Abstract
Purpose
The aim of this study is to report peripheral reperfusion of ischemic areas of the retina on ultra-widefield fluorescein angiography (UWFA) following anti-vascular endothelial growth factor (VEGF) intravitreal injections in patients treated for diabetic retinopathy.
Methods
This study is a retrospective review of 16 eyes of 15 patients with diabetic retinopathy, who received anti-VEGF intravitreal injections and underwent pre- and postinjection UWFA. The main outcome measured was the presence of reperfusion in postinjection UWFA images in areas of the retina that demonstrated nonperfusion in preinjection images. Images were analyzed for reperfusion qualitatively and quantitatively by two graders.
Results
Twelve of 16 eyes (75%) or 11 of 15 patients (73.3%) demonstrated reperfusion following anti-VEGF injection. On UWFA, reperfusion was detected both within the field of 7-standard field (7SF) fluorescein angiography and in the periphery outside the 7SF. Four of 16 eyes or 4 of 15 patients did not demonstrate reperfusion, one of which had extensive scarring from prior panretinal photocoagulation.
Conclusion
In patients with diabetic retinopathy, treatment with anti-VEGF agents can be associated with reperfusion of areas of nonperfusion, as demonstrated by UWFA.
Keywords: anti-VEGF, diabetes, diabetic retinopathy, ischemia, perfusion, reperfusion
Introduction
In diabetes mellitus, high blood glucose levels and advanced glycosylation end products cause constriction and leakage of retinal vasculature, thickening of the basement membrane, and pericyte apoptosis.1–4 This damage to the vasculature results in ischemia, promoting a cascade of molecular processes including upregulation of hypoxia inducible factor-1, which in turn upregulates cytokines and growth factors, including vascular endothelial growth factor (VEGF), which further contribute to breakdown of the blood–retinal barrier.5,6 Analyses of vitreous fluid from patients with diabetic retinopathy and proliferative diabetic retinopathy consistently demonstrate abnormally increased VEGF concentrations.7,8 In support of the clinical evidence, studies in animal models indicate a correlative role of VEGF in diabetic retinopathy. Injection of VEGF into primate vitreous fluid induces lesions consistent with diabetic retinopathy.9 Neutralizing VEGF in diabetic rats inhibits blood–retinal barrier breakdown in a dose-dependent manner.10 These findings indicate that VEGF is a major contributor to diabetic retinopathy and diabetic macular edema.
Major clinical trials have demonstrated that anti-VEGF therapies improve vision. The RISE and RIDE trials demonstrated that intravitreal injection of ranibizumab 0.3 mg (Lucentis, Hoffman-La Roche Ltd, Basel, Switzerland), a monoclonal antibody against VEGF-A, as compared with sham injections, improved all measures of vision assessed in the trials.11 The VIVID and VISTA trials demonstrated that intravitreal aflibercept 2 mg (Eylea, Regeneron Pharmaceuticals, Inc, Tarrytown, NY, USA) therapy resulted in greater gains and fewer losses in visual acuity when compared with laser.12 In The Diabetic Retinopathy Clinical Research Network’s (DRCR.net) Protocol I, treatment with ranibizumab plus prompt or deferred laser improved patient visual acuity, as compared with sham injections and laser.13 These and other studies have established anti-VEGF intravitreal injections as the standard of care for the treatment of diabetic macular edema.
In addition to improvement of visual acuity and reduction of diabetic macular edema, studies have shown that anti-VEGF therapies normalize blood vessels on 7-standard fields (7SFs) color fundus photographs. Analyses of photographs obtained in RISE and RIDE demonstrated that patients receiving intravitreal ranibizumab were more likely to have ≥2-step or ≥3-step improvement in Diabetic Retinopathy Severity Scale score than patients receiving sham injections.14 In VIVID and VISTA, a greater proportion of eyes treated with intravitreal aflibercept had ≥2-step improvement in Diabetic Retinopathy Severity Scale score compared with eyes treated with laser photocoagulation.12 A retrospective analysis of fluorescein angiography (FA) obtained in the RISE and RIDE trials demonstrated decreased rate of progression of nonperfusion in treated eyes in patients receiving monthly 0.3 mg injections of ranibizumab, or 0.5 mg injections of ranibizumab, as compared with those patients receiving monthly sham injections (with crossover to ranibizumab injections).15 While these studies showing reduced progression of nonperfusion are encouraging, there are to our knowledge no prior studies evaluating retinal reperfusion after anti-VEGF therapy in diabetic retinopathy.
