Abstract
Fungal growth inhibition on solid media has been historically measured and calculated based on the average of perpendicular diameter measurements of growth on fungicide amended media. We investigated the sensitivity of the calculated area (DA) and the measured area (MA) for assessing fungicide growth inhibition of the ascomycete, Phyllosticta citricarpa on solid media. Both the calculated, DA and the actual measured area, MA were adequate for distinguishing significant treatment effects of fungicide on fungal growth, however MA was more sensitive at identifying significant differences between the controls and fungicide concentrations below 5 ppm.
Introduction
Methods of filamentous fungal growth assessment depend upon the media in which the fungus is grown. Direct measurements of fungal growth are utilized when fungi are grown in liquid media and single time point measurements are required. Biomass production (fungal growth) can be measured directly from dry or wet weight assessment. In cases where there is difficulty in separating mycelia from media, indirect measures are utilized. Indirect measures of fungal growth include visual observation and measurement [1–6], indicator dye hydrolysis, increases in measurable component unique to the fungus such as growth linked enzymes—laccase, ergosterol and/or chitin [7–9] and absorbance [10–13]. Radial growth measurement is a popular indirect measure of filamentous growth on solid media [14–16] when assessing fungicidal properties of chemicals. Radial growth measurements are usually taken perpendicularly [17] and may be reported as the average of two or more pairs of measurements [6]. Measurements may be made utilizing pre-drawn lines prior to placement of the inoculating fungal plug or drawn at the time of assessment.
Phyllosticta citricarpa (McAlp.) is a phytopathogenic fungus causing significant economic losses in citrus. P. citricarpa is a filamentous fungus of the class ascomycetes, order Dothideales. Cultures grow well on agar media with optimal growth between 24 and 27°C [18–20]. Fungal growth on potato dextrose agar (PDA) is slow and characterized by compact dark pigmented mycelia with a lobate intermediate zone of white to grey colored mycelia and a distinct lobate translucent edge [19, 21]. In some P. citricarpa isolates, mycelia penetrate the media to form plaque-like growth [21].
The relationship between fungal colony diameter and actual growth is governed by the assumption that colony diameter is a good indicator of colony growth. For this assumption to hold true: (i) the rate of growth in all directions must be equal, such that measurements made perpendicular to each other will give the average of overall growth of the fungus and (ii) growth occurs only on the surface of the media or that growth into the media is minimal and does not affect horizontal growth. Because P. citricarpa does not conform to these parameters (Fig 1) we tested the hypotheses that calculated area (DA) using the averages of perpendicular diameter to predict inhibition will be less sensitive than the use of the measured area (MA).
Fig 1. Day 7 growth of Phyllosticta citricarpa on unamended PDA.
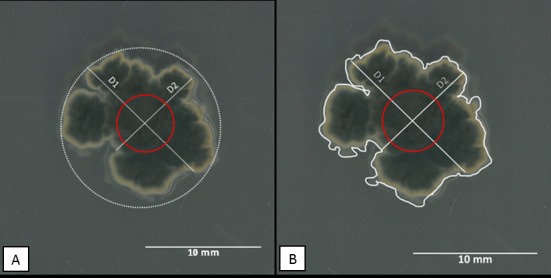
(A) Measurements D1 and D2 are the perpendicular length for estimating growth and used to compute calculated area (DA). The red circle is the approximate placement and size of the plug. (B) Measured area (MA) is represented by the area encompassed by the white outline of the colony.
Material and Methods
Fungal isolates
A selection of P. citricarpa isolates were obtained from citrus black spot lesions on fruits isolated between 2010 and 2013. Isolates were maintained on potato dextrose agar (PDA) at room temperature and under ambient light. These isolates were assumed to be clonal as there has been no evidence of both mating types in Florida to date [22] and lack of sexual structure produced in mating studies (data not shown). A total of 22 isolates were used (Table 1).
