Abstract
Background
Antibody-mediated rejection (ABMR) represents one of the cardinal causes of late allograft loss after kidney transplantation and there is great need for noninvasive tools improving early diagnosis of this rejection type. One promising strategy might be the quantification of peripheral blood DNA levels of the highly prevalent and apathogenic Torque Teno virus (TTV), which might mirror the overall level of immunosuppression and thus help determine the risk of alloimmune response.
Methods
To assess the association between TTV load in the peripheral blood and ABMR, 715 kidney transplant recipients (median 6.3 years posttransplantation) were subjected to a systematical cross-sectional ABMR screening and, in parallel, TTV quantification.
Results
Eighty-six of these recipients had donor-specific antibodies and underwent protocol biopsy, ABMR positive patients (n = 46) showed only 25% of the TTV levels measured in patients without ABMR (p = 0.003). In a generalized linear model, higher TTV levels were associated with a decreased risk for ABMR after adjustment for potential confounders (risk ratio 0.94 per TTV log level; 95% confidence interval 0.90-0.99; p = 0.02).
Conclusion
Future studies will have to clarify whether longitudinal assessment of TTV load might predict ABMR risk and help guide the type and intensity of immunosuppression to prevent antibody-mediated graft injury.
Introduction
Registry analyses have failed to demonstrate major improvements in long-term survival of kidney allografts over the last decades 1, and there is emerging evidence for a critical role of antibody-mediated rejection (ABMR) as a major cause of chronic transplant injury and loss 2. Besides distinct baseline immunological factors, such as recipient presensitization and levels of HLA incompatibility, insufficient levels of immunosuppression, which in some instances might be due to medication nonadherence, were shown to pose recipients at risk for the development of ABMR 3,4. While diagnostic criteria of this rejection type are now well established 5, there is still a need for noninvasive screening tools to timely uncover emerging rejection processes 6 and provide a useful basis for the early implementation of therapeutic interventions.
In this respect, a promising strategy might be the monitoring of peripheral blood levels of the human apathogenic Torque Teno virus (TTV), which might mirror the overall strength of innate and specific immunity 7,8. TTV DNA can be detected in up to 90% of tested healthy and diseased individuals, whereby peripheral blood levels of viral load might be closely related to the immunological status of the host 9,10. Indeed, following organ transplantation, considerable increases in TTV DNA load were reported 11. Longitudinal evaluation of TTV in lung transplant recipients has revealed an increase shortly after transplantation, with a peak within the first 3 months followed by a slow decrease over the following 2 years 12,13. Interestingly, in lung transplants, TTV load was found to associate with the type of immunosuppression, whereby patients on tacrolimus had significantly higher levels of TTV than those on cyclosporine 12. Remarkably, in heart allograft recipients, TTV was lower in patients who experienced early organ rejection 14.
To our knowledge, there is currently no study that has systematically evaluated the relationship between TTV load and ABMR occurrence after kidney transplantation. We hypothesized that, as an indicator of higher immunocompetence, low TTV DNA levels associate with a higher risk of rejection. In this study, we systematically investigated the relationship between TTV load and ABMR in a large prevalent population of long-term kidney transplant recipients, who were subjected to a cross-sectional HLA antibody and ABMR screening.
Methods
Study patients
The present study included 715 recipients of a kidney allograft. It was based on a prospective cross-sectional screening for ABMR in the context of the BORTEJECT trial (www.clinicaltrials.org; NCT01873157). The design of the BORTEJECT trial has earlier been reported in detail 15. In brief, 741 recipients were included in the cross-sectional part of the trial and subjected to Luminex-based anti-HLA antibody testing. Key inclusion criteria were a functioning graft at ≥6 month post transplantation and an estimated glomerular filtration rate (eGFR) >20 ml/minute/1.73 m2. Key exclusion criteria were acute rejection <one month before screening and acute deterioration of graft function suspicious of acute rejection. Hundred eleven of these recipients were DSA-positive and 86 of them were subjected to protocol biopsies 16. The present study included 715 of these screened individuals. Twenty-six patients were excluded, because material for TTV testing (n = 1) or protocol biopsies (n = 25; no informed consent or one or more exclusion criteria that precluded inclusion in the interventional study part of the BORTEJECT trial) were not available. Complement-dependent cytotoxicity (CDC) crossmatch conversion was performed by immunoadsorption-based desensitization (19, 20). Extended criteria donors were defined as age ≥60 years, or age >50 years with at least two of the following conditions: history of hypertension, serum creatinine >1.5 mg/dL or cause of death resulting from a stroke. Delayed graft function was defined as requirement of more than one dialysis in the first week after kidney transplantation. Kidney function was assessed using eGFR according to the MDRD formula 17.
HLA antibody characterization
As previously described in detail 16, patient sera were in a first step prescreened for anti-HLA class I and II reactivity using LABScreen® Mixed assays (One Lambda, Canoga Park, CA, USA). HLA antibody-positive sera were then heat-inactivated to prevent the prozone effect and subjected to HLA class I and/or II single antigen bead testing (HLA class I: LABScreen® Single Antigen HLA class I Antibody Detection Test Combi; HLA class II: LABScreen® Single Antigen HLA class II Antibody Detection Test-Group 1; One Lambda). The presence of DSA was determined on the basis of recorded HLA reactivity patterns assessed on single antigen bead panels [>1000 median fluorescence intensity (MFI) was considered positive] and the results of donor/recipient HLA class I and II typing. For DSA-positive recipients, MFI levels of the peak DSA were recorded. Of the 715 kidney transplant recipients included in the analysis 370 subjects were HLA antibody screening positive (48%), 270 were single antigen bead assay positive (38%), and 86 had DSA and were subjected to protocol biopsy (12%).
