Abstract
Carbon isotope fractionation (εp) between the inorganic carbon source and organic matter has been proposed to be a function of pCO2. To understand the CO2-dependency of εp and species-specific differences therein, inorganic carbon fluxes in the four dinoflagellate species Alexandrium fundyense, Scrippsiella trochoidea, Gonyaulax spinifera and Protoceratium reticulatum have been measured by means of membrane-inlet mass spectrometry. In-vivo assays were carried out at different CO2 concentrations, representing a range of pCO2 from 180 to 1200 μatm. The relative bicarbonate contribution (i.e. the ratio of bicarbonate uptake to total inorganic carbon uptake) and leakage (i.e. the ratio of CO2 efflux to total inorganic carbon uptake) varied from 0.2 to 0.5 and 0.4 to 0.7, respectively, and differed significantly between species. These ratios were fed into a single-compartment model, and εp values were calculated and compared to carbon isotope fractionation measured under the same conditions. For all investigated species, modeled and measured εp values were comparable (A. fundyense, S. trochoidea, P. reticulatum) and/or showed similar trends with pCO2 (A. fundyense, G. spinifera, P. reticulatum). Offsets are attributed to biases in inorganic flux measurements, an overestimated fractionation factor for the CO2-fixing enzyme RubisCO, or the fact that intracellular inorganic carbon fluxes were not taken into account in the model. This study demonstrates that CO2-dependency in εp can largely be explained by the inorganic carbon fluxes of the individual dinoflagellates.
Abbreviations: Ci, inorganic carbon; CCM, CO2-concentrating mechanism; Chl-a, Chlorophyll-a; εp, carbon isotope fractionation; εp-meas, measured carbon isotope fractionation; εp-mod, modeled carbon isotope fractionation; εs, equilibrium fractionation between CO2 and HCO3−; εf, kinetic fractionation associated with the CO2 fixation of RubisCO; LCO2, ratio of CO2 efflux relative to total Ci uptake; DIC, dissolved inorganic carbon; HCO3−, bicarbonate; RHCO3, ratio of HCO3− to total Ci uptake; RubisCO, ribulose-1,5-bisphosphate Carboxylase/Oxygenase; CA, carbonic anhydrase; TA, total alkalinity
Keywords: CCM, CO2 uptake, HCO3− uptake, Leakage
Highlights
-
•
To understand 13C fractionation in dinoflagellates, inorganic carbon fluxes were measured by MIMS and used for modeling.
-
•
Changes in cellular carbon fluxes, i.e. HCO3− contribution and CO2 leakage were CO2-dependent.
-
•
These CO2-dependencies could largely explain the CO2-dependent fractionation patterns observed in the four tested species.
1. Introduction
During photosynthetic carbon fixation, the lighter carbon isotope 12C is preferred over the heavier carbon isotope 13C, thereby causing carbon isotope fractionation (εp) between the inorganic carbon (Ci) source and the organic carbon. Values for εp of marine phytoplankton have been shown to be CO2-sensitive (e.g. Degens et al., 1968), and thus were discussed to serve as a proxy for past CO2 concentrations (Jasper and Hayes, 1990, Pagani, 2014, Van de Waal et al., 2013, Hoins et al., 2015). Large species-specific differences in εp have been described, which are yet poorly understood (e.g. Hinga et al., 1994, Burkhardt et al., 1999). Moreover, irrespective of the phytoplankton species investigated, most of these studies have solely described the relationship between εp and CO2, and only few have investigated the underlying physiological processes. Such mechanistic understanding is, however, needed to identify the reasons of the CO2-dependency of εp.
Carbon isotope fractionation of phytoplankton is primarily driven by the enzyme ribulose-1,5-bisphosphate Carboxylase/Oxygenase (RubisCO), which is responsible for the fixation of CO2 into organic compounds. The intrinsic fractionation associated with RubisCO (εf) has been estimated to range between ~ 22 and 30‰ (e.g. Roeske and O'Leary, 1984, Guy et al., 1993, Scott et al., 2007), even though a recent study obtained values as low as 11‰ for the RubisCO of the coccolithophore Emiliania huxleyi (Boller et al., 2011). While RubisCO principally sets the upper limit of fractionation, other processes strongly determine the degree to which RubisCO can express its fractionation (Sharkey and Berry, 1985, Burkhardt et al., 1999, Rost et al., 2002). First, there is leakage, i.e. the amount of CO2 diffusing out of the cell in relation to Ci uptake. With higher leakage, the intracellular Ci pool is ‘refreshed’, thereby preventing accumulation of 13C and allowing RubisCO to approach its upper fractionation values. Second, the relative contribution of bicarbonate (HCO3−) to total Ci uptake plays a role, as HCO3− is enriched in 13C by ~ 10‰ relative to CO2 (Mook et al., 1974). An increasing HCO3− contribution thus lowers εp. The enzyme carbonic anhydrase (CA), which accelerates the otherwise slow interconversion between CO2 and HCO3−, can also influence εp under certain conditions, e.g. by influencing leakage as well as the relative HCO3− contribution. All these processes play a role in the CO2-concentrating mechanisms (CCMs) of phytoplankton. Assessing the mode of CCMs may therefore help to understand the reasons for CO2-dependent changes in εp and species-specific differences therein.
Dinoflagellates are cosmopolitan unicellular algae that occur in many different environments, including eutrophic coastal regions and oligotrophic open oceans. In this study, we investigated whether the CO2-dependency of εp, which was found in the dinoflagellate species Alexandrium fundyense, Gonyaulax spinifera, Protoceratium reticulatum and Scrippsiella trochoidea (Burkhardt et al., 1999, Hoins et al., 2015), can be explained by changes in their Ci fluxes. Characteristics of CCMs in the tested species, including their CA activities and Ci fluxes, were measured by means of membrane-inlet mass spectrometry (MIMS). Results were fed into a single-compartment model that considers cellular leakage, the relative HCO3− contribution as well as the carbon isotope fractionation of RubisCO (Sharkey and Berry, 1985, Burkhardt et al., 1999). The calculated carbon fractionation (εp-mod) was then compared to the measured carbon fractionation (εp-meas).
