Abstract
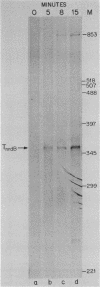
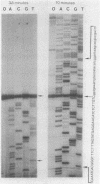
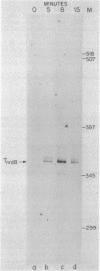
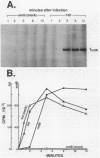
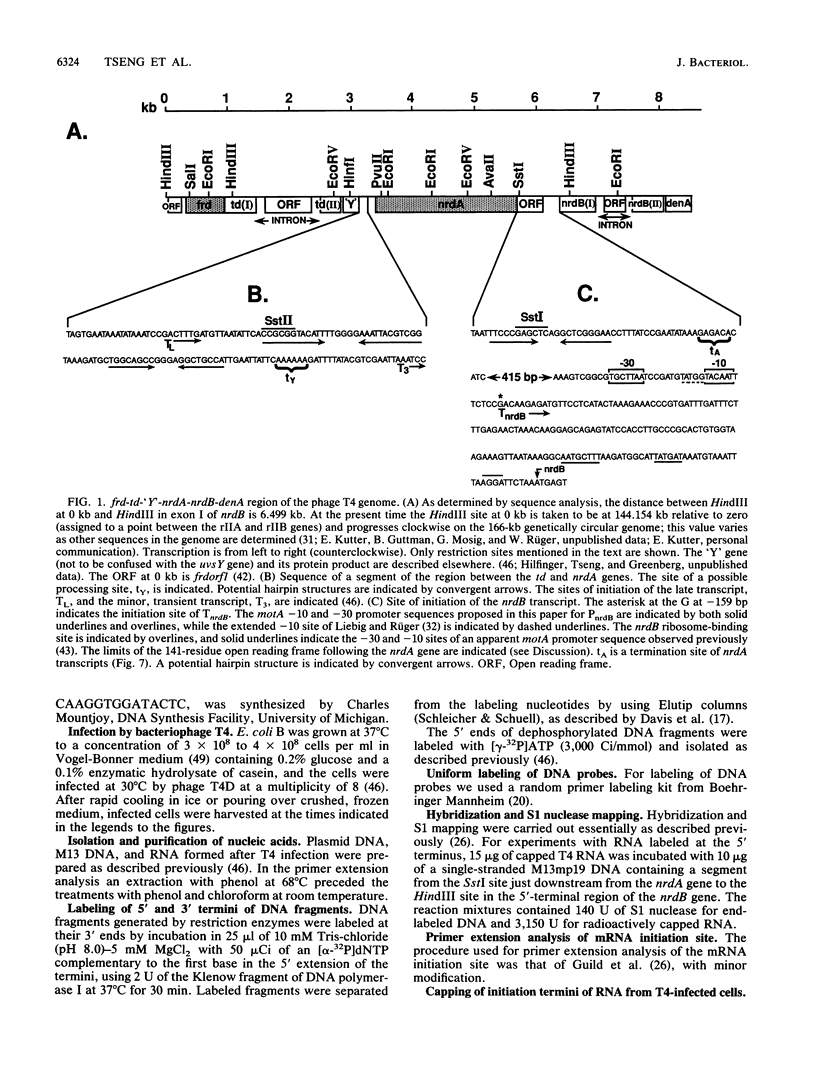
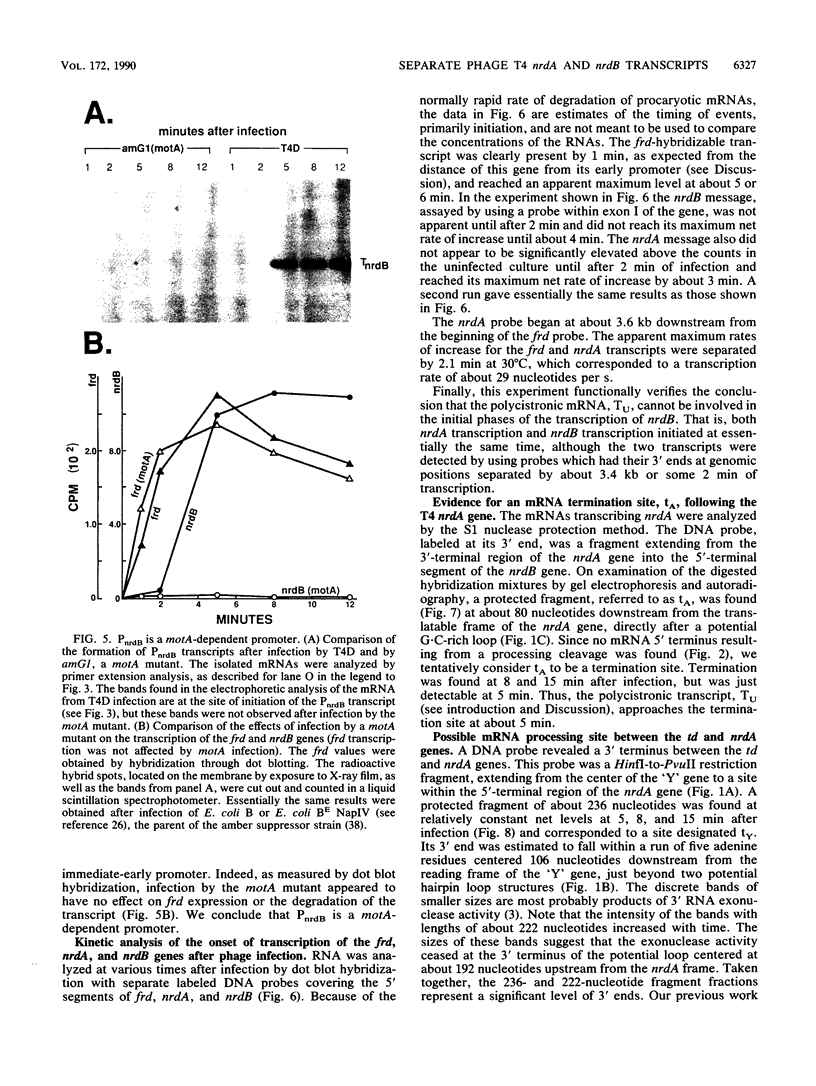
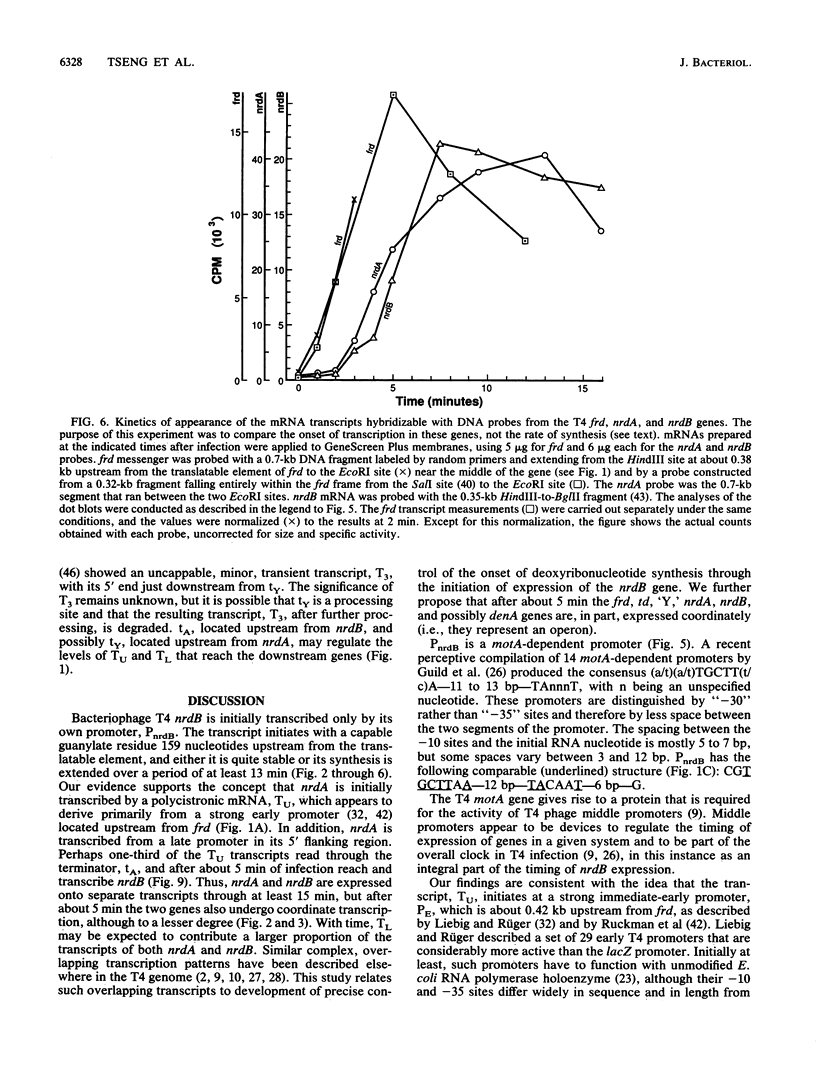
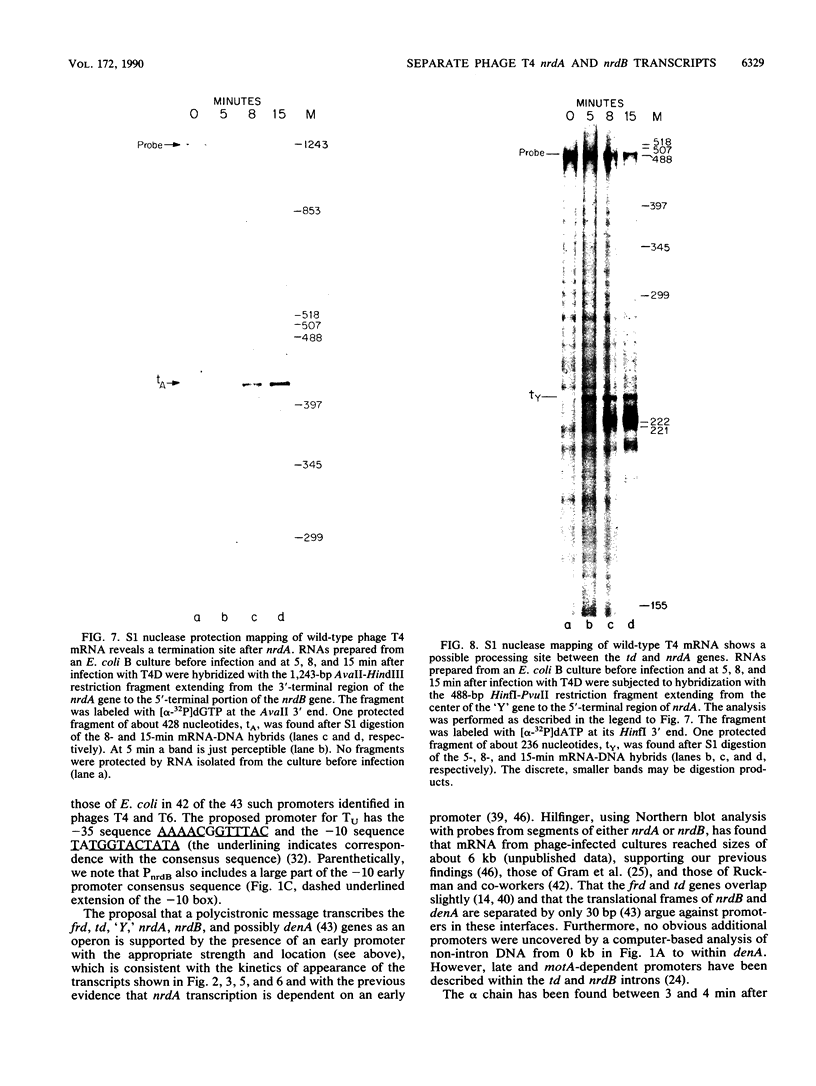
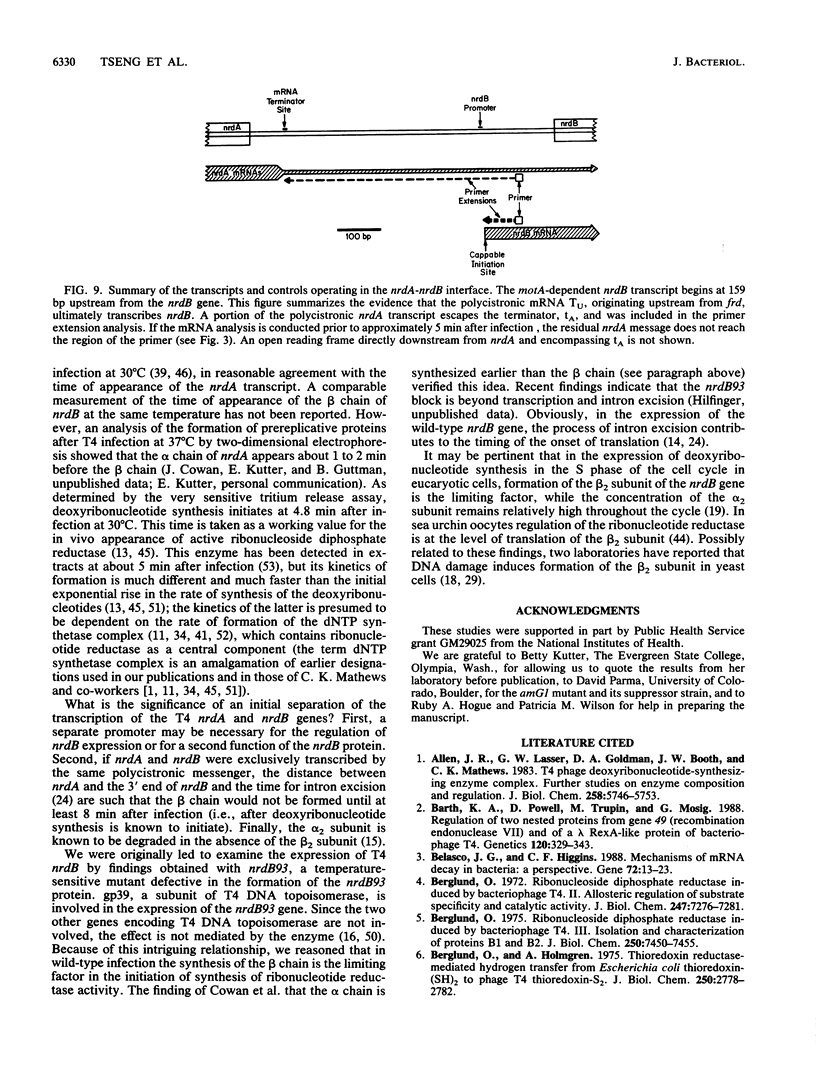
We examined the expression of the bacteriophage T4 nrdA and nrdB genes, which encode the alpha 2 and beta 2 subunits, respectively, of ribonucleoside diphosphate reductase, the first committed enzyme in the pathway of synthesis of the deoxyribonucleoside triphosphates. T4 nrdA, located 700 bp upstream from nrdB, has been shown previously to be transcribed by two major transcripts: a prereplicative, polycistronic message, TU, orginating at an immediate-early promoter, PE, that is 3.5 kb upstream from nrdA, and a postreplicative message commencing from a late promoter in its 5' flank. We have found a third promoter initiating a transcript at 159 nucleotides upstream from the reading frame of nrdB. PnrdB functions only in the presence of the T4 motA gene product, which is required for middle (time) promoters, and therefore the onset of nrdB transcription is delayed more than 2 min after infection. Because of the distance of nrdA from PE, the inception of nrdA transcription (delayed early) coincides closely with that of nrdB. An apparent termination site, tA, occurs about 80 bp downstream from nrdA. Some of the polycistronic mRNA reading through the site after 5 min contributes to nrdB transcription. nrdA and nrdB genes in an uninfected host have been reported to be transcribed only coordinately. In contrast, T4 nrdA and nrdB are initially transcribed separately onto the PE and PnrdB transcripts, respectively, but at about 5 min after