Biopsies
ABMR-typical lesions, such as glomerulitis, transplant glomerulopathy, peritubular capillaritis, capillary microthrombi, intimal arteritis and multilayering of peritubular capillary basement membranes were documented and scored following the rules of the 2013 update of the BANFF scheme. Immunohistochemical C4d staining was scored on paraffin sections whereby, according to the BANFF scheme, minimal staining (C4d1) was considered positive 5. In addition to the 44 patients diagnosed with ABMR due to acute or chronic active ABMR in the BORTEJECT trial, two patients with chronic inactive ABMR were also included in this analysis.
TTV DNA quantification
TTV DNA was extracted from 200 μL of plasma using the NucliSENS easyMAG platform (bioMerieux, France) as recommended by the manufacturer and eluted in 50 μL of elution buffer. TTV DNA was quantitated by TaqMan real time-polymerase chain reaction (PCR) as described previously 18. The quantitative PCR reactions were performed in a volume of 25 µL using 2x TaqMan Universal PCR Master Mix, containing 5 µL of extracted DNA, 400 nM of each primer and 80 nM of the probe. Thermal cycling was started for 3 minutes at 50°C, followed by 10 minutes at 95°C, and then by 45 cycles at 95°C for 15 seconds, at 55°C for 30 seconds, and at 72°C for 30 seconds using the 7300 Real Time PCR System (Applied Biosystems, Foster City, CA, USA). Results were recorded in copies/mL. TTV DNA quantitation was in the linear range from 2 to 10 log10 copies/mL as determined by the use of 10-fold dilutions of a plasmid standard. Limit of detection was 2 log10 copies/mL of plasma. In each run a TTV DNA standard, a negative control (no template) and two positive controls (patient sera; median 5.3 log10 copies/mL) were included. Comparing DNA loads of the positive controls between each of the 14 test runs, a median difference of 0.11 log10 copies/mL (IQR 0.04-0.19 log10 copies/mL) was detected. Duplicates within 11 test runs, showed a median DNA load difference of 0.24 log10 copies/mL (IQR 0.03-0.36 log10 copies/mL). None of the samples showed signs of PCR inhibition as confirmed by quantitation of known amount of control DNA spiked into the samples before DNA extraction.
Statistics
Summaries for continuous variables are presented as the median and inter quartile range (IQR). The Mann Whitney U test was used for comparing continuous data. Categorical variables are presented as absolute numbers and percentages, and group comparisons were made using the Chi square or Fisher’s exact tests. Bivariate correlations were calculated using Spearman coefficient. Associations between TTV and ABMR were initially tested applying logistic regression. We used contingency tables with a Chi square test for hypothesis testing after categorizing TTV into quintiles. Score tests were used to assess linear trends. Logistic regression models were applied to assess whether TTV was independently associated with ABMR as the outcome. TTV log level was the main covariable and other predictors of ABMR and TTV were simultaneously entered as covariables: donor and recipient age, female sex, previous kidney transplantation, a positive pretransplant CDC crossmatch, CDC panel-reactivity >10%, preformed DSA, HLA mismatch in A, B and DR, ABO incompatibility, antibody induction therapy, initial immunosuppression including tacrolimus, cyclosporine, mechanistic target of rapamycin (mTOR) inhibitor, belatacept and mycophenolic acid, immunosuppression at screening including triple immunosuppression, steroids, tacrolimus, cyclosporine and belatacept, eGFR and protein-creatinine ratio at the time of screening and years between transplantation and screening. Covariables with missing data were entered as indicator variables with ‘missing’ as a separate category. Significance of single model parameters was assessed by the Wald test. We used the likelihood ratio test for deviation from linearity, variable contribution to the models and the investigation of interactions, respectively. For the final model we used a generalized linear model with a log link function to directly estimate risk ratios (RR) with 95% confidence intervals (CI) including the most clinical relevant confounders of ABMR (female sex, previous kidney transplantation, CDC crossmatch conversion, CDC panel reactivity >10%, eGFR, and protein-creatinine ratio). We did not include pre-transplant DSA, because solid phase DSA detection was not part of the clinical routine before 2009 and therefore data were only available from 264 (37%) of the patients. A 2-sided p value <0.05 was generally considered statistically significant. Exact tests were used where applicable. We used MS EXCEL 2010 (Redmond, WA, USA), IBM SPSS Statistics 20.0 (SPSS Inc., Chicago, IL, USA) and STATA 14.1 (STAT coop., College station, TX, USA) for data management and analysis.
Results
This cross-sectional study included 715 kidney allograft recipients subjected to ABMR screening after a median of 6.3 years (IQR 2.6-12.6) posttransplantation. Forty-six of these patients (6%) were diagnosed with ABMR (acute or chronic active ABMR: n = 44; chronic inactive ABMR: n = 2). Median time interval between transplantation and screening was 4.4 years (IQR 1.8-12.8) in ABMR+ and 6.6 years (IQR 2.7-12.7; p = 0.19) in ABMR- patients. Baseline characteristics are provided in Table 1 and Table 2. ABMR+ patients were more often female and recipients of a retransplant and were more frequently sensitized to HLA antigens before transplantation (preformed DSA, >10% CDC panel reactivity and/or a positive CDC crossmatch), more often received antibody induction therapy and/or immunoadsorption-based desensitization 19,20 and less often received mycophenolic acid (MPA). At the time of screening, ABMR+ patients showed lower eGFR.
Table 1.