2. Material and methods
2.1. Incubations
Cultures of the dinoflagellate species A. fundyense (formerly Alexandrium tamarense strain Alex5; John et al., 2014), S. trochoidea (strain GeoB267; culture collection of the University of Bremen), G. spinifera (strain CCMP 409) and P. reticulatum (strain CCMP 1889) were grown in 0.2 μm filtered North Sea water (salinity 34), which was enriched with 100 μmol L− 1 nitrate and 6.25 μmol L− 1 phosphate. Metals and vitamins were added according to f/2 medium (Guillard and Ryther, 1962), except for FeCl3 (1.9 μmol L− 1), H2SeO3 (10 nmol L− 1) and NiCl2 (6.3 nmol L− 1) that were added according to K medium (Keller et al., 1987). Each of the strains was grown in 2.4 L air-tight borosilicate bottles at 15 °C and 250 ± 25 μmol photons m− 2 s− 1 at a 16:8 h light:dark cycle. Bottles were placed on roller tables in order to avoid sedimentation.
Dissolved CO2 concentrations ranged from ~ 5–50 μmol L− 1 and were reached by pre-aerating culture medium with air containing 180, 380, 800 and 1200 μatm pCO2. The carbonate chemistry was calculated based on pH and total alkalinity (TA), using the program CO2sys (Pierrot et al., 2006). pH values were measured using a WTW 3110 pH meter equipped with a SenTix 41 Plus pH electrode (WTW, Weilheim, Germany), which was calibrated prior to measurements to the National Bureau of Standards (NBS) scale. An automated TitroLine burette system (SI Analytics, Mainz, Germany) was used to determine TA. Dissolved inorganic carbon (DIC) was determined colorimetrically using a QuAAtro autoanalyser (Seal Analytical, Mequon, USA). For more details on the carbonate chemistry in the acclimations, please refer to Eberlein et al. (2014) for A. fundyense and S. trochoidea and to Hoins et al. (2015) for G. spinifera and P. reticulatum.
To determine εp values, the isotopic composition of the organic material was measured using an Automated Nitrogen Carbon Analyser mass spectrometer (ANCA-SL 20–20, SerCon Ltd., Crewe, UK), and the isotopic composition of the DIC in growth medium was measured using a GasBench-II coupled to a Thermo Delta-V advantage isotope ratio mass spectrometer (see Hoins et al., 2015 for details on isotope analysis). Prior to assays, cells were acclimated to the different CO2 concentrations for at least 7 generations (i.e. > 21 days). To prevent changes in the carbonate chemistry, i.e. keeping drawdown of DIC < 3%, incubations were terminated at low cell densities (< 400 cells mL− 1).
2.2. MIMS assays
A custom-made membrane-inlet mass spectrometer (MIMS; Isoprime, GV Instruments, Manchester, UK; see Rost et al., 2007 for details) was used to determine CA activities and Ci fluxes of A. fundyense and S. trochoidea acclimated to four different pCO2 (i.e. 180, 380, 800 and 1200 μatm; Eberlein et al., 2014), and of G. spinifera and P. reticulatum acclimated to a low and high pCO2 (i.e. 180 and 800 μatm). Assays were performed in an 8 mL temperature-controlled cuvette, equipped with a stirrer. Assay tests over ~ 1 h confirmed that conditions during the assay do not cause physiological stress (i.e. no decline in O2 production rates), and subsequent microscopic inspection did not reveal any visual effects on cell morphologies. Prior to the measurements, acclimated cells were concentrated using a 10 μm membrane filter (Millipore, Billerica, MA) by gentle vacuum filtration (< 200 mbar) and stepwise transferred into Ci-free medium buffered with a 4-(2-hydroxylethyl)-1-piperazine-ethanesulfonic acid (50 mmol− 1 HEPES) solution at 15 ± 0.3 °C and a pH of 8.0 ± 0.1. Chlorophyll a (Chl-a) concentrations were determined fluorometrically by using a TD-700 Fluorometer (Turner Designs, Sunnyvale, CA, USA) and ranged between 0.15 and 1.70 μg mL− 1 during the assays.
To quantify activities of extracellular CA (eCA), the 18O depletion rate of doubly labeled 13C18O2 in seawater was determined by measuring the transient changes in 13C18O18O (m/z = 49), 13C18O16O (m/z = 47) and 13C16O16O (m/z = 45) in the dark, following the approach of Silvermann (1982). If cells possess eCA, exchange rates of 18O are accelerated relative to the spontaneous rate. To monitor the spontaneous rate, NaH13C18O3 label was injected to the cuvette, waiting until the m/z = 49 signal reached a steady-state decline. This rate was then compared to the steady-state decline after cells were added. Following Badger and Price (1989), eCA activity is expressed as percentage decrease in 18O-atom fraction upon the addition of cells, normalized to Chl-a. Consequently, 100 units (U) correspond to a doubling in the rate of interconversion between CO2 and HCO3− per μg Chl-a.
Photosynthetic O2 and Ci fluxes were determined following Badger et al. (1994). Making use of the chemical disequilibrium, this approach estimates CO2 and HCO3− fluxes during steady-state photosynthesis. It is based on the simultaneous measurements of O2 and CO2 concentrations during consecutive light and dark intervals with increasing amounts of DIC. Oxygen fluxes in the dark and light are converted into Ci fluxes by applying a respiratory quotient of 1.0 and a photosynthetic quotient of 1.1 (Burkhardt et al., 2001, Rost et al., 2003). The light intensity in the cuvette was adjusted to the acclimation conditions (i.e. 250 ± 25 μmol photons m− 2 s− 1). Net CO2 uptake was calculated from the steady-state decline in CO2 concentration at the end of the light period, corrected for the interconversion between CO2 and HCO3−. The uptake of HCO3− was calculated by subtracting net CO2 uptake from net Ci uptake, and the CO2 efflux from the cells was estimated from the initial slope after turning off the light. Rate constants k1 and k2 were determined based on temperature, salinity and pH (Zeebe and Wolf-Gladrow, 2001, Schulz et al., 2006), yielding mean values of 0.9241 (± 0.0506) min− 1 and 0.0085 (± 0.0008) min− 1, respectively. To eliminate any eCA activity, a prerequisite to apply the rate constants, we added dextran-bound sulfonamide (DBS; 50 μmol L− 1) to the cuvette. For more details on the calculations, please refer to Badger et al. (1994) and Schulz et al. (2007).
2.3. Single-compartment model
To calculate εp-mod, results for the relative HCO3− contribution and leakage were fed into a single-compartment model after Sharkey and Berry (1985) and Burkhardt et al. (1999):
| (1) |
where RHCO3 represents the ratio of HCO3− to total Ci uptake, εs the equilibrium fractionation between CO2 and HCO3− (− 10‰; Mook et al., 1974), LCO2 the ratio of CO2 efflux relative to total Ci uptake, and εf the kinetic fractionation associated with the CO2 fixation of RubisCO, which was here assumed to be 28‰ after Raven and Johnston (1991).
