Abstract
Objective
In humans, apolipoprotein (apo) E4 is associated with elevated plasma cholesterol levels and a high risk of developing atherosclerosis, whereas apoE2 is protective. Here we investigate the mechanism by which mice expressing human apoE isoforms recapitulate this association when they also express high levels of human low-density lipoprotein receptor (LDLR).
Methods and Results
Primary hepatocytes from apoE4 mice secreted less apoE into the medium than hepatocytes from apoE2 mice. Increased LDLR expression decreased this secretion and increased degradation of apoE4. An apoE4-GFP fusion protein expressed in the liver of apoE-deficient mice accumulated on the hepatocyte surface bordering the space of Disse in an LDLR-dependent manner. Fluorescence-labeled very low–density lipoprotein (VLDL) remnants accumulated on the hepatocyte surface in apoE4 mice with high LDLR, but they were internalized poorly. In contrast, apoE2-GFP did not accumulate on the hepatocyte surface even when the LDLR expression was high, but apoE2 mice with high LDLR internalized the remnants avidly without sequestering them on the hepatocyte surface.
Conclusions
The high affinity of apoE4 to the LDLR enhances VLDL sequestration on the hepatocyte surface but delays their internalization. This delay likely increases VLDL conversion to cholesterol-enriched remnants in apoE4 mice with high LDLR, and probably to LDL in humans with apoE4.
Keywords: mouse models, lipoprotein metabolism, space of Disse, recombinant adenovirus
A polipoprotein E (apoE) and the low-density lipoprotein receptor (LDLR) play a pivotal role in the clearance of lipoproteins by the liver, thereby reducing plasma cholesterol, a leading determinant of atherosclerosis susceptibility.1,2 Uptake of triglyceride-rich lipoproteins (TRL) occurs via multiple receptors and perhaps in several steps.3 The first, most important step appears to be the sequestration of TRL on the microvilli of hepatocytes, where LDLR, heparan sulfate proteoglycans (HSPG), and LDLR related proteins (LRP) are located. These molecules bind apoE proteins secreted by the liver thereby serving as reservoirs for enriching remnant particles with apoE.4 They also serve as the receptors for internalization of apoE-enriched remnants.3
In humans, the Apoe gene is polymorphic, resulting in production of 3 common isoforms, apoE2, E3, and E4. They differ in primary structure at 2 positions, E2 having Cys at both positions 112 and 158, E3 having a Cys at 112 and an Arg at 158, and E4 having Arg at both positions. They also differ in their LDLR binding affinity; apoE4 binds LDLR with a slightly higher affinity than apoE3, whereas apoE2 has much reduced binding compared to the other 2 isoforms.5–9 Despite the low receptor binding of apoE2, the majority of individuals carrying apoE2 have lower plasma LDL cholesterol and reduced atherosclerosis risk, although 5% to 10% of apoE2 homozygotes develop type III hyperlipoproteinemia characterized by markedly elevated plasma lipid levels.2,10 Equally paradoxical is the association of the apoE4 isoform with high LDL-cholesterol, low plasma triglycerides (TG), and an increased risk of atherosclerosis.1,2,10–14 How the different apoE isoforms lead to different plasma lipoprotein profiles in vivo remains unclear, and mice with the wild-type Apoe gene replaced with human alleles do not simply replicate human phenotypes. Thus, all mice expressing apoE2 (Apoe2/2) exhibit type III hyperlipoproteinemia and develop atherosclerosis even on a normal chow containing low cholesterol and low fat, whereas those expressing apoE3 (Apoe3/3) or apoE4 (Apoe4/4) are normolipidemic and do not develop atherosclerosis.7,15,16 However, the human-like associations are replicated when the mice expressing human apoE isoforms also have 2- to 3-fold normal LDLR expression attributable to the Ldlr*h allele coding for human LDLR.17–20 Both adenovirus-mediated or global overexpression of the human LDLR in mice with apoE2 results in reduction of plasma cholesterol and TG and the absence of atherosclerosis.17,21 Mice with human apoE3 and the Ldlr*h allele (Apoe3/3Ldlrh/+) have significantly decreased HDL-cholesterol but only a small increase in remnants and do not develop atherosclerosis.18 In contrast, on a high-fat Western type diet, mice with apoE4 overexpressing the LDLR (Apoe4/4Ldlrh/+) have increased plasma very low density lipoproteins (VLDL)/chylomicron remnants, decreased HDL-cholesterol levels, and develop atherosclerosis.18
The present work was aimed to test the hypothesis that the adverse effects of the increased LDLR expression in mice with apoE4 is because the higher affinity of apoE4 for the LDLR inhibits enrichment of apoE4 on apoE-poor VLDL. We show that a substantial amount of apoE4, and to a lesser extent apoE3, but not apoE2, is colocalized with LDLR on the surface of hepatocytes. This interaction with the LDLR increases the association of apoE4 with hepatocytes, limits apoE4 secretion, and enhances its degradation in primary cultured hepatocytes. It also enhances the sequestration of VLDL remnants on the hepatocyte surface, but not their internalization.