Patient baseline characteristics in relation to ABMR category
| Characteristics | Overall cohort (n = 715) | Available data (n) | ABMR+ (n = 46) | Available data (n) | ABMR- (n = 669) | Available data (n) | p-value |
|---|---|---|---|---|---|---|---|
| Recipient data | |||||||
| Age (years), median (IQR) | 48 (37-58) | 715 | 47 (29-54) | 46 | 48 (38-58) | 669 | 0.15 |
| Female sex, % (n) | 37 (267) | 715 | 52 (24) | 46 | 36 (243) | 669 | 0.04 |
| Previous kidney transplantation, % (n) | 16 (115) | 715 | 33 (15) | 46 | 15 (100) | 669 | 0.003 |
| Donor data | |||||||
| Age (years), median (IQR) | 49 (39-58) | 682 | 46 (30-59) | 44 | 49 (39-58) | 638 | 0.51 |
| Non heart beating donor, % (n) | 5 (33) | 715 | 9 (4) | 46 | 4 (29) | 669 | 0.16 |
| Living donor, % (n) | 20 (139) | 695 | 18 (8) | 45 | 20 (131) | 650 | 0.85 |
| Extended criteria donor, % (n) | 28 (184) | 659 | 26 (11) | 42 | 28 (173) | 617 | 0.86 |
| Transplant data | |||||||
| HLA (A, B, DR) mismatch (n), median (IQR) | 3 (2-4) | 679 | 3 (2-3) | 45 | 3 (2-4) | 634 | 0.95 |
| ABO incompatibility, % (n) | 2 (16) | 715 | 0 | 46 | 16 (2) | 669 | 0.62 |
| CDCXM conversion, % (n) | 2 (17) | 715 | 13 (6) | 46 | 2 (11) | 669 | <0.001 |
| Current CDC-PRA >10%, % (n) | 9 (60) | 658 | 19 (8) | 43 | 8 (52) | 615 | 0.05 |
| Preformed DSA, % (n) | 28 (75) | 264 | 83 (20) | 24 | 23 (55) | 240 | <0.001 |
| Cold ischemia time (hours), median (IQR) | 13 (8-19) | 635 | 12 (9-18) | 43 | 13 (8-19) | 592 | 0.85 |
| Delayed graft function, % (n) | 16 (117) | 715 | 17 (8) | 46 | 16 (109) | 669 | 0.84 |
ABMR, antibody-mediated rejection; IQR, interquartile range; CDCXM, complement dependent cytotoxicity cross match; CDC-PRA, complement dependent cytotoxicity panel reactive antibody; DSA, donor specific antibodies.
Table 2.
Immunosuppression and kidney function in relation to ABMR status
| Variables | ABMR+ (n = 46) | Available data (n) | ABMR- (n = 669) | Available data (n) | p-value |
|---|---|---|---|---|---|
| Initial immunosuppression | |||||
| Induction therapy, % (n) | 80 (37) | 46 | 54 (362) | 669 | <0.001 |
| Tacrolimus, % (n) | 64 (29) | 45 | 61(393) | 644 | 0.75 |
| Cyclosporine, % (n) | 38 (17) | 45 | 35 (223) | 644 | 0.75 |
| mTOR inhibitor, % (n) | 4 (2) | 45 | 4 (27) | 644 | 0.71 |
| Belatacept, % (n) | 0 | 45 | 3 (20) | 644 | 0.63 |
| Mycophenolic acid, % (n) | 88 (37) | 42 | 96 (561) | 583 | 0.03 |
| Years between transplant and screening, median (IQR) Immunosuppression at screening | 4.4 (1.8-12.8) | 46 | 6.6 (2.7-12.7) | 669 | 0.19 |
| Triple immunosuppression, % (n) | 78 (36) | 46 | 77 (516) | 669 | >0.999 |
| Steroids, % (n) | 91 (42) | 46 | 85 (563) | 660 | 0.38 |
| Tacrolimus, % (n) | 61 (28) | 46 | 69 (459) | 668 | 0.33 |
| Tacrolimus trough level (ng/mL), median (IQR) | 5.5 (5-7.3) | 46 | 6 (5-7) | 455 | 0.57 |
| Cyclosporine, % (n) | 30 (14) | 46 | 23 (154) | 668 | 0.28 |
| Cyclosporine trough level (ng/mL), median (IQR) | 74 (61-105) | 46 | 67 (53-81) | 150 | 0.11 |
| mTOR inhibitor, % (n) | 7 (3) | 46 | 4 (27) | 668 | 0.43 |
| Belatacept, % (n) | 2 (1) | 46 | 3 (22) | 668 | >0.999 |
| Antimetabolite, % (n) | 80 (37) | 46 | 91 (606) | 664 | 0.03 |
| Kidney function at screening | |||||
| Protein-creatinine ratio (mg/g), median (IQR) | 290 (84-1054) | 46 | 149 (87-351) | 544 | 0.09 |
| eGFR (mL/minute/1.73 m2), median (IQR) | 32 (25-44) | 46 | 43 (33-52) | 465 | 0.001 |
ABMR, antibody-mediated rejection; IQR, interquartile range; mTOR, mechanistic target of rapamycin; eGFR, estimated glomerular filtration rate.
TTV load in relation to baseline characteristics
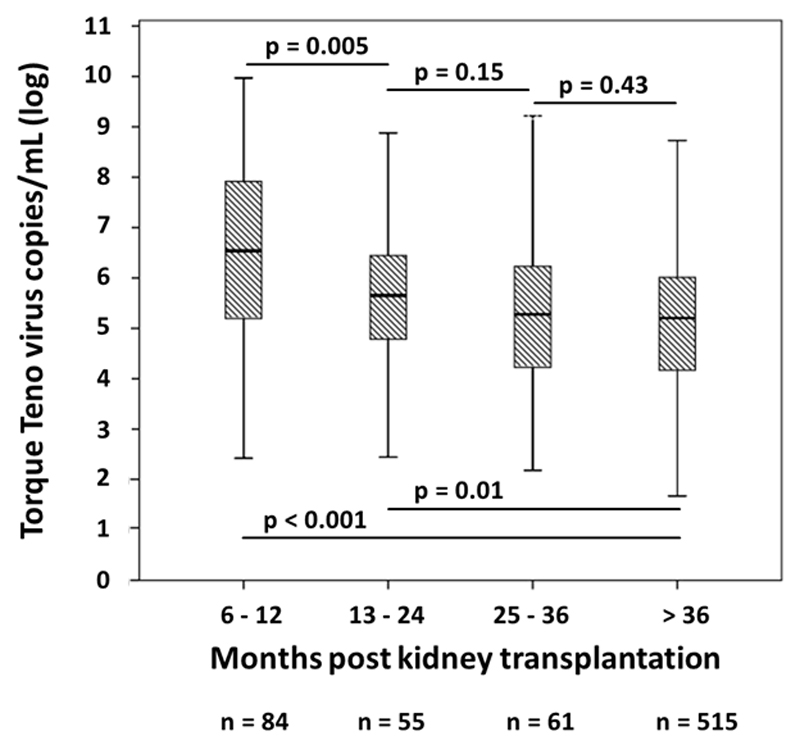
TTV DNA was detected in 678 patients (95%) with a median of 2.3x105 copies/mL (IQR 1.9x104-1.9x106). As illustrated in Figure 1, TTV load was highest in patients screened 6 to 12 months after transplantation, with a median of 3.5x106 copies/mL (IQR 1.4x105-8.7x107). While we observed a stepwise decrease of viral load in patients screened after two [4.5x105 (IQR 4.7x104-3.0x106)] and three years [1.9x105 (IQR 1.5x104-1.8x106)], there was no further decline at later time points. As shown in Table 3, TTV load was higher in male subjects and recipients of an ABO-incompatible transplant and associated with older recipient age and higher HLA mismatch. In addition TTV load was associated with higher eGFR at the time of screening (Table 4).