infection are transcribed both coordinately and on separate transcripts. Evidence is presented that TU coordinately transcribes a deoxyribonucleotide operon in the order: frd, td, gene 'Y,' nrdA, nrdB. Since the beta 2 subunit is known to be formed after the alpha 2 subunit, the expression of the nrdB gene determines the onset of deoxyribonucleoside triphosphate synthesis and thus of T4 DNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. R., Lasser G. W., Goldman D. A., Booth J. W., Mathews C. K. T4 phage deoxyribonucleotide-synthesizing enzyme complex. Further studies on enzyme composition and regulation. J Biol Chem. 1983 May 10;258(9):5746–5753. [PubMed] [Google Scholar]
- Barth K. A., Powell D., Trupin M., Mosig G. Regulation of two nested proteins from gene 49 (recombination endonuclease VII) and of a lambda RexA-like protein of bacteriophage T4. Genetics. 1988 Oct;120(2):329–343. doi: 10.1093/genetics/120.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J. G., Higgins C. F. Mechanisms of mRNA decay in bacteria: a perspective. Gene. 1988 Dec 10;72(1-2):15–23. doi: 10.1016/0378-1119(88)90123-0. [DOI] [PubMed] [Google Scholar]
- Berglund O., Holmgren A. Thioredoxin reductase-mediated hydrogen transfer from Escherichia coli thioredoxin-(SH)2 to phage T4 thioredoxin-S2. J Biol Chem. 1975 Apr 25;250(8):2778–2782. [PubMed] [Google Scholar]
- Berglund O. Ribonucleoside diphosphate reductase induced by bacteriophage T4. II. Allosteric regulation of substrate sepecificity and catalytic activity. J Biol Chem. 1972 Nov 25;247(22):7276–7281. [PubMed] [Google Scholar]
- Berglund O. Ribonucleoside diphosphate reductase induced by bacteriophage T4. III. Isolation and characterization of proteins B1 and B2. J Biol Chem. 1975 Sep 25;250(18):7450–7455. [PubMed] [Google Scholar]