Development of ultra-widefield fluorescein angiography (UWFA) has expanded visualization of the retina to 200° of the retina from 75° on the 7SF protocol, enabling more thorough assessment of peripheral nonperfusion, neovascularization, and panretinal photocoagulation.16 Ultra-widefield imaging has demonstrated that peripheral lesions not visualized with 7SF are clinically significant. A study comparing mydriatic ultra-widefield imaging versus 7SF reported that one-third of lesions detected on ultra-widefield imaging were outside the 7SF.17 The presence of these peripheral lesions, undetectable on 7SF, was associated with a 3.2-fold increased risk of progression of diabetic retinopathy and a 4.7-fold increased risk of progression to proliferative diabetic retinopathy.18 UWFA has an additional advantage over 7SF in that it enables more accurate comparison of perfusion over multiple clinic visits. A single 7SF image is produced from seven individual images that are not captured simultaneously, thus the distribution of fluorescein dye in an individual image differs from the distribution of dye in every other individual image. This difference in the points at which separate parts of the 7SF image were captured decreases the accuracy of a comparison of perfusion in two 7SF images taken at two different clinic visits. UWFA captures more area at any single time point following fluorescein dye injection, allowing for a more true comparison of perfusion over multiple visits. UWFA is essential to assessment of diabetic retinopathy.
In this study, we therefore hypothesized that 1) areas of retinal nonperfusion have the potential to reperfuse following anti-VEGF therapy and 2) UWFA can be used to identify this normalization of perfusion following anti-VEGF treatment for diabetic retinopathy. To assess this hypothesis, we performed qualitative and quantitative analysis of areas of nonperfusion noted on fluorescein angiograms obtained prior to and after treatment with anti-VEGF injections.
Methods
Patients were initially identified by screening for those with a diagnosis of diabetes with ophthalmic complications (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 250.50) along with common proficiency test coded indicating that both a fluorescein angiogram (92235) and an intravitreal injection (67028) were performed. The inclusion criteria were: diagnosis of diabetic retinopathy (nonproliferative diabetic retinopathy or proliferative diabetic retinopathy), treatment with at least one anti-VEGF injections in the study eye (ranibizumab 0.3 mg, aflibercept 2 mg, or bevacizumab 1.25 mg), and pre- and postinjection UWFA imaging available, with the postinjection UWFA being within 5 months of the last intravitreal anti-VEGF injection in the study eye. The exclusion criteria were history of intravitreal anti-VEGF therapy before the preinjection UWFA, poor quality of imaging due to media opacity, concurrent nondiabetic retinal vascular disease (retinal artery occlusion, retinal vein occlusion), or absence of retinal nonperfusion on preinjection UWFA.
The electronic medical record was reviewed and the following data were collected: age, sex, body mass index (BMI), weight, comorbidities (hypertension [HT], congestive heart failure [CHF]), HbA1c, history of smoking, indication for pre- and postinjection UWFA, dates, names, and indications for all intravitreal injections. UWFA images included in this study had been generated using Optos Optomap Panoramic 200A (Optos PLC, Dunfermline, Scotland), captured digitally with the Optos V2 Vantage Review Software, and stored as uncompressed tiff files. All patients had provided written informed consent for all fluorescein angiograms performed and for each intravitreal anti-VEGF injections performed. This retrospective study was approved by the Institutional Review Board at Weill Cornell Medical College. Written informed consent for participation in this study was deemed unnecessary by the review board due to its retrospective nature.