2.4. Statistical analysis
Shapiro–Wilk tests confirmed normality of the data. Significant differences between CO2 treatments were confirmed by a one-way ANOVA followed by post hoc comparison of the means using the Tukey HSD (α = 0.05; Table 1).
Table 1.
Experimental conditions in dilute batch culture incubations (see also Eberlein et al., 2014, Hoins et al., 2015): average CO2 concentrations (μmol L− 1), total alkalinity (TA; μmol L− 1), dissolved inorganic carbon (DIC; μmol L− 1) and pH (NBS scale). HCO3− contribution, leakage, modeled carbon isotope fractionation (εp-mod) and measured carbon isotope fractionation (εp-meas) was derived under the same conditions.
| pCO2 μatm | CO2 μmol L− 1 | TA μmol L− 1 | DIC μmol L− 1 | pH NBS | HCO3− contribution | Leakage | εp-mod ‰ | εp-meas ‰ |
|---|---|---|---|---|---|---|---|---|
| A. fundyense | ||||||||
| 180 | 5.9 ± 0.9a | 2434 ± 3 | 1992 ± 10a | 8.50 ± 0.06a | 0.22 ± 0.03 | 0.44 ± 0.01a | 10.1 ± 0.2a | 9.0 ± 0.3a |
| 380 | 11.5 ± 2.1b | 2439 ± 1 | 2117 ± 8b | 8.27 ± 0.07b | 0.24 ± 0.04 | 0.46 ± 0.02a | 10.6 ± 0.5a | 10.2 ± 0.5b |
| 800 | 25.9 ± 5.8c | 2434 ± 2 | 2245 ± 8c | 7.97 ± 0.10c | 0.24 ± 0.04 | 0.53 ± 0.02b | 12.6 ± 0.6b | 12.7 ± 0.4c |
| 1200 | 36.5 ± 9.3d | 2418 ± 1 | 2283 ± 5d | 7.83 ± 0.12d | 0.23 ± 0.08 | 0.63 ± 0.05c | 15.3 ± 0.8c | 12.1 ± 0.2c |
| S. trochoidea | ||||||||
| 180 | 6.6 ± 0.2a | 2386 ± 1 | 1872 ± 2a | 8.45 ± 0.01a | 0.53 ± 0.03a,b | 0.56 ± 0.06 | 10.4 ± 1.5 | 6.0 ± 0.5a,b |
| 380 | 13.1 ± 0.5b | 2388 ± 2 | 2096 ± 3b | 8.21 ± 0.02b | 0.55 ± 0.04a | 0.53 ± 0.06 | 9.4 ± 1.5 | 5.0 ± 0.1a |
| 800 | 28.8 ± 2.0c | 2385 ± 1 | 2223 ± 3c | 7.91 ± 0.03c | 0.48 ± 0.03b,c | 0.54 ± 0.01 | 10.3 ± 0.5 | 7.1 ± 0.7b |
| 1200 | 41.5 ± 3.6d | 2386 ± 4 | 2268 ± 9d | 7.77 ± 0.04d | 0.46 ± 0.04c | 0.48 ± 0.04 | 8.8 ± 1.1 | 11.8 ± 0.7c |
| G. spinifera | ||||||||
| 180 | 6.0 ± 1.1a | 2447 ± 5 | 1962 ± 15a | 8.50 ± 0.05a | 0.19 ± 0.11 | 0.61 ± 0.01 | 15.6 ± 0.9a | 7.8 ± 1.0a |
| 380 | 11.7 ± 2.5b | 2461 ± 12 | 2083 ± 1b | 8.27 ± 0.07b | – | – | – | 9.4 ± 0.4a |
| 800 | 27.9 ± 7.4c | 2475 ± 13 | 2224 ± 9c | 7.96 ± 0.10c | 0.19 ± 0.11 | 0.71 ± 0.01 | 18.6 ± 1.7b | 11.7 ± 0.7b |
| 1200 | 42.4 ± 7.9d | 2459 ± 4 | 2293 ± 5d | 7.78 ± 0.06d | – | – | – | 8.1 ± 0.5a |
| P. reticulatum | ||||||||
| 180 | 7.1 ± 0.5a | 2460 ± 8 | 2002 ± 2a | 8.43 ± 0.04a | 0.44 ± 0.13 | 0.50 ± 0.06 | 9.58 ± 2.0 | 8.4 ± 1.8 |
| 380 | 13.9 ± 0.8b | 2455 ± 2 | 2121 ± 4b | 8.21 ± 0.02b | – | – | – | 8.4 ± 0.7 |
| 800 | 31.0 ± 4.7c | 2461 ± 12 | 2249 ± 23c | 7.88 ± 0.08c | 0.49 ± 0.19 | 0.48 ± 0.09 | 9.2 ± 1.9 | 8.6 ± 2.0 |
| 1200 | 45.2 ± 6.9d | 2473 ± 19 | 2288 ± 16d | 7.75 ± 0.05d | – | – | – | 9.9 ± 0.8 |
Values represent the mean of triplicate incubations (n = 3; ± SD). Superscript letters indicate significant differences between pCO2 treatments (P < 0.05).
3. Results
3.1. CA activity
In A. fundyense and P. reticulatum, eCA activities were low with maximum activities of 156 U (μg Chl-a)− 1 and 44 U (μg Chl-a)− 1, respectively. In S. trochoidea and G. spinifera, eCA activities were comparably high with up to 1600 U (μg Chl-a)− 1 and 1100 U (μg Chl-a)− 1, respectively. In neither of the species, eCA activities were responding to changes in pCO2. Please note that for G. spinifera and P. reticulatum no statistics could be applied due to the lack of replication.