- Berglund O., Sjöberg B. M. A thioredoxin induced by bacteriophage T4. II. Purification and characterization. J Biol Chem. 1970 Nov 25;245(22):6030–6035. [PubMed] [Google Scholar]
- Brissenden J. E., Caras I., Thelander L., Francke U. The structural gene for the M1 subunit of ribonucleotide reductase maps to chromosome 11, band p15, in human and to chromosome 7 in mouse. Exp Cell Res. 1988 Jan;174(1):302–308. doi: 10.1016/0014-4827(88)90165-6. [DOI] [PubMed] [Google Scholar]
- Broida J., Abelson J. Sequence organization and control of transcription in the bacteriophage T4 tRNA region. J Mol Biol. 1985 Oct 5;185(3):545–563. doi: 10.1016/0022-2836(85)90071-3. [DOI] [PubMed] [Google Scholar]
- Chiu C. S., Cook K. S., Greenberg G. R. Characteristics of a bacteriophage T4-induced complex synthesizing deoxyribonucleotides. J Biol Chem. 1982 Dec 25;257(24):15087–15097. [PubMed] [Google Scholar]
- Chiu C. S., Cox S. M., Greenberg G. R. Effect of bacteriophage T4 nrd mutants on deoxyribonucleotide synthesis in vivo. J Biol Chem. 1980 Apr 10;255(7):2747–2751. [PubMed] [Google Scholar]
- Chiu C. S., Tomich P. K., Greenberg G. R. Simultaneous initiation of synthesis of bacteriophage T4 DNA and of deoxyribonucleotides. Proc Natl Acad Sci U S A. 1976 Mar;73(3):757–761. doi: 10.1073/pnas.73.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., Maley F., Belfort M. Intervening sequence in the thymidylate synthase gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1984 May;81(10):3049–3053. doi: 10.1073/pnas.81.10.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. S., Greenberg G. R. Properties of Bacteriophage T4 ribonucleoside diphosphate reductase subunits coded by nrdA and nrdB mutants. J Biol Chem. 1983 May 25;258(10):6064–6072. [PubMed] [Google Scholar]