Methods
Mice
ApoE-deficient mice (Apoe−/−), LDLR-deficient mice (Ldlr−/−), mice homozygous for replacement of the mouse apoE gene with either the human APOE*2, APOE*3, or APOE*4 allele (Apoe2/2, Apoe3/3, and Apoe4/4), and mice overexpressing the human LDLR minigene (Ldlrh/+) were individually backcrossed at least 6 generations to C57BL/6 genetic background.7,15–17 Intercross of these mutants produced mice with various combinations of the Apoe and Ldlr loci. Mice were fed a high-fat Western-type diet (HFW) containing 21% (wt/wt) fat and 0.2% (wt/wt) cholesterol (TD88137; Teklad) for at least 2 weeks before experiments. Genotype and lipid profiles of experimental mice are presented in supplemental Table I and supplemental Figure I (available online at http://atvb.ahajournals.org). The animals were handled under protocols approved by the Institutional Animal Care and Use Committees of the University of North Carolina-Chapel Hill. For additional details on methods, please refer to the supplemental materials (available online at http://atvb.ahajournals.org).
35S Labeling of Primary Mouse Hepatocytes
Primary hepatocytes were isolated as described.22 The cells were plated onto 60-mm mouse collagen IV-coated dishes (Falcon) and pulsed with 0.5 mL medium containing 35S methionine (100 μCi/mL, Amersham) for 30 or 60 minutes, and chased for 1 and 4 hours with fresh medium with excess cold methionine. ApoE was immunoprecipitated using a goat antihuman apoE antibody (Calbiochem), separated by SDS-PAGE, and visualized by a Fla-3000 phosphoimager (FujiFilm).
Adenoviruses
The plasmid vectors containing cytomegalus viral promoter-driven cDNA for fusion proteins, apoE2-GFP, apoE3-GFP, and apoE4-GFP, were provided by Dr Robert DeKroon at Duke University (Durham, NC). These vectors express fusion proteins with EGFP (enhanced green fluorescent protein) attached to the C-terminal end of each apoE isoform.23 Adenoviral vectors encoding apoE2-GFP, apoE3-GFP, and apoE4-GFP were made using the AdEasy adenoviral system (Stratagene). Recombinant adenovirus stock, stored at −80°C, was diluted with PBS and 1×109 PFU in 0.2 mL of adenovirus was injected into each mouse via the tail vein.
Isolation, DiI Labeling, and Injection of VLDL
The VLDL fraction was isolated from pooled plasma by ultracentrifugation at d <1.006 g/mL and labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI C18; Molecular Probes Inc), as described by Stephan and Yurachek.24 DiI-labeled VLDL (100 μg protein) was injected into tail veins of mice and livers were fixed with 4% paraformaldehyde 20 minutes later. DiI-labeled VLDL remaining in the plasma at 2 minutes, 10 minutes, and 20 minutes was determined using a microscope fluorometer (Olympus FV500 with a SPOT 2 digital camera) using a modification of the fluorometric procedure described.25
Microscopic Analyses
Livers were perfused through the portal vein at 2 mL/min for 2 minutes with 4% paraformaldehyde, excised from animals, and further fixed overnight in 4% paraformaldehyde. Slides with consecutive liver paraffin sections (5 μm) were used for immunohistochemistry. For confocal analysis 100-μm-thick liver sections were cut with a vibratome.
Statistical Analysis
The significance of differences between means was determined by use of 1-way ANOVA and Turkey-Kramer honestly significant difference tests (JMP software; SAS Inc).
Results
Secretion, Synthesis, and Degradation of ApoE in Primary Hepatocytes
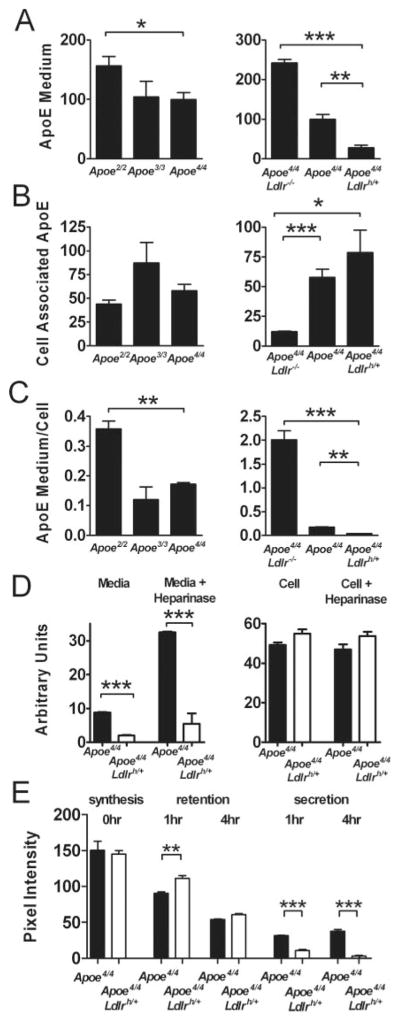
To determine the cellular metabolism of the different apoE isoforms, we isolated primary hepatocytes from mice expressing different apoE isoforms and compared the apoE protein using Western blot analysis. After culturing in DMEM without FBS for 24 hours, primary hepatocytes from Apoe2/2 mice secreted more apoE protein into the medium compared to the cells from Apoe4/4 mice (Figure 1A). The ratio of medium apoE to cell-associated apoE in the Apoe2/2 cells was twice as high as those of Apoe3/3 or Apoe4/4 cells (Figure 1C). In addition, the level of LDLR expression affected the amount of apoE secreted from the cultured primary hepatocytes (Figure 1A). The ratio of medium apoE to cell-associated apoE in the cultured Apoe4/4Ldlrh/± hepatocytes was significantly lower than in Apoe4/4 cells (Figure 1C). In turn, the ratio in hepatocytes lacking LDLR (Apoe4/4 Ldlr−/−) expression was significantly higher than in Apoe4/4 hepatocytes (Figure 1C). Heparinase treatment increased apoE4 in the medium in both Apoe4/4Ldlrh/+ and Apoe4/4 hepatocytes, but the apoE4 secretion from the Apoe4/4Ldlrh/+ remained significantly depressed (Figure 1D). These data indicate that the relative amount of apoE secretion from the liver is inversely related to the affinity of the isoforms to the receptor as well as to the levels of LDLR expression.