Pairs of pre- and postinjection UWFA images were graded for reperfusion (1= reperfusion present in postinjection image compared side-by-side with preinjection image, 0= no reperfusion) by 2 independent graders (AML and IR); both graders were masked to all characteristics of the patients and injections that corresponded to each image. Reperfusion was verified by a senior grader (SK). Because the graders could not be masked to the identities of the images as pre- or postinjection (due to obvious identifiers such as abnormal neovascularization in preinjection images), reperfusion was confirmed quantitatively using ImageJ software. During this process, none of the automatic settings, including automatic white balance, were changed. Each image that was graded positively for reperfusion by the graders was converted in ImageJ to an 8-bit grayscale image (256 levels of gray, with pure black pixels =0 and pure white pixels =1). Then, a minimum threshold of 35% was set so that pixels <35% appeared black. This threshold was collectively decided upon by 3 graders and applied consistently to every image. Each image was then binarized so that perfused areas were white on a black background. The difference in white pixel number between the pre- and postinjection images was calculated in selected areas determined by the graders to have reperfusion. We were unable to quantify pixels over the area of an entire image due to artifacts such as eyelashes, lids, glare, etc. Therefore, we had to select small areas that were judged to have reperfusion. Any increase in pixel count postinjection supported a grade positive for reperfusion. Pairs of images that were graded positively for reperfusion by the graders and demonstrated increased pixel count in the selected area were considered to demonstrate reperfusion.
Results
Forty-nine eyes of 39 patients were retrospectively identified as having a diagnosis of diabetes, having underwent intravitreal anti-VEGF injection(s), and having received pre- and postinjection UWFA, with the postinjection UWFA occurring less than 5 months after the last anti-VEGF injection. Thirty eyes of 21 patients were excluded due to nongradable images from media opacities (vitreous hemorrhage, cataract) or movement artifact, resulting in 19 eyes of 18 patients with gradable images. Of these 19, 1 eye was excluded because of concurrent acute retinal vein occlusion, and 2 eyes of 2 patients were excluded because of absence of nonperfusion in the preinjection image. A total of 16 eyes of 15 patients were thus included in the study. All patients were injection naive prior to their preinjection image.
Indication for the preinjection UWFA was standard of care diabetic retinopathy management for all 16 eyes. The UWFA revealed a diagnosis of nonproliferative diabetic retinopathy in 1 eye, proliferative diabetic retinopathy not meeting high-risk criteria in 9 eyes, proliferative diabetic retinopathy meeting high-risk criteria in 3 eyes (2 of whom subsequently had panretinal laser photocoagulation and 1 of whom did not), a history of proliferative diabetic retinopathy with prior panretinal photocoagulation, and persistent neo-vascularization requiring additional fill-in laser (which was performed during the study period) in 2 eyes, and a history of proliferative diabetic retinopathy with prior panretinal photocoagulation and improvement in neovascularization in 1 eye. Indications for anti-VEGF therapy included treatment of diabetic macular edema in 15 of 16 eyes and treatment of high-risk proliferative diabetic retinopathy in the absence of edema in 1 eye after obtaining informed consent including extensive discussion of the risks and benefits of observation versus panretinal photocoagulation versus intravitreal anti-VEGF therapy, the ongoing DRCR.net Protocol S study and as-of-yet unestablished efficacy in clinical trials of anti-VEGF therapy for diabetic retinopathy, and the off-label nature of treatment of proliferative diabetic retinopathy with anti-VEGF therapies. The mean time between the pre- and postinjection FA was 337 days (range: 50–1,173). Indications for postinjection UWFA included annual UWFA in 2 patients (one with prior FA showing nonproliferative diabetic retinopathy and one with prior UWFA showing regressing proliferative diabetic retinopathy status post panretinal photocoagulation), reassessment for progression to high-risk proliferative diabetic retinopathy in the 9 eyes that had previously demonstrated non–high-risk proliferative diabetic retinopathy, evaluation for regression of neovascularization after laser in 3 eyes, evaluation of the contralateral eye after clearance of vitreous hemorrhage in 1 eye, and reevaluation of high-risk proliferative diabetic retinopathy in 1 eye that was treated with anti-VEGF therapy alone for this condition.
Twelve out of 16 eyes (75%) demonstrated reperfusion both qualitatively and quantitatively on UWFA following anti-VEGF therapy. Representative images are included in Figures 1 and 2. The 11 patients who reperfused included 7 males and 4 females with a mean age of 60.0 years (range: 46–73) at the time that the second image was obtained, mean weight of 87.7 kg, mean BMI of 30.7, and mean HbA1c of 8.7% (range: 7.2–14.0) (Table 1). This group included 10 patients with a diagnosis of HT, 2 with a diagnosis of CHF, 7 never smokers, 1 current smoker, and 3 former smokers. Eleven eyes who reperfused received only ranibizumab injections, 1 received only bevacizumab injections, and the mean number of injections per eye was 3 (range: 1–6; Table 2). The images were obtained at a mean of 71.9 seconds (range: 38–110) after the fluorescein dye was injected, and the pre- and postimages were obtained at a mean of 4.5 seconds (range: 0–28) apart. The postinjection image was obtained at a mean of 67 days (range: 21–144) after the patient received their last anti-VEGF injection and 211 days (range: 50–544) from the preinjection UWFA.