- Cook K. S., Wirak D. O., Seasholtz A. F., Greenberg G. R. Effect of bacteriophage T4 DNA topoisomerase gene 39 on level of beta chain of ribonucleoside diphosphate reductase in a T4 nrdB mutant. J Biol Chem. 1988 May 5;263(13):6202–6208. [PubMed] [Google Scholar]
- Elledge S. J., Davis R. W. DNA damage induction of ribonucleotide reductase. Mol Cell Biol. 1989 Nov;9(11):4932–4940. doi: 10.1128/mcb.9.11.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Jildevik I., Skog S., Thelander L., Tribukait B. Cell cycle-dependent expression of mammalian ribonucleotide reductase. Differential regulation of the two subunits. J Biol Chem. 1985 Aug 5;260(16):9114–9116. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fuchs J. A. Coordinate control of the synthesis of ribonucleoside diphosphate reductase components in Escherichia coli. J Bacteriol. 1977 May;130(2):957–959. doi: 10.1128/jb.130.2.957-959.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert I., Calero S., Barbé J. Measurement of in vivo expression of nrdA and nrdB genes of Escherichia coli by using lacZ gene fusions. Mol Gen Genet. 1990 Feb;220(3):400–408. doi: 10.1007/BF00391745. [DOI] [PubMed] [Google Scholar]
- Goldfarb A., Malik S. Changed promoter specificity and antitermination properties displayed in vitro by bacteriophage T4-modified RNA polymerase. J Mol Biol. 1984 Jul 25;177(1):87–105. doi: 10.1016/0022-2836(84)90059-7. [DOI] [PubMed] [Google Scholar]
- Gott J. M., Zeeh A., Bell-Pedersen D., Ehrenman K., Belfort M., Shub D. A. Genes within genes: independent expression of phage T4 intron open reading frames and the genes in which they reside. Genes Dev. 1988 Dec;2(12B):1791–1799. doi: 10.1101/gad.2.12b.1791. [DOI] [PubMed] [Google Scholar]
- Gram H., Liebig H. D., Hack A., Niggemann E., Rüger W. A physical map of bacteriophage T4 including the positions of strong promoters and terminators recognized in vitro. Mol Gen Genet. 1984;194(1-2):232–240. doi: 10.1007/BF00383522. [DOI] [PubMed] [Google Scholar]
