Abstract
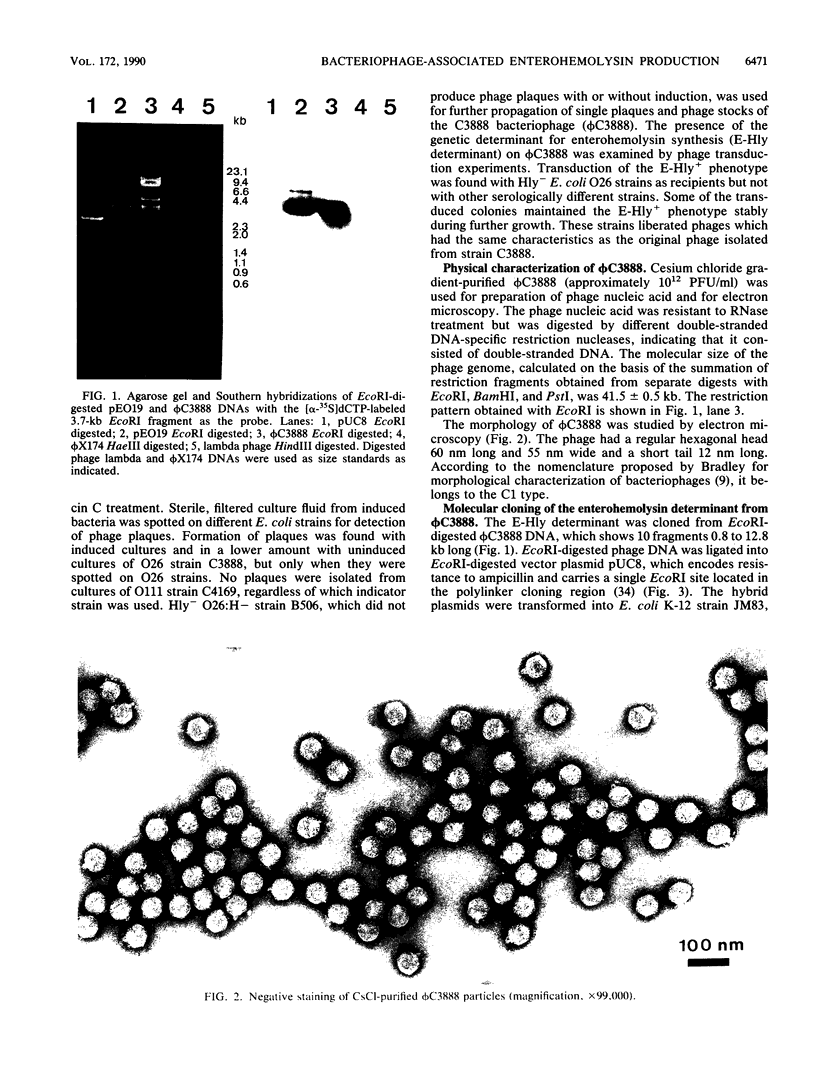
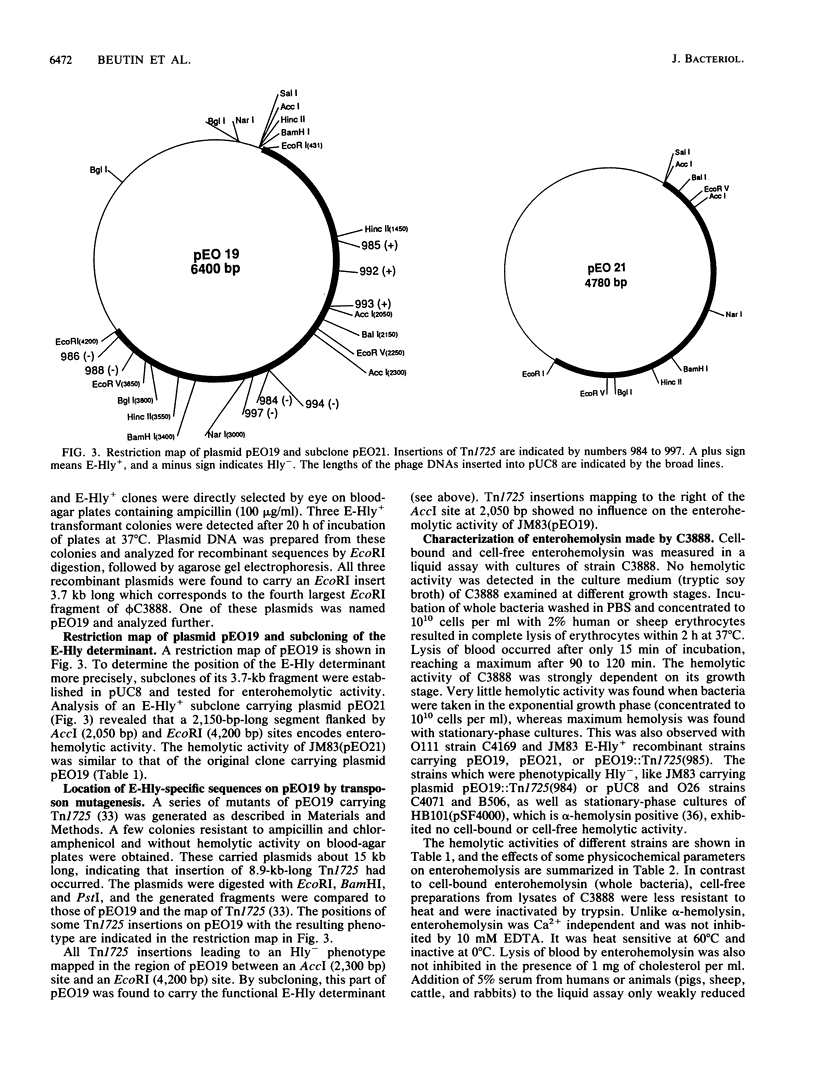
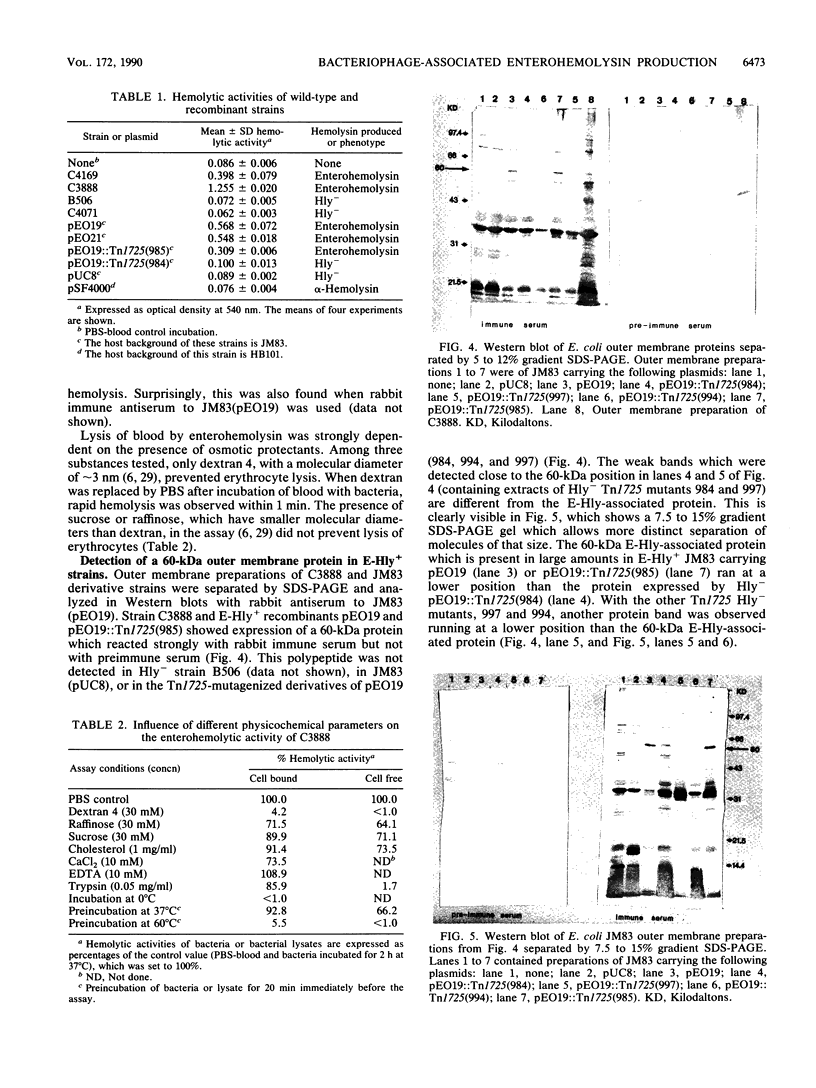
A temperate bacteriophage that determines the expression of enterohemolysin was isolated from Escherichia coli O26 strain C3888. The genetic determinant associated with enterohemolysin production (E-Hly determinant) was cloned from EcoRI-digested bacteriophage DNA in vector plasmid pUC8. pUC8 recombinant plasmid pEO19 carries a 3.7-kb EcoRI insert of phage DNA, and enterohemolysin was expressed in E. coli K-12 after transformation. Hemolysin-negative derivatives of pEO19 were generated by transposon mutagenesis with Tn1725. By subcloning, the phage E-Hly determinant was assigned to a 2,150-bp piece of DNA which is flanked by EcoRI and AccI restriction sites. The enterohemolysin-producing recombinant strains and wild-type strain C3888 express a 60-kDa protein which was detected in the bacterial outer membrane by Western immunoblotting. Biologically active enterohemolysin was detected only in bacteria grown to the stationary phase, and the hemolysin was not released into the culture medium. Lysis of erythrocytes was inhibited by 30 mM dextran 4, which functions as an osmotic protectant without destroying the enterohemolysin itself.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Bode L., Richter T., Peltre G., Stephan R. Rapid visual detection of Escherichia coli and Vibrio cholerae Heat-labile enterotoxins by nitrocellulose enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Mar;19(3):371–375. doi: 10.1128/jcm.19.3.371-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Montenegro M. A., Orskov I., Orskov F., Prada J., Zimmermann S., Stephan R. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J Clin Microbiol. 1989 Nov;27(11):2559–2564. doi: 10.1128/jcm.27.11.2559-2564.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Montenegro M., Zimmermann S., Stephan R. Characterization of hemolytic strains of Escherichia coli belonging to classical enteropathogenic O-serogroups. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 May;261(3):266–279. doi: 10.1016/s0176-6724(86)80044-x. [DOI] [PubMed] [Google Scholar]
- Beutin L., Prada J., Zimmermann S., Stephan R., Orskov I., Orskov F. Enterohemolysin, a new type of hemolysin produced by some strains of enteropathogenic E. coli (EPEC). Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Mar;267(4):576–588. doi: 10.1016/s0176-6724(88)80042-7. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L., Beutin L., Köhler H. Nitrocellulose-enzyme-linked immunosorbent assay (NC-ELISA) - a sensitive technique for the rapid visual detection of both viral antigens and antibodies. J Virol Methods. 1984 Feb;8(1-2):111–121. doi: 10.1016/0166-0934(84)90045-4. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Kathariou S., Hacker J., Hof H., Huhle B., Wagner W., Kuhn M., Goebel W. Molecular analysis of bacterial cytolysins. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S456–S466. doi: 10.1093/clinids/9.supplement_5.s456. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Hughes C. Genetics of Escherichia coli hemolysin. Curr Top Microbiol Immunol. 1985;118:139–162. doi: 10.1007/978-3-642-70586-1_8. [DOI] [PubMed] [Google Scholar]