Figure 1.
TTV load in peripheral blood in relation to time after kidney transplantation. Box plots indicate median, IQR, and range.
Table 3.
TTV level in relation to baseline patient characteristics
| TTV copies/mL, median (IQR) |
|||
|---|---|---|---|
| Characteristics | Yes | No | p-value |
| Recipient data | |||
| Age at transplantation >mediana | 2.5x105 (2.2x104-2.3x106) | 2.1x105 (1.5x104-1.7x106) | 0.01 |
| Female | 1.4x105 (6.7x103 -1.7x106) | 2.7x105 (2.5x104-2.0x106) | 0.02 |
| Previous kidney transplantation | 1.2x105 (7.0x103-2.3x106) | 2.5x105 (2.1x104-1.8x106) | 0.23 |
| Donor data | |||
| Age >mediana | 2.4x105 (1.7x104-2.4x106) | 2.1x105 (1.9x104-1.4x106) | 0.23 |
| Non-heart beating donor | 7.4x104 (2.7x104-2.3x106) | 2.4x105 (1.9x104-1.9x106) | 0.71 |
| Living donor | 2.8x105 (1.4x104-4.5x106) | 2.1x105 (1.9x104-1.7x106) | 0.35 |
| Extended criteria donor | 2.9x105 (2.1x104-3.3x106) | 2.2x105 (1.9x104-1.7x106) | 0.44 |
| Transplant data | |||
| HLA (A, B, DR) mismatch >mediana | 7.0x105 (5.1x104-1.2x107) | 1.6x105 (1.6x103-1.1x106) | <0.001 |
| ABO incompatibility | 1.5x106 (1.1x105-2.1x107) | 2.1x105 (1.9x104-1.8x106) | 0.03 |
| CDCXM conversion | 8.3x105 (7.3x102-7.8x106) | 2.9x105 (1.9x104-1.8x106) | 0.50 |
| Preformed DSA | 3.0x105 (1.6x104-3.1x106) | 3.7x105 (2.8x104-7.1x106) | 0.97 |
| Current CDC-PRA >10% | 1.3x105 (5.9x104-2.6x106) | 2.3x105 (1.9x104-1.9x106) | 0.67 |
| Cold ischemia time >mediana | 1.6x105 (1.6x104-1.2x106) | 2.4x105 (2.2x104-2.2x106) | 0.27 |
| Delayed graft function | 2.8x105 (1.7x104-2.1x106) | 2.2x105 (1.9x104-1.9x106) | 0.90 |
TTV, Torque Teno virus; IQR, interquartile range; CDCXM, complement dependent cytotoxicity cross match; CDC-PRA, complement dependent cytotoxicity panel reactive antibody; DSA, donor specific antibodies;.
For continuous data the median was selected as cut off value to define positivity (recipient age >48 years, donor age >49 years, >3 HLA mismatches, cold ischemia time >13 hours).
Table 4.
TTV load in relation to immunosuppression and kidney function
| Variables | TTV copies/mL, median (IQR) |
p-value | |
|---|---|---|---|
| Yes | No | ||
| Initial immunosuppression | |||
| Induction therapy | 3.1x105 (2.6x104-3.7x106) | 1.4x105 (9.8x103-1.0x106) | <0.001 |
| Tacrolimus | 2.9x105 (2.2x104-3.1x106) | 1.4x105 (1.3x104-9.5x105) | 0.002 |
| Cyclosporine | 1.3x105 (7.7x103-6.2x105) | 3.0x105 (2.2x104-4.4x106) | <0.001 |
| mTOR inhibitor | 3.9x104 (3.6x103-5.7x105) | 2.4x105 (2.0x104-1.9x106) | 0.03 |
| Belatacept | 9.5x106 (2.1x106-6.4x107) | 2.1x105 (1.8x104-1.6x106) | <0.001 |
| Mycophenolic acid | 2.6x105 (1.9x104-2.5x106) | 1.6x105 (8.2x103-8.7x105) | 0.39 |
| Immunosuppression at screening | |||
| Triple immunosuppression | 2.8x105 (2.2x104-2.6x106) | 1.2x105 (1.2x104-7.4x105) | 0.001 |
| Steroid | 2.7x105 (2.1x104-2.4x106) | 1.3x105 (1.4x104-6.0x105) | 0.01 |
| Tacrolimus | 2.8x105 (2.3x104-2.2x106) | 1.4x105 (1.0x104-1.2x106) | 0.01 |
| Tacrolimus through level >mediana | 2.8x105 (2.2x104-1.7x106) | 2.8x105 (2.2x104-2.2x106) | 0.20 |
| Cyclosporine | 1.0x105 (7.0x103-5.4x105) | 2.9x105 (2.2x104-2.6x106) | <0.001 |
| Cyclosporine through level >mediana | 9.5x104 (7.5x103-8.3x105) | 1.3x105 (6.7x103-5.2x105) | 0.46 |
| mTOR inhibitor | 6.4x104 (6.4x103-2.8x106) | 2.4x105 (2.1x104-1.9x106) | 0.24 |
| Belatacept | 1.0x107 (3.1x106-2.6x108) | 2.1x105 (1.9x104-1.6x106) | <0.001 |
| Antimetabolite | 2.4x105 (2.1x104-2.2x106) | 1.0x105 (3.9x103-7.7x105) | 0.03 |
| Kidney function at screening | |||
| Protein-creatinine ratio >mediana | 2.1x105 (1.8x104-1.7x106) | 2.5x105 (2.3x104-1.9x106) | 0.40 |
| eGFR >mediana | 2.7x105 (3.7x104-2.4x106) | 1.6x105 (7.9x103-1.9x106) | 0.003 |
TTV, Torque Teno virus; IQR, interquartile range; mTOR, mechanistic target of rapamycin; eGFR, estimated glomerular filtration rate.