- Guild N., Gayle M., Sweeney R., Hollingsworth T., Modeer T., Gold L. Transcriptional activation of bacteriophage T4 middle promoters by the motA protein. J Mol Biol. 1988 Jan 20;199(2):241–258. doi: 10.1016/0022-2836(88)90311-7. [DOI] [PubMed] [Google Scholar]
- Hinton D. M. Transcript analyses of the uvsX-40-41 region of bacteriophage T4. Changes in the RNA as infection proceeds. J Biol Chem. 1989 Aug 25;264(24):14432–14439. [PubMed] [Google Scholar]
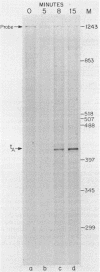
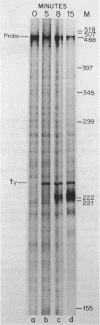
- Hsu T., Karam J. D. Transcriptional mapping of a DNA replication gene cluster in bacteriophage T4. Sites for initiation, termination, and mRNA processing. J Biol Chem. 1990 Mar 25;265(9):5303–5316. [PubMed] [Google Scholar]
- Hurd H. K., Roberts C. W., Roberts J. W. Identification of the gene for the yeast ribonucleotide reductase small subunit and its inducibility by methyl methanesulfonate. Mol Cell Biol. 1987 Oct;7(10):3673–3677. doi: 10.1128/mcb.7.10.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebig H. D., Rüger W. Bacteriophage T4 early promoter regions. Consensus sequences of promoters and ribosome-binding sites. J Mol Biol. 1989 Aug 20;208(4):517–536. doi: 10.1016/0022-2836(89)90145-9. [DOI] [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutant of bacteriophage T4. I. Ribonucleic acid metabolism. J Biol Chem. 1968 Nov 10;243(21):5610–5615. [PubMed] [Google Scholar]
- Mathews C. K., Moen L. K., Wang Y., Sargent R. G. Intracellular organization of DNA precursor biosynthetic enzymes. Trends Biochem Sci. 1988 Oct;13(10):394–397. doi: 10.1016/0968-0004(88)90182-x. [DOI] [PubMed] [Google Scholar]