Figure 1.
A, Secretion of apoE from primary hepatocytes isolated from Apoe2/2, Apoe3/3, Apoe4/4 (left), and from Apoe4/4Ldlr−/−, Apoe4/4, Apoe4/4Ldlrh/+ (right) mice into the culture medium. Cell-associated apoE (B) and the ratio of apoE in the medium/cell-associated (C) from the above primary hepatocytes. D, Effects of heparinase on apoE secretion from Apoe4/4 and Apoe4/4Ldlrh/+ primary hepatocytes. E, Pulse chase analysis of Apoe4/4 and Apoe4/4 Ldlrh/+ hepatocytes. 35S-Methionine in cell-associated apoE (synthesis, time 0) during a 30-minute pulse and after 1-hour and 4-hour chase (retention) and in medium (secretion) was determined using SDS-PAGE and phosphoimager. Values shown are the average of 3 wells. The experiment was repeated with a similar result.
**P≤0.005 and ***P≤0.0005.
The ratio of extracellular to cell-associated apoE amounts is likely determined by the uptake, but could also be attributable to changes in apoE synthesis or degradation. To determine how the LDLR expression levels affect production and degradation of apoE, we used a pulse chase system in primary hepatocytes isolated from Apoe4/4 and Apoe4/4Ldlrh/+ mice. The synthesis during a 30 minute (Figure 1E) pulse was not significantly different between Apoe4/4 (pixel intensity of 150.2±12.4) and Apoe4/4Ldlrh/+ (144.8±5.1) hepatocytes, indicating that a 2.5× higher LDLR expression had no effect on the apoE production rate. Cell-associated apoE retained after a 1-hour chase was more in Apoe4/4Ldlrh/+ cells than in Apoe4/4 cells, but they were not significantly different at 4 hours. However, consistent with the observation described above, apoE4 secreted from Apoe4/4Ldlrh/+ cells into the medium was significantly less than that from Apoe4/4 cells. The sum of the secreted and cell-retained apoE4 in Apoe4/4 and Apoe4/4Ldlrh/+ cells was 81% and 84% at 1 hour and 61% and 44% at 4 hours, respectively, of the initial synthesis suggesting that an elevated LDLR expression also increases apoE4 degradation. The results of experiments with a 60 minute pulse were very similar (supplemental Figure II).
Localization of ApoE Isoforms in the Liver
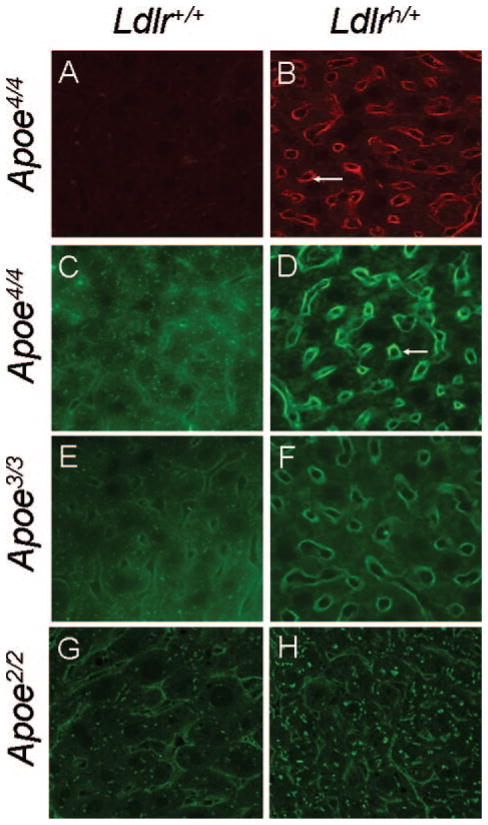
Immunostaining with an antibody against huLDLR illustrated the sinusoidal localization of the huLDLR in the liver sections of Apoe4/4Ldlrh/+ but not in Apoe4/4 mice (Figure 2A, 2B, and supplemental Figure III). ApoE4 colocalized with the LDLR in the Apoe4/4Ldlrh/+ liver as highlighted by the very intense staining of sinusoids with antibody against human apoE (Figure 2D). In contrast, apoE4 staining in the Apoe4/4 liver was diffuse and also present in the cytoplasm in a punctated pattern (Figure 2C). Very similar staining patterns were observed in the livers of Apoe3/3 and Apoe3/3Ldlrh/+ mice (Figure 2E and 2F), except that the sinusoidal staining relative to cytoplasmic staining was less intense in the Apoe3/3Ldlrh/+ liver than in the Apoe4/4Ldlrh/+ liver. This suggests that the LDLR levels influence sinusoidal localization of apoE4 and, to a lesser extent, of apoE3. Apoe2/2 and Apoe2/2Ldlrh/+ livers both had more pronounced intrahepatic staining than other apoE isoforms (Figure 2G and 2H), and the Apoe2/2Ldlrh/+ liver had more intracellular staining than the Apoe2/2 liver with no increase in sinusoidal staining. Total LDLR proteins in the membrane fraction of Ldlrh/+ livers detected by Western blots with antibodies specific for human or mouse LDLRs were similar regardless of apoE isoforms (supplemental Figure IV). Absolute amount of each LDLR was not determined.