For continuous data the median was selected as cut off value to define positivity (tacrolimus level >6 ng/mL, cyclosporine >67 ng/mL, protein-creatinine ratio ratio >154 mg/g, eGFR >42 mL/minute/1.73 m2).
TTV load in relation to immunosuppressive therapy
As shown in Table 4, antibody induction therapy and tacrolimus-based initial immunosuppression were associated with twofold higher TTV levels. In contrast, TTV levels were significantly lower in patients on cyclosporine- or mTOR inhibitor-based treatment. A remarkable finding was that initial treatment with belatacept was associated with a 45-fold increase in viral load. Analyzing immunosuppressive therapy at the time of screening, we found that patients on triple immunosuppression had twofold higher TTV levels compared to patients with dual immunosuppression or single therapy, and lower levels were detected in patients who were off steroids or antimetabolite. Again, tacrolimus was associated with higher and cyclosporine with lower levels of TTV, without any relationship to trough levels. Finally, TTV load in patients on belatacept at the time of screening was 35 times higher.
Subgroup analyses of patients treated with costimulation blockade revealed a higher percentage of living donor recipients among patients on belatacept at the time of transplantation (45% vs. 19%, p = 0.009). Patients treated with belatacept initially or at the time of screening more often received induction therapy (95% vs. 56%; p < 0.001 and 100% vs. 54%; p < 0.001). Finally patients on belatacept at the time of screening had a higher eGFR [54 mL/minute/1.73 m2 (IQR 36-60) vs. 42 mL/minute/1.73 m2 (IQR 32-51); p = 0.047].
TTV load in relation to humoral alloresponse
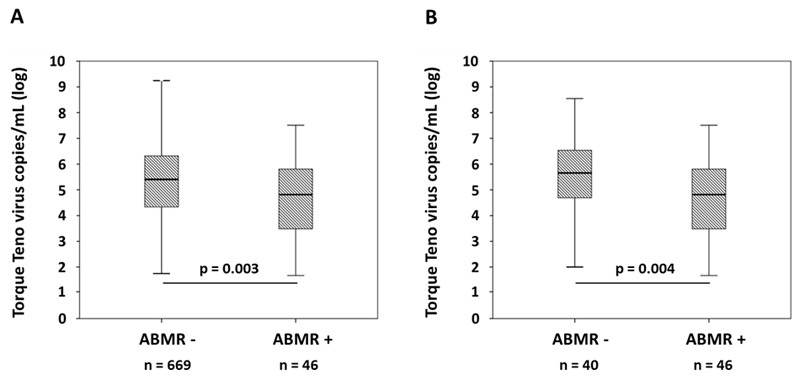
As shown in Figure 2, TTV load among ABMR+ recipients was 4-fold lower than in patients who had no ABMR [6.6x104 (IQR 3.0x103-7.2x105) vs. 2.6x105 (IQR 2.2x104-2.1x106); p = 0.003; Figure 2A]. A robust and linear inverse association between TTV load and ABMR was confirmed using score tests. Univariate logistic regression revealed a decrease in risk for ABMR of 0.91 per TTV log level [95% CI 0.87-0.96; p = 0.001]. Logistic regression models demonstrated that associations between TTV load and ABMR were independent of potential confounders. Similarly, also a generalized linear model revealed an inverse independent association with TTV log level and ABMR risk [RR 0.94 per TTV log level; 95% CI 0.90-0.99; p = 0.02; Table 5].
Figure 2.
TTV load in peripheral blood in relation to ABMR diagnosis (A: overall cohort; B: DSA+ recipients subjected to protocol biopsy). Box plots indicate median, IQR, and range.
Table 5.
Crude and multivariable adjusted risk ratio for ABMR (n = 715)
| Model | Risk Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Crude | |||
| TTV PCR (log) | 0.91 | 0.87-0.96 | 0.001 |
| Multivariable adjusted | |||
| TTV PCR (log) | 0.94 | 0.90-0.99 | 0.02 |
| Recipient female sex | 1.29 | 0.71-2.32 | 0.40 |
| Previous transplantation | 1.20 | 0.92-1.56 | 0.17 |
| CDCXM conversion | 4.78 | 2.94-7.78 | <0.001 |
| Current CDC-PRA >10% | 1.51 | 0.76-3.01 | 0.24 |
| eGFRa category 0 | Reference | ||
| eGFR category 1 | 0.38 | 0.16-0.87 | 0.02 |
| eGFR category 2 | 0.42 | 0.16-1.05 | 0.06 |
| Protein-creatinine ratiob category 0 | Reference | ||
| Protein-creatinine ratio category 1 | 0.98 | 0.39-2.45 | 0.96 |
| Protein-creatinine ratio category 2 | 2.03 | 0.92-4.46 | 0.08 |
TTV, Torque Teno virus; PCR, polymerase chain reaction; CDCXM, complement dependent cytotoxicity cross match; CDC-PRA, complement dependent cytotoxicity panel reactive antibody; eGFR, estimated glomerular filtration rate.
eGFR category 0, n = 172, 13.1-35.0 mL/minute/1.73 m2; category 1, n = 172, 35.1-48.2 mL/minute/1.73 m2; category 2, n = 173; 48.3-78.2 mL/minute/1.73 m2.