Table 1. List of Phyllosticta citricarpa isolates obtained from Citrus sinensis fruits in Florida and their characteristic included in this study.
| Year isolateda | Locationb | Isolate no. | Other namec | Symptomsd | ITS Sequencee | Yellow pigmentf |
|---|---|---|---|---|---|---|
| 2010 | 1 | 2010_01 | FLGC1 | Hard spot | yes | Present |
| 2010 | 1 | 2010_03 | FLGC3 | Hard spot | yes | Present |
| 2010 | 2 | 2010_04 | FLGC4 | Hard spot | no | Present |
| 2010 | 2 | 2010_06 | FLGC6 | False melanose | no | Present |
| 2010 | 2 | 2010_08 | FLGC8 | False melanose | no | Present |
| 2010 | 2 | 2010_10 | FLGC10 | False melanose | no | Present |
| 2011 | 3 | 2011_02 | - | - | yes | Present |
| 2011 | 2 | 2011_15 | FLGC15 | - | no | Present |
| 2011 | 2 | 2011_63 | - | False melanose | no | Present |
| 2011 | 2 | 2011_67 | - | Hard spot | no | Present |
| 2011 | 2 | 2011_88 | - | - | no | Present |
| 2012 | 2 | 2012_051 | - | Hard spot | no | Present |
| 2012 | 2 | 2012_086 | - | Hard spot | no | Present |
| 2012 | 2 | 2012_117 | - | Hard spot | no | Present |
| 2012 | 2 | 2012_125 | - | Hard spot | no | Present |
| 2012 | 2 | 2012_135 | - | Hard spot | no | Present |
| 2013 | 3 | 2013_01 | - | - | no | Present |
| 2013 | 3 | 2013_03 | - | - | no | Present |
| 2013 | 3 | 2013_04 | - | - | no | Present |
| 2013 | 3 | 2013_11 | - | - | no | Present |
| 2013 | 3 | 2013_18 | - | - | no | Present |
| 2013 | 3 | 2013_20 | - | - | no | Present |
aYear fruit with symptoms were collected and isolates isolated from lesions.
bUnique locations (groves) from which diseased fruit were obtained.
cOther isolate identification codes, these were used in the study of copper on growth characteristics of Phyllosticta citricarpa [23].
dLesion from which the fungus was isolated; a dash (-) indicates no information on lesion used for isolation, however isolations where typically made from hard-spots, false melanose and virulent spots.
eSequencing of the ITS region for molecular identification using CITRI1/ITS4 primers [24].
Fungal growth
Fungal growth was assessed using (i) that calculated area (DA) and (ii) measured colony area (MA) on Day 7, 14 and 21 on PDA with and without fungicide. Each isolate was grown on PDA with and without the demethylation inhibitor (DMI) fungicide, fenbuconazole (dissolved in acetone) to obtain a final concentration of 0.1, 1, 5, and 10 ppm (μg/ml). Controls were PDA and PDA amended with acetone (1 ml/L). Plates were maintained at 25°C for 21 days under a 12 hr light-dark cycle and scans of each plate were taken using a on days 7, 14, and 21 post inoculation. Scans were made using a Konica Minolta Bizhub C652 (Konica Minolta Business Solutions U.S.A., Inc.) and saved as JPEG images (600 x 600 dpi, 24 bit depth, file size ranged from 2.19 to 3.01 MB). Scans were analyzed using the National Institutes of Health ImageJ v1.4 software, measure tool (rsb.info.nih.gov/ij/index.html) [26]. Two replicated were carried out per isolate per fungicide concentration and the entire experiment was repeated twice.
Methods of measurement were compared to determine the differences in analyzed results and whether these differences changed the final conclusion made at the treatment level. In order to compare measured area (MA) to diameter (D), average diameter was converted to area of a circle,
| (1) |
and then compared to measured area, MA. Inhibition ratios, Ratio0 and RatioA were calculated using the controls–PDA and PDA amended with acetone (1 ml/L) respectively using the formula:
| (2) |
Statistical analysis
Under normal circumstances an EC50 approach (the fungicide concertation required to reduce growth by 50% of the control) would be used to evaluate in vitro fungicide sensitivity of the fungus of interest. In this case, since the concentrations tested resulted either in no effect or greater than 50% reduction this approach was not used. Instead inhibition ratios were analyzed using a mixed model with fungicide concentration*day*method of fungal growth assessment (Media*Day*Method) as the fixed effect and repetition was the random effect. A Beta distribution for the ratio was assumed. Since the Beta distribution does not allow for values to be either 0 or 1, in the few instances where the observed ratio equaled 1 it was converted to 0.995 for analyses. All analysis was done in SAS 9.4 (SAS Institute Inc., NC) using PROC GLIMMIX. The Tukey method was used to control the family-wise error rate for multiple comparisons. A p-value ≤ 0.05 was considered statistically significant.