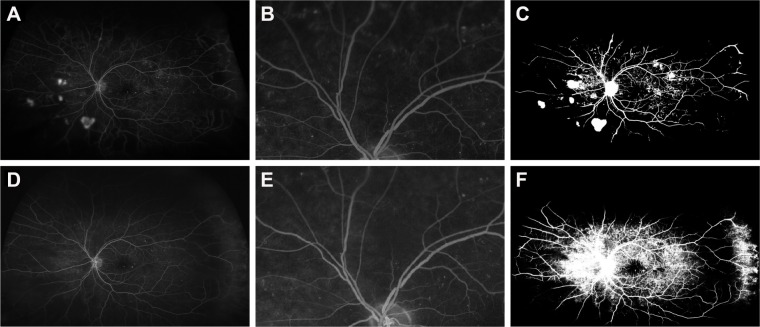
Figure 1.
Reperfusion in eye of a 61-year-old male, following treatment with 5 injections of ranibizumab.
Notes: Preinjection fluorescein angiography (A–C) shows ischemia and abnormal neovascularization (A, preinjection fluorescein angiogram; B, magnified view of preinjection fluorescein angiogram; and C, binary preinjection image). Postinjection images (D–F) show reperfusion with improved perfusion as compared with preinjection image A (D, postinjection fluorescein angiogram; E, magnified view of preinjection fluorescein angiogram; and F, binary postinjection image).
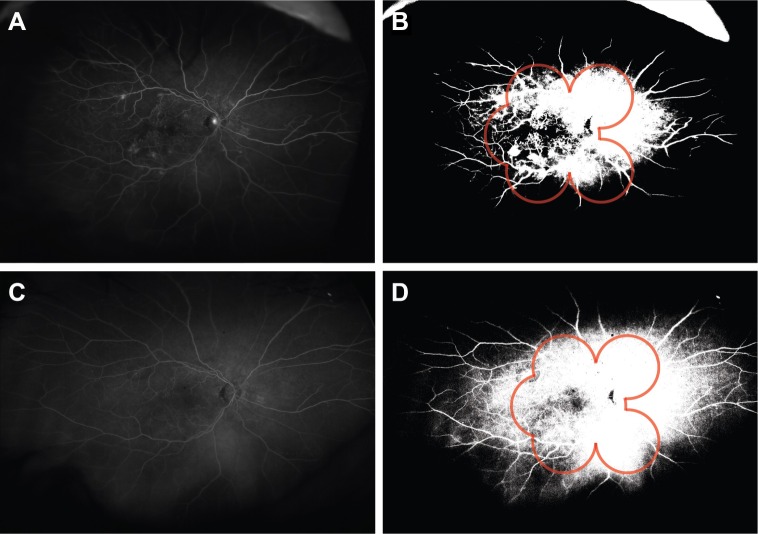
Figure 2.
Reperfusion in eye of a 60-year-old female, following treatment with two injections of ranibizumab.
Notes: The preinjection fluorescein angiography (A, preinjection fluorescein angiogram and B, binary preinjection image) shows peripheral ischemia both inside and outside the field of 7SF FA (B, red outline). The postinjection image (C, postinjection fluorescein angiogram and D, binary postinjection image) shows reperfusion both inside and outside the field of 7SF FA (D, red outline).
Abbreviations: 7SF, 7-standard field; FA, fluorescein angiography.
Table 1.
Patient demographics (n=15 patients)
| Variable | Positive reperfusion (n=11) |
Negative reperfusion (n=4) |
|---|---|---|
| Sex (n) | ||
| Male | 7 | 2 |
| Female | 4 | 2 |
| Diabetes mellitus type (n) | ||
| 1 | 0 | 0 |
| 2 | 11 | 4 |
| Age (years) | ||
| Mean | 60 | 61.5 |
| Range | 46–73 | 47–71 |
| Weight (kg) | ||
| Mean | 87.7 | N/A |
| Range | 54.9–141.5 | |
| Body mass index (kg/m2) | ||
| Mean | 30.7 | N/A |
| Range | 21.9–48.1 | |
| HbA1c (%) | ||
| Mean | 8.68 | N/A |
| Range | 7.2–14 | |
| Comorbidities (n)* | ||
| HT | 10 | 2 |
| CHF | 2 | 2 |
| Smoker (n) | ||
| Never | 7 | 3 |
| Former | 3 | 1 |
| Current | 1 | 0 |
Note:
One patient in the positive reperfusion group had both HT and CHF.