Figure 2.
Immunohistochemical localization of apoE in the liver of mice. Anti-human LDLR staining in Apoe4/4 (A) and Apoe4/4 Ldlrh/+ (B). Staining for anti-human apoE in Apoe4/4 (C), Apoe4/4 Ldlrh/+ (D), Apoe3/3 (E), Apoe3/3Ldlrh/+ (F), Apoe2/2 (G), Apoe2/2Ldlrh/+ (H). (600X).
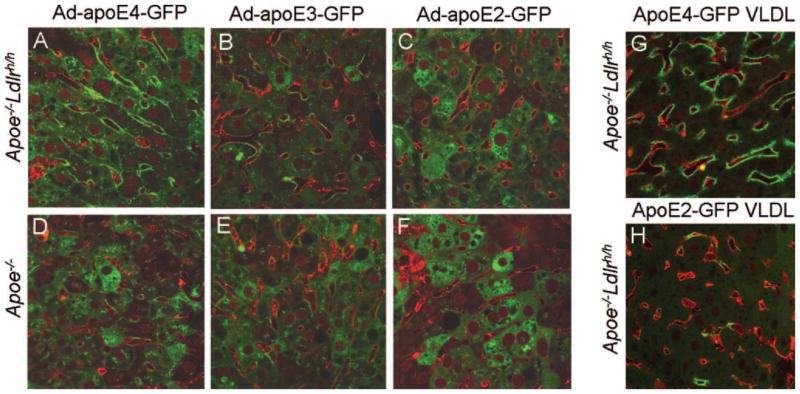
To confirm the hepatic localization of apoE isoforms and the effect of increased LDLR without using antibodies, we injected Ad-apoE4-GFP, Ad-apoE3-GFP, and Ad-apoE2-GFP into Apoe−/− mice. ApoE-GFP fusion proteins appear to function normally and retain isoform specific characteristics as all lowered cholesterol levels in Apoe−/− mice (supplemental Figure V). Using confocal microscopy of the liver sections stained with Alexafluor633-labeled lectin to demarcate liver sinusoids, endothelial cells, and the space of Disse (SD),26 the apoE-GFP fusion proteins were visible as bright green hepatocytes with perinuclear concentration in the cytoplasm of 70% to 80% of the cells (Figure 3A through 3F). Notably, apoE4-GFP and apoE3-GFP also demarked sinusoids. Inspection under a higher magnification revealed that GFP and Alexafluor633 labeled lectin signals were not overlapping (supplemental Figure VI), suggesting that the accumulation of apoE4-GFP is subendothelial in the SD, likely on the hepatocyte surface. The subendothelial accumulation of apoE4-GFP or apoE3-GFP was more pronounced in the liver of the Apoe−/−Ldlrh/h mice injected with Ad-apoE4-GFP or Ad-apoE3-GFP (Figure 3A and 3B). In marked contrast, Ad-apoE2-GFP showed little accumulation of apoE2-GFP on the hepatocyte surface even in the Apoe−/−Ldlrh/h mice (Figure 3C). These data demonstrate that the in vivo accumulation of apoE in the SD is dependent on its affinity to the LDLR and the expression levels of the LDLR.
Figure 3.
Localization of apoE-GFP fusion proteins in the liver. Ad-apoE4-GFP (A, D), Ad-apoE3-GFP (B, E), and Ad-apoE2-GFP (C, F) were injected into Apoe−/−Ldlrh/h (A, B, C) and Apoe−/− (D, E, F) mice. After 5 days liver sections (100 μm) were stained with Alexafluor633 labeled lectin (red), and GFP (green) signals were examined under a confocal microscopy (600×). VLDL isolated from Apoe−/− mice injected with Ad-apoE4-GFP or apoE2-GFP was injected into tail veins of Apoe−/−Ldlrh/h mice. ApoE4-GFP 5 minutes after i.v. injection (G), and apoE2-GFP (H).