- Hacker J., Schröter G., Schrettenbrunner A., Hughes C., Goebel W. Hemolytic Escherichia coli strains in the human fecal flora as potential urinary pathogens. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 May;254(3):370–378. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hugo F., Arvand M., Reichwein J., Mackman N., Holland I. B., Bhakdi S. Identification with monoclonal antibodies of hemolysin produced by clinical isolates of Escherichia coli. J Clin Microbiol. 1987 Jan;25(1):26–30. doi: 10.1128/jcm.25.1.26-30.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Newland J. W., Strockbine N. A., Miller S. F., O'Brien A. D., Holmes R. K. Cloning of Shiga-like toxin structural genes from a toxin converting phage of Escherichia coli. Science. 1985 Oct 11;230(4722):179–181. doi: 10.1126/science.2994228. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Regulation of haemolysin synthesis in E. coli determined by HLY genes of human origin. Mol Gen Genet. 1985;199(1):111–116. doi: 10.1007/BF00327519. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (Lond) 1985 Dec;95(3):551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Green P., Parsell Z. Vero cell toxins in Escherichia coli and related bacteria: transfer by phage and conjugation and toxic action in laboratory animals, chickens and pigs. J Gen Microbiol. 1983 Oct;129(10):3121–3137. doi: 10.1099/00221287-129-10-3121. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Ubben D., Schmitt R. Tn1721 derivatives for transposon mutagenesis, restriction mapping and nucleotide sequence analysis. Gene. 1986;41(2-3):145–152. doi: 10.1016/0378-1119(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Hull R., Falkow S. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect Immun. 1983 Oct;42(1):178–186. doi: 10.1128/iai.42.1.178-186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]