Results and Discussion
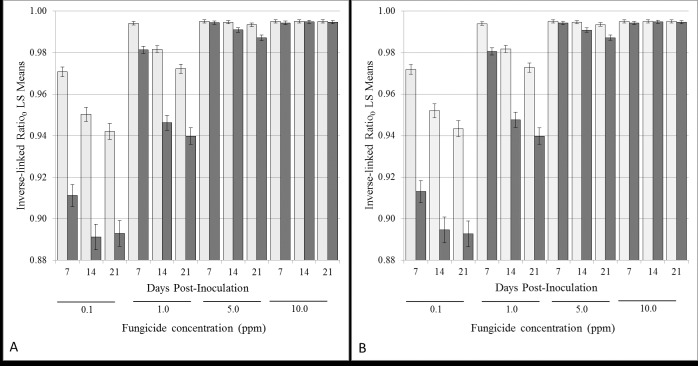
In comparing methods of assessment of fungal growth on control media and media amended with fenbuconazole using inhibition ratios, the fixed effect Media*Day*Method was highly significant for Ratio0 and RatioA, having p-values of p<0.0001 (for Ratio0, F23, 2033 = 259.27; for RatioA, F23, 2033 = 273.35). Measurements of fungal growth inhibition by measured area (MA) were significantly different from calculated area, DA at lower concentrations of fungicide (0.1 to 5 ppm) on all days measured with the exception of 5 ppm on Day 7 (Fig 2A). This can be attributed to the initial lag or inhibition in growth caused by higher concentration of the fungicide. There was no difference between methods used to assess fungicide growth inhibition for the 10 ppm on all days assessed (Fig 3; S1 Table) and this is attributed to complete inhibition of growth by the highest fungicide concentration. This was true for ratios calculated based on either PDA (Ratio0) or PDA amended with acetone (RatioA) as controls (Fig 2A and 2B).
Fig 2. Comparison of LS-Means for the three-way interaction, Media*Day*Method, where dark bars represent measured Area (MA) and light bars represent area calculated from Diameter (DA).
A. Inhibition ratios calculated using un-amended PDA as control (Ratio0) for each time point, Days 7, 14 and 21 for each fungicide level tested. B. Inhibition ratios calculated using acetone amended PDA as control (RatioA) for each time point, Days 7, 14 and 21 for each fungicide level tested. The error-bars are the 95% confidence intervals for each mean.
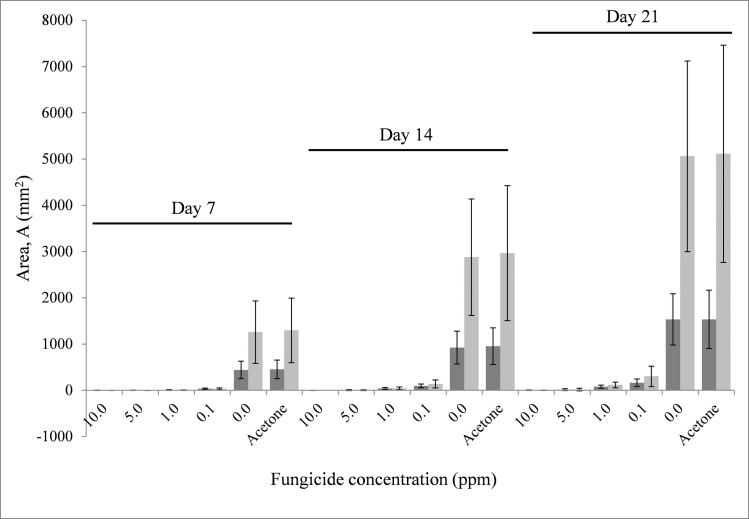
Fig 3. Area of fungal growth measured, MA (dark bar) or calculated, DA (light bar) from average diameter for Days 7, 14 and 21 for each fungicide level tested.
The error-bars are the 95% confidence intervals for each mean.
The measured area, MA is a more sensitive means of distinguishing subtle changes in fungal growth inhibition at lower fungicide concentrations. This is further reflected in the ability of MA to distinguish small changes in fungal growth inhibition between Day 7 and 14 (p = 0.0094), Day 7 and 21 (p<0.0001), and Day 14 and 21(p = 0.0262) at 5 ppm (Ratio0) which were indistinguishable using calculated area, DA (p>0.05). Similar findings were found with ratios calculated using PDA amended with acetone (RatioA) as control (Fig 2B–Measured Area, MA—Day 7 vs 14 (p = 0.0104), Day 7 vs 21 (p<0.0001) and Day 14 vs 21(p = 0.0314)).