Neither immunostaining nor Ad-apoE-GFP expression allows us to dissociate whether apoE accumulating on the hepatocyte surface is newly synthesized by the hepatocyte or originates from lipoproteins in circulation. However, we note that not all the cells demarcated by intense sinusoidal apoE-GFP signals have strong intracellular GFP signals. This suggests a substantial part of the apoE-GFP may be derived from apoE-GFP synthesized by other transfected hepatocytes. To confirm this, we isolated VLDL fractions enriched with apoE2-GFP or apoE4-GFP from Apoe−/−Ldlr−/− mice 5 days after transfection with the Ad-apoE2-GFP or Ad-apoE4-GFP, and injected them into Apoe−/−Ldlrh/h mice. When livers were examined under confocal microscopy 5 minutes after the injection through tail veins, apoE4-GFP was already localized on hepatocyte surfaces (Figure 3G). This suggests that lipoproteins that acquire apoE4 during the circulation can accumulate in the SD very efficiently. In contrast, very little apoE2-GFP was accumulated on the hepatocyte surface during this time (Figure 3H).
Sinusoidal Sequestration of ApoE and Internalization of Lipoprotein Remnants
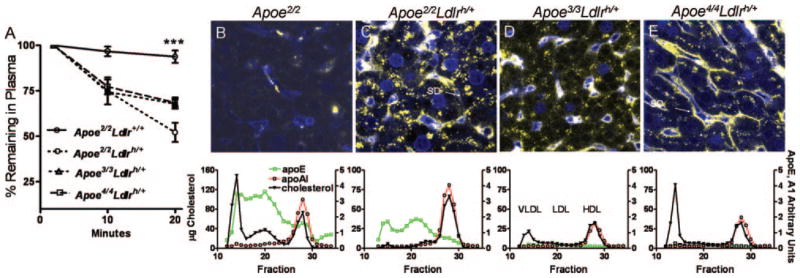
The striking differences in the cellular localizations of the different apoE isoforms in the livers of mice with high levels of LDLR expression raise a question regarding the physiological relevance of apoE accumulated on the sinusoidal surface to remnant clearance. We therefore examined how the apoE isoforms affect plasma VLDL clearance and liver uptake by injecting DiI-labeled Apoe−/− VLDL into Apoe2/2, Apoe2/2Ldlrh/+, Apoe3/3Ldlrh/+, and Apoe4/4Ldlrh/+ mice. Decay of DiI-labeled Apoe−/− VLDL particles from plasma showed that the Apoe2/2 mice had the slowest clearance of Apoe−/− VLDL (Figure 4A). The clearance in the Apoe2/2Ldlrh/+, Apoe3/3Ldlrh/+, and Apoe4/4Ldlrh/+ mice were not different at 10 minutes after injection. However, the VLDL was removed from circulation during the next 10 minutes significantly faster in the Apoe2/2Ldlrh/+ mice than in both Apoe3/3Ldlrh/+ and Apoe4/4Ldlrh/+mice. At 20 minutes after injection, DiI-VLDL was barely detectable in the liver of Apoe2/2 mice (Figure 4B), but was avidly internalized in the Apoe2/2Ldlrh/+ liver (Figure 4C). Most of the signal was intracellular and surface-bound DiI-VLDL was negligible (arrow, SD). In marked contrast, strong DiI-signal was on the hepatic surface of the Apoe4/4Ldlrh/+ liver (arrow, SD), whereas intracellular DiI-VLDL was less than in the Apoe2/2Ldlrh/+ liver (Figure 4C and 4E). Heparinase treatment of the Apoe4/4Ldlrh/+ mice 5 minutes before the injection of DiI-VLDL did not alter the localization of apoE and VLDL appreciably (supplemental Figure VII).
Figure 4.
Localization and clearance of VLDL. A, DiI-labeled Apoe−/− VLDL was injected into Apoe2/2, Apoe2/2Ldlrh/+, Apoe3/3Ldlrh/+, and Apoe4/4Ldlrh/+ mice and fluorescent signals in plasma were measured at 2, 10, and 20 minutes. 2 minutes was taken as 100% (n=4). B through E, Livers at 20 minutes were analyzed by confocal microscopy for DiI-VLDL (yellow) and Alexafluor633 labeled lectin (blue): Apoe2/2 (B), Apoe2/2Ldlrh/+ (C), Apoe3/3Ldlrh/+ (D), Apoe4/4Ldlrh/+ (E). Below each panel are their lipoprotein profiles. Cholesterol (black), and relative amounts of apoE (green) and apoA1 (red) in the lipoproteins separated by fast protein liquid chromatography (FPLC) from plasma of mice before the injection of DiI-labeled VLDL.
The reduced ability to internalize VLDL by the liver was associated with the accumulation of cholesterol-rich but apoE-poor VLDL remnants and 10-fold less total apoE in the plasma of Apoe4/4Ldlrh/+ mice compared to the Apoe2/2Ldlrh/+ mice (Figure 4B through 4E, lower panels). Plasma apoprotein to lipid ratios indicate that the Apoe2/2Ldlrh/+ mice have the most apoE enriched lipoproteins in all subclasses. Consistent with their higher HDL-C, Apoe2/2Ldlrh/+ mice also had more plasma apoA1 than Apoe4/4Ldlrh/+ mice, but the ratios of cholesterol to apoA1 in the HDL particles in these mice were not different. Hepatic expression of apoE, LRP, and SRB-1 that could influence lipid/cholesterol flux were not significantly different among experimental mice (supplemental Figure VIII).