Protein-creatinine ratio category 0, n = 198, 36-107 mg/g; category 1, n = 204, 108-277 mg/g; category 2, n = 201; 278-1250 mg/g.
In a next step we focused on the subgroup of 86 patients subjected to protocol biopsy on the basis of a positive DSA result. A major finding was that only 46 (53%) of these recipients were diagnosed with ABMR and as many as 40 subjects (47%) did not meet the diagnostic criteria of this rejection type. Remarkably, as illustrated in Figure 2, TTV load in DSA+ABMR+ patients (n = 40) was by far lower than in DSA+ABMR- recipients [6.6x104(IQR 3.0x103-7.2x105) vs. 4.5x105 (IQR 4.3x104-3.9x106); p = 0.004; Figure 2B].
Multivariate analysis revealed a robust and linear inverse independent association between TTV load and ABMR (RR 0.96 per TTV log level; 95% CI 0.93-0.99; p = 0.01). Similarly, patients with a positive C4d staining in peritubular capillaries showed lower levels of TTV compared to C4d-negative patients [1.5x104 (IQR 1.7x102-1.4x106) vs. 2.7x105 (IQR 2.8x104-1.4x106); p = 0.04], regardless of the presence or absence of morphological ABMR features.
Finally, TTV load was found to inversely correlate with MFI of peak DSA (R = -0.27; p = 0.01), a marker, which in the studied cohort was shown to associate with ABMR diagnosis 16. Patients with DSA MFI above the median had lower TTV levels compared to patients with DSA MFI below the median [6.2x104 (IQR 2.3x102-9.6x105) vs. 3.7x105 (IQR 4.1x104-2.8x106); p = 0.007].
Discussion
The most important finding of our study was the inverse association of TTV load in the peripheral blood of kidney transplant recipients with the occurrence of late ABMR. A major strength of our study, which included a cross-sectional cohort of long-term transplant recipients subjected to a systematic rejection screening, is its large sample size, which allowed for a comprehensive multivariate analysis. Remarkably, even after adjustment for a variety of relevant confounders, TTV load remained independently associated with ABMR diagnosis. Our data support the results of an earlier smaller study suggesting a relationship between viral load and transplant rejection in thoracic organ transplant recipients 14.
Within a sensitivity analysis restricted to biopsied recipients, ABMR also associated with lower TTV levels in peripheral blood, providing further evidence for a role of TTV load in the detection of humoral alloresponses after kidney transplantation. Similarly, viral load inversely associated with C4d staining in peritubular capillaries, a marker that has been repeatedly shown to predict a more severe course of rejection 21,22. In this respect, it is important to note that TTV viral load was also associated with the fluorescence intensity of detected DSA, which might reflect the pathogenic potential of the alloresponse and predict the extent of antibody mediated graft injury 16,23.
Prevalence of TTV in our cohort was 95% with a median TTV load of 2.3x105 copies/mL. While similar results were obtained in liver transplant recipients, higher TTV levels were reported for lung allograft recipients, presumably a result of higher levels of immunosuppression 12,13,24. In our cross-sectional analysis we found the highest TTV levels in recipients screened after 6 to 12 months posttransplantation, with a stepwise decrease in levels at later time points. Dynamics of TTV load over time might reflect changing levels of immunosuppression. In this respect, our data are consistent with earlier studies. For example in patients after lung transplant peak levels of TTV were found between day 30 and 90 posttransplant with a decrease thereafter and in a cohort of patients after liver transplant including long term data up to year 15 TTV load constantly declined over time 12,24.
In accordance with previous studies performed in healthy individuals 25,26, analysis of our cohort revealed higher TTV levels in older subjects. This might be explained by immunologic senescence, with less ability of the elderly to cope with viral replication and infections 27. Moreover, in line with earlier results obtained in nontransplant cohorts, TTV load was found to be lower in female recipients 25,26. In this context, a more effective adaptive immune response to viral pathogens in women was suggested, which might be promoted by estrogen 28. In addition higher levels were detected in patients with a high HLA mismatch. Similar associations were described in kidney transplant patients with polyoma viraemia 29. A higher HLA mismatch might lead to a reduced virus clearance by escape of virus-specific immunity due to the recipient MHC restriction of the T cell response 30.
A major finding was that TTV load associated with the intensity of immunosuppressive regimen at the time of transplantation and rejection screening, respectively. Recipients who had been subjected to induction and or desensitization therapy and patients on triple immunosuppression and steroids at the time of screening showed higher levels of TTV. Similarly, higher levels were detected in recipients of an ABO-incompatible transplant, presumably an indirect consequence of intensified immunosuppression in these distinct patient cohorts. Notably, our study could not establish an association between TTV load and calcineurin inhibitor trough levels, which might be in contrast to a smaller study in lung and heart transplant patients showing a relationship between tacrolimus trough levels at early time points and a relative shift in genomic abundance within the virome towards TTV 14.