3.2. HCO3− contribution and leakage
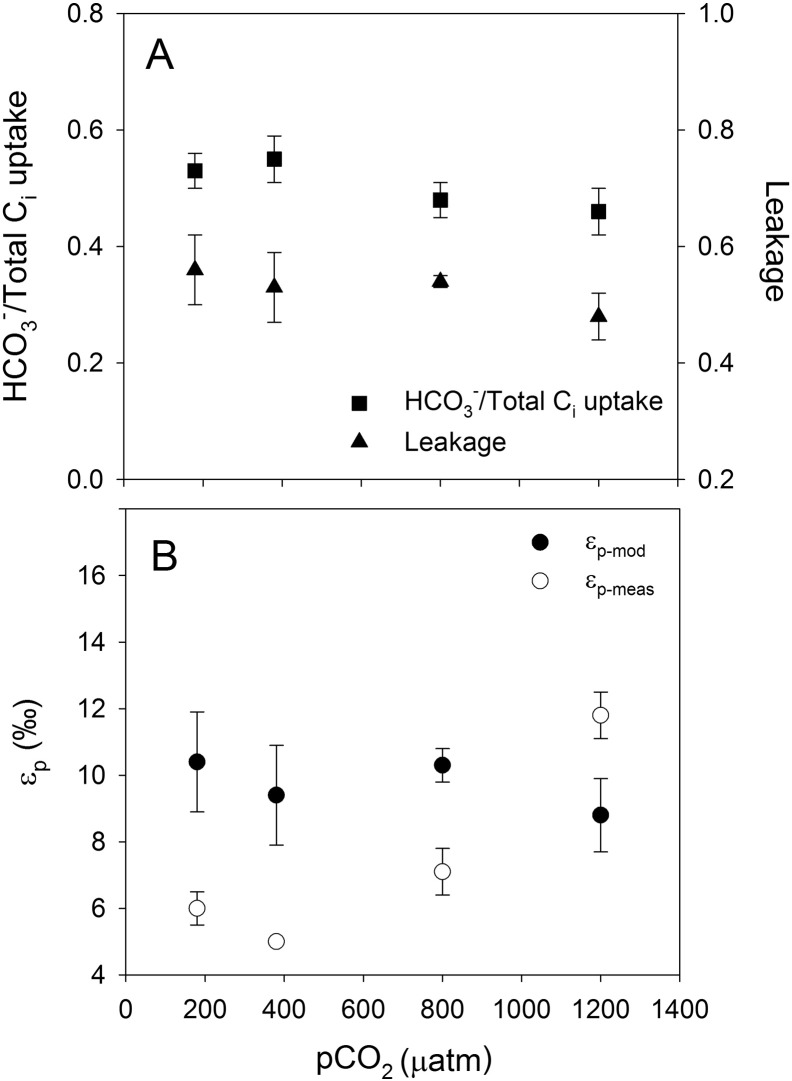
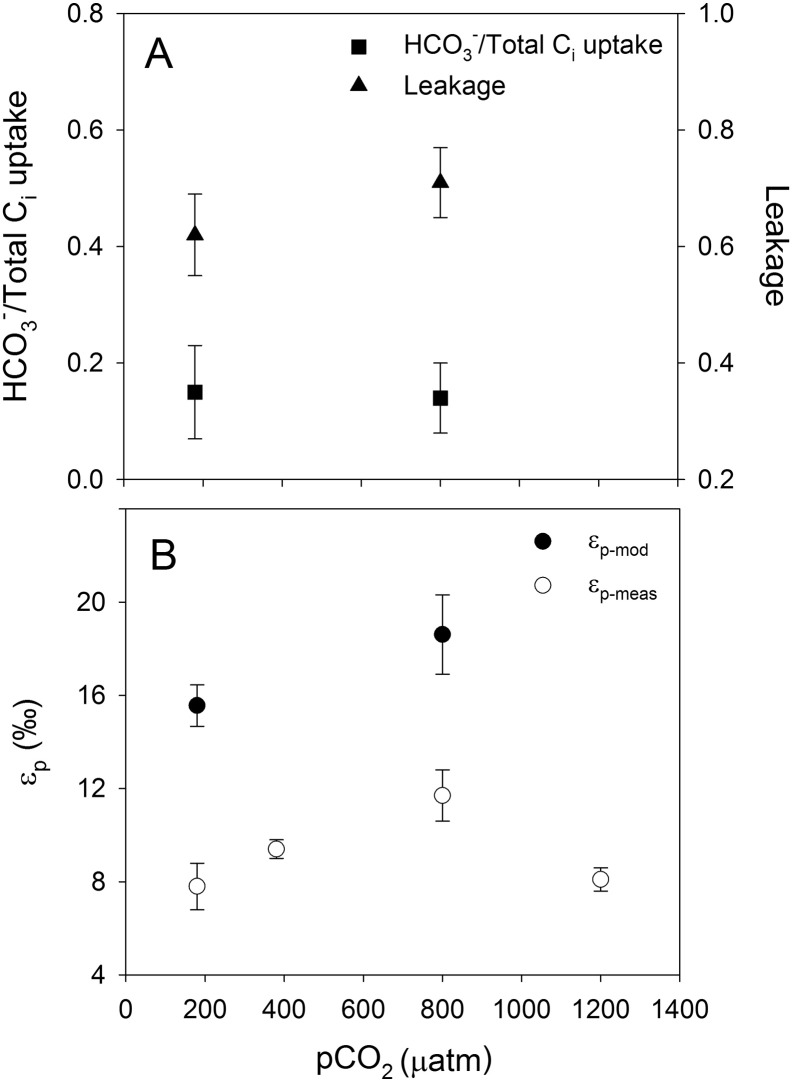
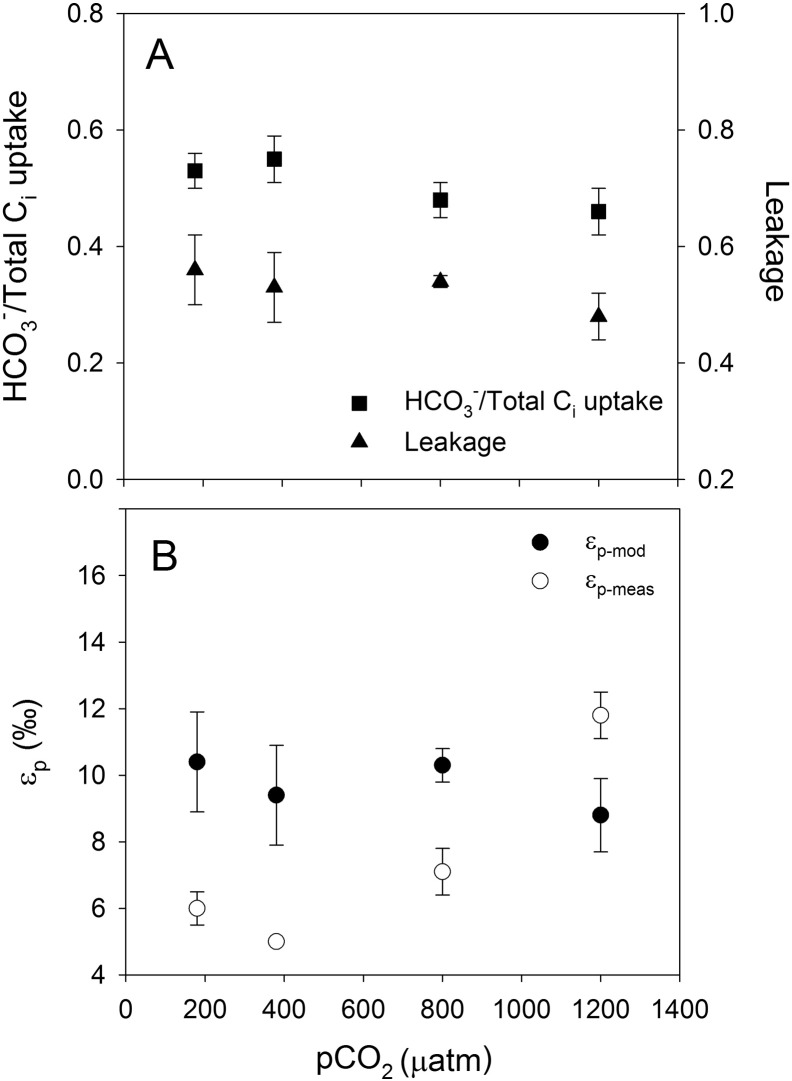
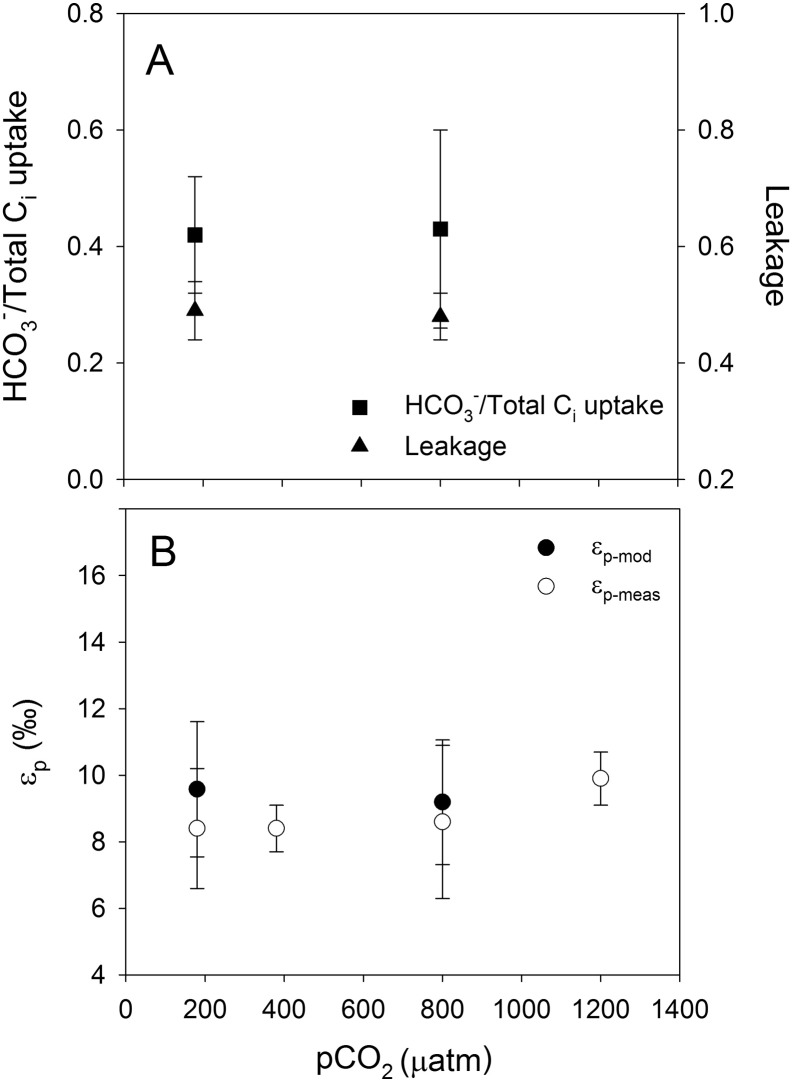
Relative HCO3− contribution was around 0.2 in A. fundyense and G. spinifera (Figs. 1A and 3A; Table 1), whereas S. trochoidea and P. reticulatum showed higher values of ~ 0.5 (Figs. 2A and 4A; Table 1). In other words, in A. fundyense and G. spinifera approximately 80% of the Ci taken up is in the form of CO2, whereas in S. trochoidea and P. reticulatum this was 50%. There was a significant decrease in HCO3− contribution with increasing CO2 concentration in S. trochoidea, while no such CO2-dependency was observed in any of the other tested species. Leakage differed significantly between the tested species, with values of up to 0.7 at 800 μatm pCO2 in G. spinifera (Fig. 3A; Table 1) and lowest average values of ~ 0.5 in S. trochoidea and P. reticulatum, respectively (Figs. 2A and 4A; Table 1). Only in A. fundyense, leakage was significantly CO2-dependent and increased from 0.44 to 0.63 (Fig. 1A; Table 1). For details on the kinetics of O2, CO2 and HCO3− fluxes in A. fundyense and S. trochoidea, please refer to Eberlein et al. (2014).
Fig. 1.
Relative HCO3− contribution, leakage and εp-mod and εp-meas in A. fundyense. Each data point represents the mean ± standard deviation (n = 3).
Fig. 3.
A) Relative HCO3− contribution, leakage and B) εp-mod and εp-meas in G. spinifera. Each data point represents the mean ± standard deviation (n = 3).
Fig. 2.
A) Relative HCO3− contribution, leakage and B) εp-mod and εp-meas in S. trochoidea. Each data point represents the mean ± standard deviation (n = 3).
Fig. 4.
A) Relative HCO3− contribution, leakage and B) εp-mod and εp-meas in P. reticulatum. Each data point represents the mean ± standard deviation (n = 3).