Abbreviations: CHF, congestive heart failure; HT, hypertension; N/A, data not available.
Table 2.
Treatment and UWFA imaging (n=16 eyes)
| Variable | Positive reperfusion (n=12) |
Negative reperfusion (n=4) |
|---|---|---|
| Drug (n)* | ||
| Ranibizumab | 11 | 2 |
| Bevacizumab | 1 | 2 |
| Aflibercept | 0 | 1 |
| No injections | ||
| Mean | 3 | 7.25 |
| Range | 1.0–6.0 | 5.0–14.0 |
| Time between last injection and postinjection UWFA (days) | ||
| Mean | 67 | 42 |
| Range | 21.0–144.0 | 21.0–70.0 |
| Time of FA after dye injected (seconds) | ||
| Mean | 72 | 58 |
| Range | 38.0–110.0 | 38.0–91.0 |
| Time difference between pre- and postinjection images evaluated (seconds) | ||
| Mean | 4.5 | 15.5 |
| Range | 0.0–28.0 | 1.0–31.0 |
Note:
One patient in the negative reperfusion group was treated with more than one drug.
Abbreviations: FA, fluorescein angiography; UWFA, ultra-widefield fluorescein angiography.
Four out of 16 eyes (25%) did not demonstrate reperfusion. These 4 patients included 2 males and 2 females with a mean age of 61.5 years (range: 47–71) at the time that the second image was obtained (Table 1). There were not enough available data to calculate mean weight, BMI, or HbA1c. This group included 2 patients with a diagnosis of HT, 2 with a diagnosis of CHF, 3 never smokers, and 1 former smoker. One patients received only ranibizumab injections, 1 received ranibizumab and aflibercept, 2 received bevacizumab injections only, and the mean number of injections was 7.25 (range: 5–14; Table 2). The images were obtained at a mean of 58 seconds (range: 38–91) after the fluorescein dye was injected, and the pre- and postimages were obtained at a mean of 15.5 seconds (range: 1–31) apart. The postinjection image was obtained at a mean of 42 days (range: 21–70) after the patient received their last anti-VEGF injection and 642 days (range: 217–1173) from the preinjection UWFA.
Discussion
In this small retrospective case series, we demonstrate that treatment of diabetic retinopathy with anti-VEGF injections has the potential to reverse retinal ischemia as noted on UWFA. These findings suggest that areas of ischemia on UWFA can possess viable, salvageable tissue with the potential to reperfuse. Though our study is not designed to demonstrate cause and effect between anti-VEGF injections and reperfusion, we believe that injections likely contribute to reperfusion by stabilizing healthier blood vessels. Reperfusion of vessels might be one reason that clinical trials have shown reversal of diabetic retinopathy after anti-VEGF therapy.
The molecular mechanism by which anti-VEGF therapies normalize retinal blood vessels remains unknown, but studies of other retinal diseases and extensive studies of anti-VEGF effects on tumors might give insight into the role of anti-VEGF therapy in the retina. One prior study demonstrated reversal of retinal nonperfusion in eyes treated with anti-VEGF agents for retinal vein occlusion. The authors of the study hypothesized that anti-VEGF agents interrupt a positive feedback loop in which injury induces VEGF signaling, resulting in further injury.19 Large clinical trials of cancer treatments have demonstrated that anti-VEGF therapy enhances the effect of chemotherapy.20–22 Additional studies have contrasted the efficacy of anti-VEGF combined with chemotherapy with the relative ineffectiveness of anti-VEGF monotherapy.23 This contrast led to the hypothesis that anti-VEGF stabilizes tumor vessels, thus augmenting chemotherapy delivery. Investigations of this hypothesis elucidated specific processes involved in the normalization of vessels treated with anti-VEGF, including reversal of hyperpermeability, restoration of pericytes, and normalization of the basement membrane.24,25 Normalized basement membrane could serve as a framework for vessel regrowth in the retina as well.
