA recognition sequence), but not within telomeric regions. Digested DNA was then separated by 1% agarose gel electrophoresis at 55 V for 18 hours in 1X TAE. Separated DNA was then transferred to a nylon membrane and immobilized by UV-crosslinking using Stratalinker (Stratagene). Hybridization was done using either a DIG- or 32P-labeled probe of (TTTAGGG)4 sequence. Radioactive signals were scanned using Pharos FX Plus Molecular Imager (Bio-Rad) and data were analyzed with Quantity One software (Bio-Rad). After additional steps (see the accompanying Protocol in Supplementary Data), non-radioactive telomeric signal intensity was scanned with high resolution hemiluminescence settings using a Molecular Imager Chemidoc XRS+ scanner (Bio Rad) with Image Lab™ Software (Bio Rad). To calculate average telomere length, signal intensity was analyzed using TeloTool program, which allows data standardization to obtain statistically valid values [4].
3 Results and discussion
Some previous reports indicated that DIG-labeled probes can be equally or even more sensitive than 32P-labeled probes when detecting repetitive genomic DNA sequences [5]. Since then, several different approaches to improve DIG-method sensitivity were implemented, including the recent proposal to incorporate several DIG molecules into bridged nucleic acid-containing oligonucleotides [6]. To improve the ability of current non-radioactive Southern blot protocol to detect telomere sequences in plants, we compared the efficiencies of (TTTAGGG)4 probes that were labeled either radioactively or with a digoxigenin moiety.
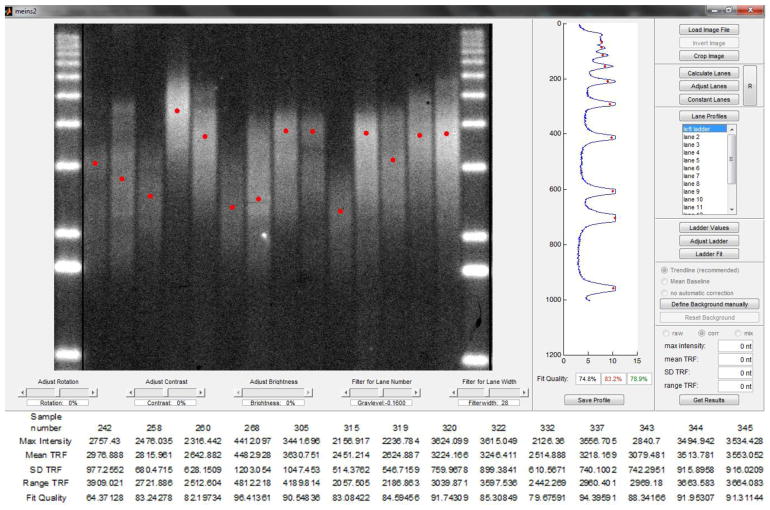
First, we checked the 5′- and 3′-terminal positions of digoxigenin on the DNA oligonucleotide. Only 5′ labeled DIG probe (Figure 1) produced a visible signal on the Southern blot, suggesting that digoxigenin should be located on the 5′ side of DNA probe to produce the most signal. Several other modifications over the standard hybridization techniques with non-radioactive probes [7] were made. We found that a decrease in hybridization temperature from a standard 55°C to 42°C greatly improves DIG-probe affinity to the plant telomeric DNA without affecting specificity. Furthermore, an increase in incubation time by 10–15 minutes over manufacturer’s recommendations for all steps following hybridization with DIG probe results in improved signal. Specifically, longer blocking time appears to reduce non-specific binding of antibodies, while increasing the time of washing steps may decrease background. Side-by-side comparison of TRF assays performed with radioactive and non-radioactive versions of the TRF assay indicates that the efficiency of both approaches is similar, in both cases producing well-defined and clear telomeric smears (Figure 2). Furthermore, as analyzed by the recently developed TeloTool software [4], the quality of the telomeric signal produced by the non-radioactive assay is sufficient for precise quantification of mean, range and maximum telomere length (Figure 3). In summary, the modified non-radioactive TRF method presented here appears to be suitable for measuring telomere length in Arabidopsis thaliana and other plants with telomeres in the range of 1–10 kb, though its suitability for measuring telomeres in the extremely long (>50 kb) or short (<1 kb) range will need to be tested.