In addition, our results provide evidence that TTV load might be related to the type of immunosuppression. In line with a recent study performed in lung transplant recipients, we detected significantly higher TTV levels in patients on tacrolimus- and lower levels in patients on cyclosporine-based immunosuppression 12. One might speculate that these data reflect the higher immunosuppressive potential of tacrolimus as suggested by the results of large systematic intervention trials 31. Remarkably, mTOR inhibitor-based immunosuppression was associated with comparably low TTV levels. This might be of particular interest considering the increased risk of rejection reported from mTOR inhibitor use 32,33. Notably, mounting evidence indicates that mTOR inhibitors might decrease the incidence of cytomegalovirus infection in solid-organ recipients 34,35 and 1 might speculate that there could also be an additional direct effect of this compound on TTV replication. Interestingly, the highest levels of TTV were observed in patients on belatacept-based immunosuppression. Belatacept is well established to preserve kidney function and, as suggested by a recent analysis of long-term results of the BENEFIT trial, improve transplant survival 36. Moreover, in support of its high immunosuppressive potential, belatacept was shown to prevent the formation of DSA, an effect that might have considerably contributed to favorable outcomes 36. Potent suppression of immune surveillance by costimulation blockade might at least in part have contributed to a markedly enhanced TTV replication observed in our cohort. There is emerging evidence, that costimulation via CD28 might be crucial for generation of antiviral T cells 37. One might speculate that co-stimulation blockade might have led to insufficient formation of TTV specific T cells and thus directly influenced viral control in patients with belatacept-based immunosuppression. Finally, a more intense immunosuppression due to an increased rate of induction therapy in the subgroup of patients treated with belatacept might have at least partly contributed to higher TTV levels.
Calcineurin inhibitor trough levels at the time of ABMR screening did not associate with rejection diagnosis. We are aware that our study, which did not include details regarding cumulative dosage or longitudinal concentration measurements, was not designed to determine the predictive value of calcineurin inhibitor trough level monitoring. Likewise the study design did not allow testing the predictive value of TTV load. However, our results might provide a valuable basis for future prospective studies designed to clarify the value of longitudinal TTV load assessment in predicting ABMR occurrence. Further studies will have to assess the independent value of TTV quantification as a tool for guidance of individualized immunosuppressive treatment. Factors that would support the use of TTV detection as a monitoring tool are the high prevalence of TTV infection in human populations, as well as the noninvasive, PCR-based detection method, which allows for sufficient standardization between centers.
Our study has several limitations. First and most importantly, TTV was analyzed as a single snap shot at heterogeneous time points and data on TTV dynamics preceding ABMR diagnosis are not available. Moreover, our study design does not prove a causative relationship between the intensity of immunosuppression and levels of TTV replication. Longitudinal assessment of TTV load in individual patients and prospective protocols of TTV guided immunosuppression are needed to determine whether TTV quantification has any advantage over currently available monitoring strategies. Secondly, we did not include screening for non-HLA antibodies and only DSA+ patients were subjected to a protocol biopsy. One might speculate that our strategy might have led to some underestimation of ABMR prevalence in our cohort. Thirdly, screening was restricted to patients with a functioning graft more than 6 months after kidney transplantation and no acute deterioration of graft function. Accordingly, we are not able to provide data on the relationship between TTV load and early rejection or rejection associated with a rapid deterioration of kidney function. Finally, our study did not establish an association between TTV load and cellular rejection. With cellular rejection being the prominent rejection type early after kidney transplantation while rarely described in late biopsies, it is not surprising that T cell-mediated rejection was diagnosed in none of our biopsied patients.
In conclusion, our data demonstrate an independent association between TTV load and late ABMR in recipients of a kidney transplant. Future studies might investigate monitoring of TTV load for risk prediction of ABMR and TTV guided immunosuppression to prevent antibody-mediated graft injury.
Acknowledgments
The authors wish to thank Lena Marinova and Daniela Böhm for excellent technical assistance.
Funding
This work was funded by a grant of the major of the city of Vienna (to GB; grant No. 14058) and by a grant from the Austrian Science Fund (to GAB; grant No. KLI 190). This study was carried out with support from the Medical University Vienna Biobank.
Abbreviations
- ABMR
antibody-mediated rejection
- CDC
complement-dependent cytotoxicity
- CI
confidence interval
- DSA
donor-specific antibody
- eGFR
estimated glomerular filtration rate
- IQR
inter quartile range
- MFI
median fluorescence intensity
- MPA
mycophenolic acid
- mTOR
mechanistic target of rapamycin
- PCR
polymerase chain reaction
- PRA
panel reactive antibody
- RR
risk ratio
- TTV
Torque Teno virus
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Tranplant. 2011;11(3):450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 2.Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev. 2012;8(6):348–357. doi: 10.1038/nrneph.2012.81. [DOI] [PubMed] [Google Scholar]
- 3.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Tranplant. 2012;12(2):388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe C, Nickerson P. Posttransplant monitoring of de novo human leukocyte antigen donor-specific antibodies in kidney transplantation. Curr Opin Organ Transplant. 2013;18(4):470–477. doi: 10.1097/MOT.0b013e3283626149. [DOI] [PubMed] [Google Scholar]
- 5.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Tranplant. 2014;14(2):272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 6.Cravedi P, Heeger PS. Immunologic monitoring in transplantation revisited. Curr Opin Organ Transplant. 2012;17(1):26–32. doi: 10.1097/MOT.0b013e32834ee402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T, Vaisanen E, Mattila PS, Hedman K, Soderlund-Venermo M. Antigenic diversity and seroprevalences of Torque teno viruses in children and adults by ORF2-based immunoassays. J Gen Virol. 2013;94(Pt 2):409–417. doi: 10.1099/vir.0.046862-0. [DOI] [PubMed] [Google Scholar]