Taken together, these data demonstrate that apoE2, which is elevated in the plasma on circulating lipoproteins and minimally associated with the hepatocyte surface, can facilitate internalization of apoE−/− VLDL remnants in the mice expressing a high level of LDLR. In contrast, apoE4 that is accumulated on the hepatocyte surface appears to enhance sequestration of VLDL remnants, but has only a limited capacity to participate in the internalization of lipoprotein remnants.
Discussion
There is little debate that the LDLR is important for clearance of remnant lipoproteins under normal physiological conditions, and that this process is mediated by the high-affinity binding of multiple apoE proteins on a lipoprotein particle to the LDLR.3 However, the common apoE isoforms in humans having affinities for the LDLR in the order of apoE4>apoE3>apoE2 significantly impact plasma cholesterol levels in an inverse order in humans and in mouse models.2,10,13,14,17,18,27 Our current study using humanized mice provides evidence that the extent of localization of the human apoE-isoforms in the liver inversely correlates with their efficacy in lipoprotein remnant internalization in vivo.
Previous studies have demonstrated the presence of apoE immunoreactivity along the sinusoidal front of hepatocytes together with some punctate cytoplasmic staining in the liver of wild type rats and mice.4,28,29 Studies have also shown that apoE is clustered on hepatocyte microvilli projecting into the space of Disse of the rat liver,28 and are used for hepatic endocytosis of remnants.4 Also, liver-derived and localized apoE has been shown to be more effective than nonhepatic derived apoE in the receptor-mediated internalization of remnants by the liver.30–32 Similarly, Linton et al showed in the space of Disse of the Apoe−/−Ldlr−/− mice that received wild-type bone marrows that an intense immunoreativity for extrahepatic apoE was present on the cell surface but cytoplasmic staining was not detected, indicating that no uptake of apoE-containing lipoproteins was occurring.29 These observations, using wild-type rodent apoE, clearly underscore the important role of apoE localized in the sinusoids of the liver in remnant uptake.
Multiple experiments have also shown that a substantial amount of apoE internalized with TRLs by the liver are recycled back to the cell-surface and resecreted, and that this resecretion is isoform specific.33–35 Considering that apoE4 and apoE3 have a higher affinity to the LDLR, our results showing enhanced accumulation of apoE4 and apoE3 in the space of Disse compared to apoE2 in mice overexpressing the human LDLR are not surprising. Although absolute amount of LDLR protein in the liver in these mice has not been determined, previous experiments by us and by others have unequivocally shown that the increases in LDLR expression lead to lowering of plasma cholesterol in mice.36,37 Surprisingly, this surface sequestration of apoE4 does not directly translate to enhanced internalization of VLDL remnants into the cells. Thus, we observed that internalization of DiI-labeled VLDL was slower in the Apoe4/4Ldlrh/+ mice than in the Apoe2/2Ldlrh/+ mice. Remarkably, there were no indications that the increased LDLR enhances either LDLR-mediated or LDLR-independent uptake of TRL in the Apoe4/4 Ldlrh/+ mice. Although we cannot eliminate the possibility that the DiI-labeled VLDL may have exchanged with the plasma lipoproteins in the recipient mice before it reached their hepatic surface, our results suggest that the sinusoidal enrichment of apoE does not necessarily ensure that apoE is transferred to TRL particles.
Jones et al recently demonstrated that mice with mutations in an adaptor protein involved in LDLR internalization can still clear VLDL.38 The authors suggested the possibility that VLDL remnants bind the LDLR on the hepatocyte cell surface but that their internalization may be mediated by another cell surface component that is also independent of LRP or HSPG. Although the nature of this process is yet to be elucidated, the concept that remnants are handed from one cell surface molecule to the other while accumulating apoE molecules before their final internalization is attractive. Because LDLR serves as a regulator of apoE availability more than just a receptor, a higher affinity of apoE4 to the LDLR could delay the handing-off process, and thereby delay the clearance of remnants in the Apoe4/4Ldlrh/+ mice. In contrast, the reduced affinity of apoE2 for the LDLR, and to a lesser extent for HSPG, results in limited apoE2 sequestration on the surface of hepatocytes allowing their secretion into the circulation.39 As circulating lipoproteins in the Apoe2/2Ldlrh/+ mice have high apoE contents, a significant portion of the apoE2-enrichment of remnants may take place in the circulation and contribute to the subsequent remnant clearance in the liver of these mice. (A hypothetical model of interactions between apoE2 and apoE4 with the LDLR within the sinusoidal space is presented in supplemental Figure IX).
The genetic interaction between the apoE-isoforms and LDLR gene expression observed in mice translates well to humans, although not completely. For instance humans with APOE*4 have elevated LDL remnants and Apoe4/4Ldlrh/+ mice accumulate VLDL remnants.12 Nevertheless, an enhanced sequestration but delayed clearance of VLDL could prolong their exposure to surface bound lipases which could then accelerate lipolysis and conversion of VLDL to smaller remnants in the livers expressing apoE4 of both species. In contrast, limited interaction of apoE2 with the LDLR enhances VLDL clearance and reduces remnant production in apoE2 livers.21,40 Consequently, the overall degrees of reduction in LDL-cholesterol resulting from the increase in LDLR expression in humans is likely to be apoE-isoform–dependent; with more pronounced reduction in individuals with apoE2 but lesser reduction with apoE4.