- Mattson T., Van Houwe G., Epstein R. H. Isolation and characterization of conditional lethal mutations in the mot gene of bacteriophage T4. J Mol Biol. 1978 Dec 15;126(3):551–570. doi: 10.1016/0022-2836(78)90058-x. [DOI] [PubMed] [Google Scholar]
- Mileham A. J., Revel H. R., Murray N. E. Molecular cloning of the T4 genome; organization and expression of the frd--DNA ligase region. Mol Gen Genet. 1980;179(2):227–239. doi: 10.1007/BF00425449. [DOI] [PubMed] [Google Scholar]
- Moss B., Koczot F. Sequence of methylated nucleotides at the 5'-terminus of adenovirus-specific RNA. J Virol. 1976 Feb;17(2):385–392. doi: 10.1128/jvi.17.2.385-392.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- Purohit S., Mathews C. K. Nucleotide sequence reveals overlap between T4 phage genes encoding dihydrofolate reductase and thymidylate synthase. J Biol Chem. 1984 May 25;259(10):6261–6266. [PubMed] [Google Scholar]
- Reddy G. P., Singh A., Stafford M. E., Mathews C. K. Enzyme associations in T4 phage DNA precursor synthesis. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3152–3156. doi: 10.1073/pnas.74.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckman J., Parma D., Tuerk C., Hall D. H., Gold L. Identification of a T4 gene required for bacteriophage mRNA processing. New Biol. 1989 Oct;1(1):54–65. [PubMed] [Google Scholar]
- Sjöberg B. M., Hahne S., Mathews C. Z., Mathews C. K., Rand K. N., Gait M. J. The bacteriophage T4 gene for the small subunit of ribonucleotide reductase contains an intron. EMBO J. 1986 Aug;5(8):2031–2036. doi: 10.1002/j.1460-2075.1986.tb04460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standart N. M., Bray S. J., George E. L., Hunt T., Ruderman J. V. The small subunit of ribonucleotide reductase is encoded by one of the most abundant translationally regulated maternal RNAs in clam and sea urchin eggs. J Cell Biol. 1985 Jun;100(6):1968–1976. doi: 10.1083/jcb.100.