In our study, some eyes demonstrated expansive reper-fusion, while other eyes reperfused in only limited areas of the retina, while some eyes did not reperfuse at all. One possibility is that ischemic areas that do not reperfuse following anti-VEGF injections might be completely infarcted or might involve living tissue that is not responsive to the given doses of anti-VEGF injections. One patient who did not reperfuse had expansive scarring from panretinal photocoagulation that may have prevented reperfusion or detection of reperfusion. Our finding that not all patients reperfused is supported by a previous study that showed spontaneous reperfusion in some but not all untreated eyes diagnosed with diabetic retinopathy. This previous study defined reperfusion as either neovascularization or recanalization, and found recanalization in one-third of eyes.26 Our study was not powered to compare the demographics of the populations that either reperfused or did not reperfuse, as only 5 patients did not reperfuse. A recent analysis of RISE and RIDE reported decreased rates of progression of nonperfusion in patients treated with ranibizumab.15 While no patients were reported to have reperfused in that analysis, the study was not designed to measure reperfusion. In the RISE and RIDE analyses of retinal nonperfusion, nonperfusion was measured categorically by the number of disk areas (DA) containing nonperfused regions (eg, >0 to <1 DA of nonperfusion versus >1 to <2 DA, etc). This method was not tailored for measuring subtle reperfusion, as an image with >1 to <2 DA of nonperfusion could partially reperfuse while still demonstrating regions of nonperfusion, albeit smaller regions, in >1 to <2 DA. In our study, some of our patients reperfused outside the area captured by 7SF FA. In patients who reperfused only outside the area captured by 7SF FA, reperfusion would not have been detected in the RISE and RIDE images. Our population of patients who reperfused was fairly similar to patients in RISE and RISE receiving injections: mean ages in all RISE and RIDE groups receiving injections ranged from 61.7–62.8 years (our study: 60.0 years), mean BMI 31.3–32.9 kg/m2 (30.7 kg/m2), history of smoking 45.6%–51.6% (36%), and mean HbA1c 7.6%–7.7% (8.7%).11 Our specific goal of measuring reperfusion and our use of UWFA may be the reason that our study detected reperfusion that was not reported in previous analyses of diabetic retinopathy.
Less than 6 months prior to the start of our study, Protocol T published by the Diabetic Retinopathy Clinical Research Group demonstrated that patients treated with aflibercept required fewer injections and less laser therapy to achieve greater visual acuity than patients treated with ranibizumab or bevacizumab.27 Therefore, it would be of interest to study reperfusion in patients treated only with aflibercept. Although the number of patients receiving aflibercept in our clinic increased following the publication of Protocol T, these patients have yet to receive a postinjection UWFA and do not qualify for this study.
The strengths of this study include the use of UWFA to capture peripheral nonperfusion and reperfusion, and the confirmation of qualitative analysis by retina specialists with quantitative measurements of reperfusion. Quantitative analysis prevented bias resulting from recognition of an image as pre- or postinjection and from the assessment of an area of reperfusion as darker or lighter based on the brightness of surrounding vasculature. A limitation of this study is that the images were not all captured at exactly the same time point at exactly the same angle, because the time and angle depended on uncontrolled variables such as the patient’s precise position during imaging. This variation between images is to be expected in any retrospective review of FA images, as well as in a physician’s analysis of images during a patient encounter. This study is also limited by the inability to confirm that areas of increased fluorescence represent areas of reperfusion. Some areas of assumed reperfusion clearly show definite vascular outlines, and these areas can be interpreted most confidently as reperfusion. Some areas show increased fluorescence, but outlines of vessels are blurred either because the equipment is not sensitive enough to clearly capture the outlines or because the patient was moving. Follow-up studies are needed to demonstrate reperfusion in patients treated with anti-VEGF agents and to better understand the characteristics of patients who reperfuse. A future prospective study could elucidate the relationship between treatment regimens, demographic factors, imaging and examination findings, and the potential for reperfusion. Whether there is any correlation in reperfusion and effect of the anti-VEGF therapy on macular thickness should also be specifically assessed. In our retrospective study, the macular edema in these patients waxed and waned and the number of injections over the study period was similarly variable, while perfusion was only assessed at a single pre- and postinjection standpoint, therefore making it difficult to assess correlations between perfusion and macular thickness.