Supplementary Material
Acknowledgments
We thank Dr Robert Bagnell for help with microscopy and image acquisition and Svetlana Zihilcheva for technical help.
Sources of Funding
This work was supported by a grant HL42630. M.A. was supported by T32HL69768.
Footnotes
Disclosures
None.
References
- 1.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 2.Mahley RW, Rall SC., Jr Apolipoprotein E. far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 3.Mahley RW, Huang Y. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing. J Clin Invest. 2007;117:94–98. doi: 10.1172/JCI30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimano H, Namba Y, Ohsuga J, Kawamura M, Yamamoto K, Shimada M, Gotoda T, Harada K, Yazaki Y, Yamada N. Secretion-recapture process of apolipoprotein E in hepatic uptake of chylomicron remnants in transgenic mice. J Clin Invest. 1994;93:2215–2223. doi: 10.1172/JCI117218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisgraber KH, Innerarity TL, Mahley RW. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982;257:2518–2521. [PubMed] [Google Scholar]
- 6.Bohnet K, Pillot T, Visvikis S, Sabolovic N, Siest G. Apolipoprotein (apo) E genotype and apoE concentration determine binding of normal very low density lipoproteins to HepG2 cell surface receptors. J Lipid Res. 1996;37:1316–1324. [PubMed] [Google Scholar]
- 7.Knouff C, Hinsdale ME, Mezdour H, Altenburg MK, Watanabe M, Quarfordt SH, Sullivan PM, Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579–1586. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider WJ, Kovanen PT, Brown MS, Goldstein JL, Utermann G, Weber W, Havel RJ, Kotite L, Kane JP, Innerarity TL, Mahley RW. Familial dysbetalipoproteinemia. Abnormal binding of mutant apoprotein E to low density lipoprotein receptors of human fibroblasts and membranes from liver and adrenal of rats, rabbits, and cows. J Clin Invest. 1981;68:1075–1085. doi: 10.1172/JCI110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamotte CD, Sturm M, Foo JI, van Bockxmeer FM, Taylor RR. Comparison of the LDL-receptor binding of VLDL and LDL from apoE4 and apoE3 homozygotes. Am J Physiol. 1999;276:E553–E557. doi: 10.1152/ajpendo.1999.276.3.E553. [DOI] [PubMed] [Google Scholar]
- 10.Gylling H, Kontula K, Miettinen TA. Cholesterol absorption and metabolism and LDL kinetics in healthy men with different apoprotein E phenotypes and apoprotein B Xba I and LDL receptor Pvu II genotypes. Arterioscler Thromb Vasc Biol. 1995;15:208–213. doi: 10.1161/01.atv.15.2.208. [DOI] [PubMed] [Google Scholar]
- 11.Curtiss LK, Boisvert WA. Apolipoprotein E and atherosclerosis. Curr Opin Lipidol. 2000;11:243–251. doi: 10.1097/00041433-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Gregg RE, Zech LA, Schaefer EJ, Stark D, Wilson D, Brewer HB., Jr Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest. 1986;78:815–821. doi: 10.1172/JCI112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boerwinkle E, Utermann G. Simultaneous effects of the apolipoprotein E polymorphism on apolipoprotein E, apolipoprotein B, and cholesterol metabolism. Am J Hum Genet. 1988;42:104–112. [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PM, Mezdour H, Quarfordt SH, Maeda N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J Clin Invest. 1998;102:130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knouff C, Malloy S, Wilder J, Altenburg MK, Maeda N. Doubling expression of the low density lipoprotein receptor by truncation of the 3′-untranslated region sequence ameliorates type III hyperlipoproteinemia in mice expressing the human apoe2 isoform. J Biol Chem. 2001;276:3856–3862. doi: 10.1074/jbc.M009423200. [DOI] [PubMed] [Google Scholar]
- 18.Malloy SI, Altenburg MK, Knouff C, Lanningham-Foster L, Parks JS, Maeda N. Harmful effects of increased LDLR expression in mice with human APOE*4 but not APOE*3. Arterioscler Thromb Vasc Biol. 2004;24:91–97. doi: 10.1161/01.ATV.0000094963.07902.FB. [DOI] [PubMed] [Google Scholar]
- 19.Altenburg MK, Johnson LA, Wilder JC, Maeda N. Apolipoprotein E4 in macrophages enhances atherogenesis in an LDL receptor dependent manner. J Biol Chem. 2007 doi: 10.1074/jbc.M610712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucic D, Huang ZH, Gu de S, Altenburg MK, Maeda N, Mazzone T. Regulation of macrophage apoE secretion and sterol efflux by the LDL receptor. J Lipid Res. 2007;48:366–372. doi: 10.1194/jlr.M600259-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.van Dijk KW, van Vlijmen BJ, de Winther MP, van ’t Hof B, van der Zee A, van der Boom H, Havekes LM, Hofker MH. Hyperlipidemia of ApoE2(Arg(158)-Cys) and ApoE3-Leiden transgenic mice is modulated predominantly by LDL receptor expression. Arterioscler Thromb Vasc Biol. 1999;19:2945–2951. doi: 10.1161/01.atv.19.12.2945. [DOI] [PubMed] [Google Scholar]