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Tseng M. J., Hilfinger J. M., Walsh A., Greenberg G. R. Total sequence, flanking regions, and transcripts of bacteriophage T4 nrdA gene, coding for alpha chain of ribonucleoside diphosphate reductase. J Biol Chem. 1988 Nov 5;263(31):16242–16251. [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Regulation of the operon encoding ribonucleotide reductase in Escherichia coli: evidence for both positive and negative control. EMBO J. 1986 May;5(5):1077–1085. doi: 10.1002/j.1460-2075.1986.tb04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Regulation of the operon encoding ribonucleotide reductase: role of the negative sites in nrd repression. J Bacteriol. 1990 Apr;172(4):1711–1718. doi: 10.1128/jb.172.4.1711-1718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirak D. O., Cook K. S., Greenberg G. R. Defect in synthesis of deoxyribonucleotides by a bacteriophage T4 nrdB mutant is suppressed on mutation of T4 DNA topoisomerase gene. J Biol Chem. 1988 May 5;263(13):6193–6201. [PubMed] [Google Scholar]
- Wirak D. O., Greenberg G. R. Role of bacteriophage T4 DNA-delay gene products in deoxyribonucleotide synthesis. J Biol Chem. 1980 Mar 10;255(5):1896–1904. [PubMed] [Google Scholar]
- Wovcha M. G., Chiu C. S., Tomich P. K., Greenberg G. R. Replicative bacteriophage DNA synthesis in plasmolyzed T4-infected cells: evidence for two independent pathways to DNA. J Virol. 1976 Oct;20(1):142–156. doi: 10.1128/jvi.20.1.142-156.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y. C., Tessman I. Control of pyrimidine biosynthesis by phage T4. II. In vitro complementation between ribonucleotide reductase mutants. Virology. 1972 Mar;47(3):767–772. doi: 10.1016/0042-6822(72)90567-3. [DOI] [PubMed] [Google Scholar]