Conclusion
Treatment with anti-VEGF agents can be associated with reperfusion of areas of nonperfusion. Prospective studies evaluating the effect of anti-VEGF therapy on perfusion should incorporate correlations to macular thickness response and other imaging features in the study design. Future studies could also explore the molecular mechanism of reperfusion and the potential role of different anti-VEGF therapies.
Acknowledgments
This work is supported in part by Research to Prevent Blindness Physician-Scientist Award (SK), The Henry Adelman Medical Student Scholarship Program in Geriatrics (AML), Patricia M. Dunnington and The Seth Sprague Educational and Charitable Foundation.
Footnotes
Disclosure
Szilárd Kiss serves as a consultant for Alimera, Allergan, Avalanche, Genentech/Roche, Optos, and Regeneron. Ariana M Levin, Irene Rusu, Anton Orlin, Mrinali Gupta, Peter Coombs, and Donald J D’Amico report no conflicts of interest in this work.
References
- 1.Early Treatment Diabetic Retinopathy Study Research Group ETDRS Report No. 12: Fundus photographic risk factors for progression of diabetic retinopathy. Ophthalmology. 1991;98(5 Suppl):823–833. [PubMed] [Google Scholar]
- 2.Liew G, Sim DA, Keane PA, et al. Diabetic macular ischaemia is associated with narrower retinal arterioles in patients with type 2 diabetes. Acta Ophthalmol. 2015;93(1):e45–e51. doi: 10.1111/aos.12519. [DOI] [PubMed] [Google Scholar]
- 3.Roy S, Maiello M, Lorenzi M. Increased expression of basement membrane collagen in human diabetic retinopathy. J Clin Invest. 1994;93(1):438–442. doi: 10.1172/JCI116979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamagishi S, Amano S, Inagaki Y, et al. Advanced glycosylation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem Biophys Res Commun. 2002;290(3):973–978. doi: 10.1006/bbrc.2001.6312. [DOI] [PubMed] [Google Scholar]
- 5.Ozaki H, Yu AY, Della N, et al. Hypoxia inducible factor-1-alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40(1):182–189. [PubMed] [Google Scholar]
- 6.Kelly BD, Hackett SF, Hirota K, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93(11):1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 7.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 8.Adamis AP, Miller JW, Bernal MT, et al. Increased endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 9.Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103(11):1820–1828. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- 10.Qaum T, Xu Q, Joussan AM, et al. VEGF-initiated blood-retinal barrier break-down in early diabetes. Invest Ophthalmol Vis Sci. 2001;42(10):2408–2413. [PubMed] [Google Scholar]
- 11.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Elman MJ, Bressler NM, Qin H, et al. The Diabetic Retinopathy Clinical Research Network Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–614. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122(2):367–374. doi: 10.1016/j.ophtha.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121(9):1783–1789. doi: 10.1016/j.ophtha.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Wessel MM, Aaker GD, Parlitsis G, Cho M, D’Amico DJ, Kiss S. Ultra-widefield angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785–791. doi: 10.1097/IAE.0b013e3182278b64. [DOI] [PubMed] [Google Scholar]
- 17.Silva PS, Cavallerano JD, Sun JK, Soliman AZ, Aiello LM, Aiello LP. Peripheral lesions identified by mydriatic ultra-wide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120:2587–2595. doi: 10.1016/j.ophtha.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Silva PS, Cavallerano JD, Haddad NMN, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122(5):949–956. doi: 10.1016/j.ophtha.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013;120(4):795–802. doi: 10.1016/j.ophtha.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Novotny N, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 21.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 22.Tebbutt NC, Wilson K, Gebski VJ, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28(19):3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 23.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 24.Dickson PV, Hamner JB, Sims TL, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13(13):3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 25.Tetsuichiro I, Mancuso M, Hashizume H, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165(1):35–3952. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Kishi S, Muraoka K, Shimizu K. Reperfusion of occluded capillary beds in diabetic retinopathy. Am J Ophthalmol. 1998;126(6):791–797. doi: 10.1016/s0002-9394(98)00242-6. [DOI] [PubMed] [Google Scholar]
- 27.The Diabetic Retinopathy Clinical Research Network Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]