3.3. Ci flux based εp calculations
Estimates for εp-mod are in the same range as εp-meas in A. fundyense and P. reticulatum (Figs. 1B and 4B; Table 1), while the model overestimated the fractionation by up to 5‰ and 8‰ in S. trochoidea and G. spinifera, respectively (Figs. 2B and 3B; Table 1). Except for S. trochoidea, εp-mod generally matches trends observed in εp-meas. In A. fundyense, for instance, εp-mod increased significantly from 10.1 to 15.3‰, thereby closely matching εp-meas values (Fig. 1B; Table 1). Also in G. spinifera, εp-mod matches trends observed in εp-meas, if the highest pCO2 treatment of εp-meas is excluded. In this treatment, carbon isotope fractionation dropped significantly, most likely due to 2.5-fold increased cellular carbon contents (see discussion in Hoins et al., 2015). In S. trochoidea, neither the relative HCO3− contribution nor leakage showed a CO2-dependency; hence εp-mod did not match the increase in εp-meas with increasing CO2 concentration (Fig. 2B; Table 1).
4. Discussion
4.1. CA activity plays a minor role in Ci fluxes
By expressing CA, many marine algae species accelerate the otherwise slow interconversion between CO2 and HCO3−, thereby possibly facilitating the Ci uptake and internal Ci fluxes. In line with previous studies on dinoflagellates (Rost et al., 2006, Ratti et al., 2007), A. fundyense and P. reticulatum exhibit rather low eCA activities, even under low CO2 concentrations. In view of this, eCA is not expected to significantly influence Ci fluxes or εp in these species. In S. trochoidea and G. spinifera, however, eCA activities were high at all tested CO2 concentrations, comparable to values observed for temperate diatoms (Trimborn et al., 2009). Hence, the inhibition of eCA by the inhibitor DBS during the MIMS assay might have biased the Ci fluxes, i.e. underestimated CO2 uptake (Rost et al., 2003), thereby potentially causing an underestimation of εp. As these were the species for which the model overestimated εp values, however, it can be concluded that eCA (and its inhibition by DBS during the Ci flux measurements) did not influence the Ci fluxes much.
4.2. Species-specific differences in Ci fluxes
The HCO3− contribution differed considerably between the tested species. While A. fundyense and G. spinifera showed a strong preference for CO2, S. trochoidea and P. reticulatum used CO2 and HCO3− in equal proportions. The latter contradicts with the findings of an endpoint pH-drift experiment, suggesting that P. reticulatum is not able to efficiently use HCO3− (Ratti et al., 2007). Testing other dinoflagellates with a modified pH-drift method, including the genus Protoceratium, demonstrated that the high pH itself can affect growth and thus interpretations about the used Ci source based on pH-drift experiments must be considered with caution (Hansen et al., 2007). From an energetic point of view, high CO2 usage would be of advantage as CO2 can be taken up passively by diffusion, while HCO3− is charged and thus has to be taken up by active uptake. And yet, the tested species covered a large part of their Ci demand by HCO3−, as observed in S. trochoidea and P. reticulatum (Figs. 2A and 4A).
Similarly high HCO3− contributions were observed for other dinoflagellate species (Rost et al., 2006) and cyanobacteria (Price et al., 2008, Kranz et al., 2011). This preference for HCO3− has been associated with the very low CO2-affinity of RubisCO type IB, which is expressed in cyanobacteria, and RubisCO type II expressed in dinoflagellates (Badger et al., 1998, Whitney and Andrews, 1998). To compensate for this low affinity, high amounts of Ci have to be accumulated, which in seawater can more easily be accomplished with the abundant HCO3− ion rather than with CO2. In addition, HCO3− is about 1000-fold less permeable to lipid membranes than CO2, making it the preferred Ci form to be accumulated within the cell (Price et al., 2008). In the case where species covered the majority of their Ci demand by CO2, as observed in A. fundyense and G. spinifera (Figs. 1A and 3A), one could therefore speculate about chloroplast-based Ci accumulation rather than pumping of HCO3− at the cell wall (Badger et al., 1998). The observed differences in the preferred Ci source and likely consequences for internal Ci fluxes may also affect the overall leakage of cells.
Also leakage differed considerably among the species. A. fundyense showed a relatively low leakage of 0.44 at 180 μatm pCO2, which increased to 0.63 at 1200 μatm pCO2. Leakage in G. spinifera varied between 0.60 at 180 μatm pCO2 and 0.70 at 800 μatm pCO2. Leakage estimates in S. trochoidea and P. reticulatum were lower with ~ 0.50 and remained constant over the applied range of pCO2. These differences may be caused by different membrane permeabilities, which again potentially relate to the preferred Ci source. In fact, A. fundyense and G. spinifera both preferred CO2 over HCO3− and likewise showed the highest degrees of leakage, thereby suggesting highly permeable membranes with respect to CO2. In these species, also CO2-related changes in the membrane permeability are indicated as they show significantly increased leakage under higher pCO2 (see also Eberlein et al., 2014 for A. fundyense, formerly A. tamarense).
4.3. Patterns in carbon isotope fractionation can be explained by Ci fluxes
Using results for HCO3− contribution and leakage obtained in this study, carbon isotope fractionation was calculated and compared to previous measurements (Figs. 1B–4B; see also Hoins et al., 2015). Generally, there is a good agreement as εp-mod and εp-meas values were in the same range (A. fundyense, S. trochoidea, P. reticulatum) and/or followed the same trend (A. fundyense, G. spinifera, P. reticulatum; Figs. 1B-4B). Despite the overall agreement between flux-based estimates and directly measured carbon isotope fractionation, εp-mod was overestimated in S. trochoidea and G. spinifera. Such offsets could principally be attributed to biases in the Ci flux measurements, i.e. uncertainties in the estimation of HCO3− contribution and/or leakage. It has been argued, however, that the MIMS approach tends to overestimate the HCO3− contribution (due to the constant pH of 8.0 during the assay, see Burkhardt et al., 2001), and rather underestimates cellular leakage (due to fact that CO2 fixation does not cease instantly upon darkening, see Badger et al., 1994). Hence, by correcting for these potential biases, i.e. assuming slightly lower HCO3− contribution and higher leakage values, we would actually overestimate the fractionation even more for S. trochoidea and G. spinifera.