- 8.Rocchi J, Ricci V, Albani M, et al. Torquetenovirus DNA drives proinflammatory cytokines production and secretion by immune cells via toll-like receptor 9. Virology. 2009;394(2):235–242. doi: 10.1016/j.virol.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto H. History of discoveries and pathogenicity of TT viruses. Curr Top Microbiol Immunol. 2009;331:1–20. doi: 10.1007/978-3-540-70972-5_1. [DOI] [PubMed] [Google Scholar]
- 10.Burra P, Masier A, Boldrin C, et al. Torque Teno Virus: any pathological role in liver transplanted patients? Transplant Int. 2008;21(10):972–979. doi: 10.1111/j.1432-2277.2008.00714.x. [DOI] [PubMed] [Google Scholar]
- 11.Moen EM, Sagedal S, Bjoro K, Degre M, Opstad PK, Grinde B. Effect of immune modulation on TT virus (TTV) and TTV-like-mini-virus (TLMV) viremia. J Med Virol. 2003;70(1):177–182. doi: 10.1002/jmv.10356. [DOI] [PubMed] [Google Scholar]
- 12.Gorzer I, Haloschan M, Jaksch P, Klepetko W, Puchhammer-Stockl E. Plasma DNA levels of Torque teno virus and immunosuppression after lung transplantation. J Heart Lung Transplant. 2014;33(3):320–323. doi: 10.1016/j.healun.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Gorzer I, Jaksch P, Kundi M, Seitz T, Klepetko W, Puchhammer-Stockl E. Pre-transplant plasma Torque Teno virus load and increase dynamics after lung transplantation. PloS one. 2015;10(3):e0122975. doi: 10.1371/journal.pone.0122975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vlaminck I, Khush KK, Strehl C, et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155(5):1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskandary F, Bond G, Schwaiger E, et al. Bortezomib in late antibody-mediated kidney transplant rejection (BORTEJECT Study): study protocol for a randomized controlled trial. Trials. 2014;15:107. doi: 10.1186/1745-6215-15-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskandary F, Bond G, Kozakowski N, et al. Diagnostic Contribution of Donor-Specific Antibody Characteristics to Uncover Late Silent Antibody-Mediated Rejection-Results of a Cross-Sectional Screening Study. Transplantation. A2016 doi: 10.1097/TP.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Int Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Maggi F, Pifferi M, Fornai C, et al. TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity. J Virol. 2003;77(4):2418–2425. doi: 10.1128/JVI.77.4.2418-2425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartel G, Wahrmann M, Regele H, et al. Peritransplant immunoadsorption for positive crossmatch deceased donor kidney transplantation. Am J Tranplant. 2010;10(9):2033–2042. doi: 10.1111/j.1600-6143.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwaiger E, Eskandary E, Kozakowski N, et al. Deceased donor kidney transplantation across donor-specific antibody barriers: predictors of antibody-mediated rejection. Nephrol Dial Transplant. 2016 Mar 24; doi: 10.1093/ndt/gfw027. [published online ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Sapir-Pichhadze R, Curran SP, John R, et al. A systematic review of the role of C4d in the diagnosis of acute antibody-mediated rejection. Kidney Int. 2015;87(1):182–194. doi: 10.1038/ki.2014.166. [DOI] [PubMed] [Google Scholar]
- 22.Kikic Z, Kainz A, Kozakowski N, et al. Capillary C4d and Kidney Allograft Outcome in Relation to Morphologic Lesions Suggestive of Antibody-Mediated Rejection. Clin J Am Soc Nephrol. 2015;10(8):1435–1443. doi: 10.2215/CJN.09901014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizutani K, Terasaki P, Hamdani E, et al. The importance of anti-HLA-specific antibody strength in monitoring kidney transplant patients. Am J Tranplant. 2007;7(4):1027–1031. doi: 10.1111/j.1600-6143.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 24.Beland K, Dore-Nguyen M, Gagne MJ, et al. Torque Teno virus in children who underwent orthotopic liver transplantation: new insights about a common pathogen. J Infect Dis. 2014;209(2):247–254. doi: 10.1093/infdis/jit423. [DOI] [PubMed] [Google Scholar]
- 25.Haloschan M, Bettesch R, Gorzer I, Weseslindtner L, Kundi M, Puchhammer-Stockl E. TTV DNA plasma load and its association with age, gender, and HCMV IgG serostatus in healthy adults. Age. 2014;36(5):9716. doi: 10.1007/s11357-014-9716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brassard J, Gagne MJ, Leblanc D, et al. Association of age and gender with Torque teno virus detection in stools from diarrheic and non-diarrheic people. J Clin Virol. 2015;72:55–59. doi: 10.1016/j.jcv.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Pera A, Campos C, Lopez N, et al. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas. 2015;82(1):50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nature reviews Endocrinology. 2013;9(1):56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 29.Hassig A, Roos M, Etter A, et al. Association of BK viremia with human leukocyte antigen mismatches and acute rejection, but not with type of calcineurin inhibitor. Transplant Infect Dis. 2014;16(1):44–54. doi: 10.1111/tid.12153. [DOI] [PubMed] [Google Scholar]
- 30.Han Lee ED, Kemball CC, Wang J, et al. A mouse model for polyomavirus-associated nephropathy of kidney transplants. Am J Tranplant. 2006 May;6(5 Pt 1):913–922. doi: 10.1111/j.1600-6143.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- 31.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007 Dec 20;357(25):2562–2575. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 32.Lim WH, Eris J, Kanellis J, et al. A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. Am J Tranplant. 2014;14(9):2106–2119. doi: 10.1111/ajt.12795. [DOI] [PubMed] [Google Scholar]
- 33.Liefeldt L, Brakemeier S, Glander P, et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Tranplant. 2012;12(5):1192–1198. doi: 10.1111/j.1600-6143.2011.03961.x. [DOI] [PubMed] [Google Scholar]
- 34.Nashan B, Gaston R, Emery V, et al. Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor-based immunosuppressive therapy in de novo renal transplant recipients. Transplantation. 2012;93(11):1075–1085. doi: 10.1097/TP.0b013e31824810e6. [DOI] [PubMed] [Google Scholar]
- 35.Poglitsch M, Weichhart T, Hecking M, et al. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am J Tranplant. 2012;12(6):1458–1468. doi: 10.1111/j.1600-6143.2012.04002.x. [DOI] [PubMed] [Google Scholar]
- 36.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374(4):333–343. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 37.Christensen JE, Christensen JP, Kristensen NN, Hansen NJ, Stryhn A, Thomsen AR. Role of CD28 co-stimulation in generation and maintenance of virus-specific T cells. Int Immunol. 2002;14(7):701–711. doi: 10.1093/intimm/dxf037. [DOI] [PubMed] [Google Scholar]