- 22.Farkas MH, Swift LL, Hasty AH, Linton MF, Fazio S. The recycling of apolipoprotein E in primary cultures of mouse hepatocytes. Evidence for a physiologic connection to high density lipoprotein metabolism. J Biol Chem. 2003;278:9412–9417. doi: 10.1074/jbc.M208026200. [DOI] [PubMed] [Google Scholar]
- 23.Dekroon RM, Armati PJ. Endocytosis of apoE-EGFP by primary human brain cultures. Cell Biol Int. 2002;26:761–770. doi: 10.1016/s1065-6995(02)90930-3. [DOI] [PubMed] [Google Scholar]
- 24.Stephan ZF, Yurachek EC. Rapid fluorometric assay of LDL receptor activity by DiI-labeled LDL. J Lipid Res. 1993;34:325–330. [PubMed] [Google Scholar]
- 25.Lorenz JN, Gruenstein E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol. 1999;276:F172–F177. doi: 10.1152/ajprenal.1999.276.1.F172. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami H, Hirano H. Lectin-binding patterns on the plasma membranes of dissociated rat liver cells. Histochemistry. 1984;80:415–420. doi: 10.1007/BF00495428. [DOI] [PubMed] [Google Scholar]
- 27.Gregg RE, Zech LA, Brewer HB., Jr Apolipoprotein E alleles in severe hypertriglyceridaemia. Lancet. 1983;1:353. doi: 10.1016/s0140-6736(83)91653-7. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton RL, Wong JS, Guo LS, Krisans S, Havel RJ. Apolipoprotein E localization in rat hepatocytes by immunogold labeling of cryothin sections. J Lipid Res. 1990;31:1589–1603. [PubMed] [Google Scholar]
- 29.Linton MF, Hasty AH, Babaev VR, Fazio S. Hepatic apo E expression is required for remnant lipoprotein clearance in the absence of the low density lipoprotein receptor. J Clin Invest. 1998;101:1726–1736. doi: 10.1172/JCI2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffai RL, Hasty AH, Wang Y, Mettler SE, Sanan DA, Linton MF, Fazio S, Weisgraber KH. Hepatocyte-derived ApoE is more effective than non-hepatocyte-derived ApoE in remnant lipoprotein clearance. J Biol Chem. 2003;278:11670–11675. doi: 10.1074/jbc.M212873200. [DOI] [PubMed] [Google Scholar]
- 31.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–2192. [PubMed] [Google Scholar]
- 32.Yu KC, Jiang Y, Chen W, Cooper AD. Rapid initial removal of chylomicron remnants by the mouse liver does not require hepatically localized apolipoprotein E. J Lipid Res. 2000;41:1715–1727. [PubMed] [Google Scholar]
- 33.Heeren J, Grewal T, Jackle S, Beisiegel U. Recycling of apolipoprotein E and lipoprotein lipase through endosomal compartments in vivo. J Biol Chem. 2001;276:42333–42338. doi: 10.1074/jbc.M107461200. [DOI] [PubMed] [Google Scholar]
- 34.Heeren J, Grewal T, Laatsch A, Becker N, Rinninger F, Rye KA, Beisiegel U. Impaired recycling of apolipoprotein E4 is associated with intracellular cholesterol accumulation. J Biol Chem. 2004;279:55483–55492. doi: 10.1074/jbc.M409324200. [DOI] [PubMed] [Google Scholar]
- 35.Swift LL, Farkas MH, Major AS, Valyi-Nagy K, Linton MF, Fazio S. A recycling pathway for resecretion of internalized apolipoprotein E in liver cells. J Biol Chem. 2001;276:22965–22970. doi: 10.1074/jbc.M100172200. [DOI] [PubMed] [Google Scholar]
- 36.Herz J, Gerard RD. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokode M, Hammer RE, Ishibashi S, Brown MS, Goldstein JL. Diet-induced hypercholesterolemia in mice: prevention by overexpression of LDL receptors. Science. 1990;250:1273–1275. doi: 10.1126/science.2244210. [DOI] [PubMed] [Google Scholar]
- 38.Jones C, Garuti R, Michaely P, Li WP, Maeda N, Cohen JC, Herz J, Hobbs HH. Disruption of LDL but not VLDL clearance in autosomal recessive hypercholesterolemia. J Clin Invest. 2007;117:165–174. doi: 10.1172/JCI29415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji ZS, Fazio S, Mahley RW. Variable heparan sulfate proteoglycan binding of apolipoprotein E variants may modulate the expression of type III hyperlipoproteinemia. J Biol Chem. 1994;269:13421–13428. [PubMed] [Google Scholar]
- 40.Huang Y, Liu XQ, Rall SC, Jr, Mahley RW. Apolipoprotein E2 reduces the low density lipoprotein level in transgenic mice by impairing lipoprotein lipase-mediated lipolysis of triglyceride-rich lipoproteins. J Biol Chem. 1998;273:17483–17490. doi: 10.1074/jbc.273.28.17483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.