An alternative explanation for the overestimation by the model may be attributed to the fractionation factor of RubisCO, which we assumed to be 28‰ (Raven and Johnston, 1991). Recent studies have found lower values, even as low as 11‰ as in the case of the coccolithophore Emiliania huxleyi (Boller et al., 2011). Even though there are no indications for such low fractionation values in the highly conserved type II RubisCO, a lower fractionation would bring modeled and measured εp values closer in S. trochoidea and G. spinifera. In A. fundyense and P. reticulatum, however, it would lead to underestimated εp-mod. Hence, we would refrain from assuming much lower fractionation values for RubisCO type II in dinoflagellates in our calculations. Lastly, the fact that internal Ci fluxes were not taken into account might have also contributed to the offsets between εp-mod and εp-meas. Models that incorporate internal Ci cycling have, however, caused even higher εp-mod, as these processes work against the 13C accumulation within the chloroplasts (Cassar et al., 2006, Schulz et al., 2007) or the carboxysome (Eichner et al., 2015). Therefore, although the values and trends in carbon isotope fractionation are relatively well understood based on our physiological experiments, differences between theory and measurements are at present not fully resolved.
5. Conclusions
Our study demonstrates that carbon isotope fractionation in dinoflagellates can, to a large degree, be explained by considering their Ci fluxes. Relative HCO3− contribution and/or leakage were CO2-dependent in A. fundyense, S. trochoidea and G. spinifera, which in turn can explain the CO2-dependency of their εp observed in previous studies (Hoins et al., 2015). To further advance our understanding of the εp patterns in dinoflagellates, Ci fluxes measurements should be performed at in situ pH (Kottmeier et al., 2014, Kottmeier et al., 2016) and ideally differentiate between 13C and 12C fluxes (McNevin et al., 2006).
Acknowledgments
This research was funded through the Darwin Centre for Biogeosciences Grant 3021, awarded to GJR and AS, and the European Research Council under the Seventh Framework Program of the European Community through ERC Starting Grants #259627 to AS and #205150 to BR. DBvdW and BR thank BIOACID, financed by the German Ministry of Education and Research. This work was carried out under the program of the Netherlands Earth System Science Centre (NESSC). We thank Urban Tillmann (Alfred Wegener Institute) and Karin Zonneveld (Marum, Bremen University) for providing the dinoflagellate species Alexandrium fundyense Alex5 and Scrippsiella trochoidea GeoB267, respectively, and Ulrike Richter, Laura Wischnewski, Jana Hölscher (Alfred Wegener Institute) and Arnold van Dijk (Utrecht University) for technical support. [SS]
Contributor Information
Mirja Hoins, Email: mhoins@awi.de.
Tim Eberlein, Email: tim.eberlein@awi.de.
Dedmer B. Van de Waal, Email: D.vandeWaal@nioo.knaw.nl.
Appy Sluijs, Email: a.sluijs@uu.nl.
Gert-Jan Reichart, Email: g.j.reichart@uu.nl.
Björn Rost, Email: bjoern.rost@awi.de.
References
- Badger M.R., Price G.D. Carbonic anhydrase activity associated with the cyanobacterium Synechococcus PCC7942. Plant Physiol. 1989;89(1):51–60. doi: 10.1104/pp.89.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M.R., Palmqvist K., Yu J.W. Measurement of CO2 and HCO3− fluxes in cyanobacteria and microalgae during steady‐state photosynthesis. Physiol. Plant. 1994;90(3):529–536. [Google Scholar]
- Badger M.R., Andrews T.J., Whitney S.M., Ludwig M., Yellowlees D.C., Leggat W., Price G.D. The diversity and coevolution of RubisCO, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. 1998;76(6):1052–1071. [Google Scholar]
- Boller A.J., Thomas P.J., Cavanaugh C.M., Scott K.M. Low stable carbon isotope fractionation by coccolithophore RubisCO. Geochim. Cosmochim. Acta. 2011;75(22):7200–7207. [Google Scholar]
- Burkhardt S., Riebesell U., Zondervan I. Effects of growth rate, CO2 concentration, and cell size on the stable carbon isotope fractionation in marine phytoplankton. Geochim. Cosmochim. Acta. 1999;63:3729–3741. [Google Scholar]
- Burkhardt S., Amoroso G., Riebesell U., Sültemeyer D. CO2 and HCO3− uptake in marine diatoms acclimated to different CO2 concentrations. Limnol. Oceanogr. 2001;46(6):1378–1391. [Google Scholar]
- Cassar N., Laws E.A., Popp B.N. Carbon isotopic fractionation by the marine diatom Phaeodactylum tricornutum under nutrient- and light-limited growth conditions. Geochim. Cosmochim. Acta. 2006;70(21):5323–5335. [Google Scholar]
- Degens E.T., Behrendt M., Gotthardt B., Reppmann E. Metabolic fractionation of carbon isotopes in marine plankton—II. Data on samples collected off the coasts of Peru and Ecuador. Deep Sea Res. Oceanogr. Abstr. 1968;15(1):11–20. [Google Scholar]
- Eberlein T., Van de Waal D.B., Rost B. Differential effects of ocean acidification on carbon acquisition in two bloom-forming dinoflagellate species. Physiol. Plant. 2014 doi: 10.1111/ppl.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner M., Thoms S., Kranz S.A., Rost B. Cellular inorganic carbon fluxes in Trichodesmium: a combined approach using measurements and modelling. J. Exp. Bot. 2015;66(3):749–759. doi: 10.1093/jxb/eru427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard R.R.L., Ryther J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Guy R.D., Fogel M.L., Berry J.A. Photosynthetic fractionation of the stable isotopes of oxygen and carbon. Plant Physiol. 1993;101(1):37–47. doi: 10.1104/pp.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen P.J., Lundholm N., Rost B. Growth limitation in marine red-tide dinoflagellates: effects of pH versus inorganic carbon availability. Mar. Ecol. Prog. Ser. 2007;334:63–71. [Google Scholar]
- Hinga K.R., Arthur M.A., Pilson M.E., Whitaker D. Carbon isotope fractionation by marine phytoplankton in culture: the effects of CO2 concentration, pH, temperature, and species. Glob. Biogeochem. Cyc. 1994;8:91–102. [Google Scholar]