The lowest fungicide concentration (0.1 ppm) inhibited growth by an average of 80% (85%—Day 7; 79%—Day 14 and 76% -Day 21; p<0.05) when using the diameter of fungal growth and an average of 91% (92%—Day 7; 90%—Day 14 and 89% -Day 21; p<0.05) when using area to measure fungal inhibition. Overall, in this study DA and/or MA was adequate to distinguish significant treatment effects of fungicide on fungal growth, however MA was more sensitive.
In this study, analysis of area calculated from colony diameter (DA) and measured area (MA) were adequate to distinguish significant differences in fungicide growth inhibition for Phyllosticta citricarpa. However, measured area (MA) proved to be more sensitive in distinguishing subtle changes in growth at and below 5 ppm fenbuconazole between days measured. This was expected as calculating the area of a circle using average diameter overestimates the true area (Fig 1).
A drawback of using two-dimensional measures such as area or diameter is that the third-dimension of colony density is not taken into consideration [27]. Additionally, there is evidence to suggest that as fungi age there is a greater rate of hyphal extension which is not reflected in changes in colony diameter [27], however the impact of fenbuconazole on this phenomenon was not examined in this study.
Supporting Information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Florida Specialty Crop Block Grant #22915, Title: Production and Postharvest Treatments to Control Citrus Black Spot (Guignardia citricarpa) on Florida Citrus. Authors: Mark Ritenour, Associate Professor; Pamela D. Roberts, Professor; Jan Narciso, Research Microbiologist. URL: http://www.freshfromflorida.com/Business-Services/Grant-Opportunities/2016-Florida-Specialty-Crop-Block-Grant-Program-Farm-Bill-SCBGP-FB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Majchrowicz I, Poprawski TJ. Effects in vitro of nine fungicides on growth of entomopathogenic fungi. Biocontrol Sci Technol. 1993;3(3):321–36. [Google Scholar]
- 2.Flores FJ, Garzon CD. Detection and assessment of chemical hormesis on the radial growth in vitro of oomycetes and fungal plant pathogens. Dose-Response. 2013;11(3):361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feliziani E, Santini M, Landi L, Romanazzi G. Pre- and postharvest treatment with alternatives to synthetic fungicides to control postharvest decay of sweet cherry. Postharvest Biology and Technology. 2013;78:133–8. [Google Scholar]
- 4.Bertelsen JR, De Neergaard E, Smedegaard-Petersen V. Fungicidal effects of azoxystrobin and epoxiconazole on phyllosphere fungi, senescence and yield of winter wheat. Plant Pathol. 2001;50(2):190–205. [Google Scholar]
- 5.Meyer L, Slippers B, Korsten L, Kotzé J, Wingfield M. Two distinct Guignardia species associated with citrus in South Africa. S Afr J Sci. 2001;97(5/6):191–4. [Google Scholar]
- 6.Hincapie M, Wang N-Y, Peres NA, Dewdney MM. Baseline sensitivity of Guignardia citricarpa isolates from Florida to azoxystrobin and pyraclostrobin. Plant Dis. 2013;98(6):780–9. [DOI] [PubMed] [Google Scholar]
- 7.Matcham SE, Wood DA, Jordan BR. The measurement of fungal growth in solid substrates. Appl Biochem Biotechnol. 1984;9(4):387–8. [Google Scholar]
- 8.Mohd As’wad AW, Sariah M, Paterson RRM, Zainal Abidin MA, Lima N. Ergosterol analyses of oil palm seedlings and plants infected with Ganoderma. Crop Prot. 2011;30(11):1438–42. [Google Scholar]
- 9.van Kuijk SJA, Sonnenberg ASM, Baars JJP, Hendriks WH, Cone JW. Fungal treatment of lignocellulosic biomass: Importance of fungal species, colonization and time on chemical composition and in vitro rumen degradability. Anim Feed Sci Technol. 2015;209:40–50. [Google Scholar]