Figure 1.
5′ DIG telomeric probe structure.
Figure 2.
Comparison of a typical radioactive (A) and non-radioactive (B) Southern blot images. Each lane contains DNA from one individual plant of different A. thaliana ecotypes. Radioactive (A) or DIG-labeled (B) molecular weight DNA markers are shown on each side of the gel. 1 kb+ - standard DNA markers not visible after DIG signal visualization.
Figure 3.
Telomere length measurement and quantification by TeloTool Software. Plant telomeric signal was detected using non-radioactive TRF assay with DIG probe. Top - lane analysis by TeloTool. Bottom - numerical values (maximum intensity, mean telomere length, standard deviation, range of telomere signal, fit quality) for each sample.
4 Conclusions
Some concerns have been raised in the past that a non-radioactive version of Southern blotting assay could be less sensitive and accurate than its radioactive counterpart owing to the presence of extra protocol steps, additional DNA probe modifications and complicated antibody optimization scheme [8]. Here we provide a modified protocol to greatly improve efficiency of non-radioactive TRF assay for measuring plant telomeric DNA using a 5′-end DIG labeled probe. We show that the quality of the resulting data is sufficient for precise plant telomere length measurement using the TeloTool software, the current gold standard in the field.
Supplementary Material
Acknowledgments
We thank Xiaoyuan Xie, Callie Kobayashi and other members of the Dorothy Shippen laboratory (Texas A&M University) for help with setting up radioactive TRF assays. This work was supported by National Institutes of Health (grant number 1R03AG052891-01 to EVS) and by the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities (project No 14-83 to MRS). LRN was supported in part by the Foundation for the Promotion of Innovation (Small Innovative Enterprises in Science and Technology Assistance Fund, UMNIK project No 0020895). This work was performed in accordance with the Russian Government Program of Competitive Growth of Kazan Federal University.
References
- 1.Shakirov EV, Song X, Joseph JA, Shippen DE. POT1 proteins in green algae and land plants: DNA-binding properties and evidence of co-evolution with telomeric DNA. Nucleic Acids Res. 2009;37(22):7455–67. doi: 10.1093/nar/gkp785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sedivy JM, Shippen DE, Shakirov EV. Surprise ending. Nature Genetics. 2003;33:114–6. doi: 10.1038/ng0203-114. [DOI] [PubMed] [Google Scholar]
- 3.Cocciolone SM, Cone KC. Pl-Bh, an anthocyanin egulatory gene of maize that leads to variegated pigmentation. Genetics. 1993;135:575–88. doi: 10.1093/genetics/135.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Göhring J, Fulcher N, Jacak J, Riha K. A New Tool for Telomere Length Measurement from terminal restriction fragment analysis with improved probe intensity correction. Nucleic Acids Res. 2014;42(3):21. doi: 10.1093/nar/gkt1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zischler H, Nanda I, Schafer R, Schmid M, Epplen JT. Digoxigenated oligonucleotide probes specific for simple repeats in DNA fingerprinting and hybridization in situ. Hum Genet. 1989;82:227–233. doi: 10.1007/BF00291160. [DOI] [PubMed] [Google Scholar]
- 6.Lai Tsung-Po, Wright WE, Shay JW. TX Bio Techniques. 6. Vol. 60. Department of Cell Biology, University of Texas Southwestern Medical Center; Dallas: 2016. Generation of digoxigenin-incorporated probes to enhance DNA detection sensitivity; pp. 306–9. [DOI] [PubMed] [Google Scholar]
- 7.Solanas M, Escrich E. An improved protocol to increase sensitivity of Southern blot using dig-labelled DNA probes. Journal of Biochemical and Biophysical Methods. 1997;35(3):153–9. doi: 10.1016/s0165-022x(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 8.de Muro MA, Walker JM, Rapley R. Molecular Biomethods Handbook. Humana Press; New York: 2008. Probe design, production, and applications; pp. 41–53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.