- Hoins M., Van de Waal D.B., Eberlein T., Reichart G.-J., Rost B., Sluijs A. Stable carbon isotope fractionation of organic cyst-forming dinoflagellates: evaluating the potential for a CO2 proxy. Geochim. Cosmochim. Acta. 2015;160:267–276. [Google Scholar]
- Jasper J.P., Hayes J.M. A carbon isotope record of CO2 levels during the late quaternary. Nature. 1990;347(6292):462–464. doi: 10.1038/347462a0. [DOI] [PubMed] [Google Scholar]
- John U., Litaker R.W., Montresor M., Murray S., Brosnahan M.L., Anderson D.M. Formal revision of the Alexandrium tamarense species complex (Dinophyceae) taxonomy: the introduction of five species with emphasis on molecular-based (rDNA) classification. Protist. 2014;165:779–804. doi: 10.1016/j.protis.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.D., Selvin R.C., Claus W., Guillard R.R.L. Media for the culture of oceanic ultraplankton. J. Phycol. 1987;23:633–638. [Google Scholar]
- Kottmeier D.M., Rokitta S.D., Tortell P.D., Rost B. Strong shift from HCO3− to CO2 uptake in Emiliania huxleyi with acidification: new approach unravels acclimation versus short-term pH effects. Photosynth. Res. 2014;121(2–3):265–275. doi: 10.1007/s11120-014-9984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottmeier D.M., Rokitta S.D., Rost B. Acidification, not carbonation, is the major regulator of carbon fluxes in the coccolithophore Emiliania huxleyi. New Phytol. 2016 doi: 10.1111/nph.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz S.A., Eichner M., Rost B. Interactions between CCM and N2 fixation in Trichodesmium. Photosynth. Res. 2011;109(1–3):73–84. doi: 10.1007/s11120-010-9611-3. [DOI] [PubMed] [Google Scholar]
- McNevin D.B., Badger M.R., Kane H.J., Farquhar G.D. Measurement of (carbon) kinetic isotope effect by Rayleigh fractionation using membrane inlet mass spectrometry for CO2-consuming reactions. Funct. Plant Biol. 2006;33(12):1115–1128. doi: 10.1071/FP06201. [DOI] [PubMed] [Google Scholar]
- Mook W.G., Bommerson J.C., Staverman W.H. Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet. Sci. Lett. 1974;22:169–176. [Google Scholar]
- Pagani M. Treatise on Geochemistry. second ed. 2014. Biomarker-based inferences of past climate: the alkenone pCO2 proxy. [Google Scholar]
- Pierrot D., Lewis E., Wallace D.W.R. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy; Oak Ridge, Tennessee: 2006. MS excel program developed for CO2 system calculations. [Google Scholar]
- Price G.D., Badger M.R., Woodger F.J., Long B.M. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 2008;59(7):1441–1461. doi: 10.1093/jxb/erm112. [DOI] [PubMed] [Google Scholar]
- Ratti S., Giordano M., Morse D. CO2-concentrating mechanisms of the potentially toxic dinoflagellate Protoceratium reticulatum (Dinophyceae, Gonyaulacales) J. Phycol. 2007;43(4):693–701. [Google Scholar]
- Raven J.A., Johnston A.M. Mechanisms of inorganic-carbon acquisition in marine phytoplankton and their implications for the use of other resources. Limnol. Oceanogr. 1991;36:1701–1714. [Google Scholar]
- Roeske C.A., O'Leary M.H. Carbon isotope effects on enzyme-catalyzed carboxylation of ribulose bisphosphate. Biochemistry. 1984;23(25):6275–6284. doi: 10.1021/bi00328a005. [DOI] [PubMed] [Google Scholar]
- Rost B., Zondervan I., Riebesell U. Light dependent carbon isotope fractionation in the coccolithophorid Emiliania huxleyi. Limnol. Oceanogr. 2002;47:120–128. [Google Scholar]
- Rost B., Riebesell U., Burkhardt S., Sültemeyer D. Carbon acquisition of bloom‐forming marine phytoplankton. Limnol. Oceanogr. 2003;48(1):55–67. [Google Scholar]
- Rost B., Richter K.-U., Riebesell U., Hansen P.J. Inorganic carbon acquisition in red tide dinoflagellates. Plant Cell Environ. 2006;29:810–822. doi: 10.1111/j.1365-3040.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- Rost B., Kranz S.A., Richter K.-U., Tortell P.D. Isotope disequilibrium and mass spectrometric studies of inorganic carbon acquisition by phytoplankton. Limnol. Oceanogr. Methods. 2007;5(10):328–337. [Google Scholar]
- Schulz K.G., Riebesell U., Rost B., Thoms S., Zeebe R.E. Determination of the rate constants for the carbon dioxide to bicarbonate interconversion in pH-buffered seawater systems. Mar. Chem. 2006;100:53–65. [Google Scholar]
- Schulz K.G., Rost B., Burkhardt S., Riebesell U., Thoms S., Wolf-Gladrow D.A. The effect of iron availability on the regulation of inorganic carbon acquisition in the coccolithophore Emiliania huxleyi and the significance of cellular compartmentation for stable carbon isotope fractionation. Geochim. Cosmochim. Acta. 2007;71(22):5301–5312. [Google Scholar]
- Scott K.M., Henn-Sax M., Harmer T.L., Longo D.L., Frame C.H., Cavanaugh C.M. Kinetic isotope effect and biochemical characterization of form IA RubisCO from the marine cyanobacterium Prochlorococcus marinus MIT9313. Limnol. Oceanogr. 2007;52(5):2199–2204. [Google Scholar]
- Sharkey T.D., Berry J.A. Carbon isotope fractionation of algae as influenced by an inducible CO2 concentrating mechanism. In: Lucas W.J., Berry J.A., editors. Inorganic Carbon Uptake by Aquatic Photosynthetic Organisms. The American Society of Plant Physiologists (ASPP); 1985. pp. 389–401. [Google Scholar]
- Silvermann D.N. Carbonic anhydrase. Oxygen-18 exchange catalyzed by an enzyme with rate-contributing proton-transfer steps. Methods Enzymol. 1982;87:732–752. doi: 10.1016/s0076-6879(82)87037-7. [DOI] [PubMed] [Google Scholar]
- Trimborn S., Wolf-Gladrow D., Richter K.U., Rost B. The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J. Exp. Mar. Biol. Ecol. 2009;376(1):26–36. [Google Scholar]
- Van de Waal D.B., John U., Ziveri P., Reichart G.J., Hoins M., Sluijs A., Rost B. Ocean acidification reduces growth and calcification in a marine dinoflagellate. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney S.M., Andrews T.J. The CO2/O2 specificity of single subunit ribulose bisphosphate carboxylase from the dinoflagellate Amphidinium carterae. Aust. J. Plant Physiol. 1998;25:131–138. [Google Scholar]
- Zeebe R.E., Wolf-Gladrow D.A. Elsevier Science; Amsterdam, The Netherlands: 2001. CO2 in Seawater: Equilibrium, Kinetics, Isotopes. [Google Scholar]