- 10.Langvad F. A rapid and efficient method for growth measurement of filamentous fungi. J Microbiol Methods. 1999;37(1):97–100. [DOI] [PubMed] [Google Scholar]
- 11.Broekaert WF, Terras FRG, Cammue BPA, Vanderleyden J. An automated quantitative assay for fungal growth inhibition. FEMS Microbiol Lett. 1990;69(1–2):55–9. [Google Scholar]
- 12.Kerbs S, Hutton RD, Hollister JW. Visual micromethod for assay of fungal growth. Can J Microbiol. 1978;24(5):574–8. [DOI] [PubMed] [Google Scholar]
- 13.Teichert A, Schmidt J, Porzel A, Arnold N, Wessjohann L. (Iso)-Quinoline alkaloids from fungal fruiting bodies of Cortinarius subtortus. Journal of Natural Products. 2008;71(6):1092–4. 10.1021/np8000859 [DOI] [PubMed] [Google Scholar]
- 14.Iacomi-Vasilescu B, Avenot H, Bataillé-Simoneau N, Laurent E, Guénard M, Simoneau P. In vitro fungicide sensitivity of Alternaria species pathogenic to crucifers and identification of Alternaria brassicicola field isolates highly resistant to both dicarboximides and phenylpyrroles. Crop Prot. 2004;23(6):481–8. [Google Scholar]
- 15.Brancato FP, Golding NS. The diameter of the mold colony as a reliable measure of growth. Mycologia. 1953;45(6):848–64. [Google Scholar]
- 16.Mohammedi Z, Atik F. Fungitoxic effect of natural extracts on mycelial growth, spore germination and aflatoxin B1 production of Aspergillus flavus. Australian Journal of Crop Science. 2013;7(3):293–8. [Google Scholar]
- 17.Trinci APJ. A Kinetic Study of Growth of Aspergillus nidulans and Other Fungi. J Gen Microbiol. 1969;57:11-&. 10.1099/00221287-57-1-11 [DOI] [PubMed] [Google Scholar]
- 18.Van der Aa HAvd. Studies in Phyllosticta I. Stud Mycol. 1973;5:1–110. [Google Scholar]
- 19.Baldassari R, Wickert E, de Goes A. Pathogenicity, colony morphology and diversity of isolates of Guignardia citricarpa and G. mangiferae isolated from Citrus spp. Eur J Plant Pathol. 2008;120(2):103–10. [Google Scholar]
- 20.Wager VA. The black spot disease of Citrus in South Africa. Science Bulletin Department of Agriculture and Forestry, Union of South Africa. 1952;(303):52. [Google Scholar]
- 21.Baayen RP, Bonants PJM, Verkley G, Carroll GC, van der Aa HA, de Weerdt M, et al. Nonpathogenic isolates of the citrus black spot fungus, Guignardia citricarpa, identified as a cosmopolitan endophyte of woody plants, G. mangiferae (Phyllosticta capitalensis). Phytopathology. 2002;92(5):464–77. 10.1094/PHYTO.2002.92.5.464 [DOI] [PubMed] [Google Scholar]
- 22.Wang N- Y, Zhang K, Huguet-Tapia JC, Rollins JA, Dewdney MM. Mating type and simple sequence repeat markers indicate a clonal population of Phyllosticta citricarpa in Florida. Phytopathology. 2016. [DOI] [PubMed] [Google Scholar]
- 23.Hendricks KEM, Donahoo RS, Roberts PD, Christman MC. Effect of copper on growth characteristics and disease control of the recently introduced Guignardia citricarpa on citrus in Florida. American Journal of Plant Sciences. 2013;4(2):282–90. [Google Scholar]
- 24.Meyer L, Sanders GM, Jacobs R, Korsten L. A one-day sensitive method to detect and distinguish between the citrus black spot pathogen Guignardia citricarpa and the endophyte Guignardia mangiferae. Plant Dis. 2006;90(1):97–101. [DOI] [PubMed] [Google Scholar]
- 25.Glienke C, Pereira OL, Stringari D, Fabris J, Kava-Cordeiro V, Galli-Terasawa L, et al. Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot. Persoonia. 2011;26:47–56. 10.3767/003158511X569169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniwaki MH, Pitt JI, Hocking AD, Fleet GH. Comparison of hyphal length, ergosterol, mycelium dry weight, and colony diameter for quantifying growth of fungi from foods Advances in food mycology: Springer; 2006. p. 49–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.