Abstract
Streptococcus mutans is the leading cause of dental caries worldwide by accumulating a glycogen-like internal polysaccharide (IPS) that contributes to cariogenicity when sugars are in excess. Sodium monofluorophosphate (MFP) is an active anticariogenic compound in toothpastes. Herein, we show that MFP inhibits (with an I0.5 of 1.5 mM) the S. mutans ADP-glucose pyrophosphorylase (EC 2.7.7.27), which catalyzes the key step in IPS biosynthesis. Enzyme inhibition by MFP is similar to orthophosphate (Pi), except that the effect caused by MFP is not reverted by fructose-1,6-bisP, as occurs with Pi. Inhibition was correlated with a decrease in acidogenesis and IPS accumulation in S. mutans cells cultured with 2 mM sodium MFP. These effects were not mimicked by sodium fluoride. Considering that glycogen synthesis occurs by different pathways in mammals and bacteria, ADP-glucose pyrophosphorylase could be visualized as a molecular target for controlling S. mutans virulence. Our results strongly suggest that MFP is a suitable compound to affect such a target, inducing an anticariogenic effect primarily by inhibiting a key step in IPS synthesis.
Introduction
It has been demonstrated that S. mutans, a resident of the normal flora of the oral cavity, is the main etiologic agent of dental caries [1–3]. Cariogenicity relies on the capacity of the bacterium to: (i) build up biofilm, (ii) acidify the extracellular milieu, and (iii) persist in an adverse environment, three uncommon feasibilities in other bacteria. These capabilities are related to the accumulation of a glycogen-like internal polysaccharide (IPS), whose attributed role is to provide a carbon source during periods of shortage with the concomitant production of acidity [4]. The role of sugars on the pathogenesis of dental biofilm formation determining cariogenicity has been established in good detail [5]; and a direct relationship has been evidenced between the capacity to accumulate IPS and the in vivo cariogenic potential of S. mutans [6]. Besides, the biosynthesis and catabolism of glycogen in prokaryotes have been identified to be critical for virulence and ability of bacteria to build up biofilm [7,8].
The pathways for synthesis of glycogen in prokaryotes and mammals are remarkably different [7–10]. Indeed, the respective enzymes are not homologous and the glucosyl donor used to elongate the α-1,4-glucan is either UDP-Glc (eukaryotes) or ADP-Glc (bacteria). In addition, their regulations are different. In bacteria, the synthesis of ADP-Glc is controlled, but in eukaryotes the regulatory step is the glucan elongation [9]. In prokaryotes, production of ADP-Glc (a metabolite that is not found in mammals) takes place by the reaction catalyzed by ADP-Glc pyrophosphorylase (EC 2.7.7.27; ADP-Glc PPase): ATP + Glc-1P ↔ADP-Glc + PPi. ADP-Glc PPases are enzymes finely regulated by metabolites with the characteristic that, even when varying according to the source, the activator is a key intermediate in the major carbon assimilatory pathway in the respective organism [9,10]. Distinctively from other bacteria, the ADP-Glc PPase from Firmicutes is composed by subunits GlgC and GlgD that give rise different oligomeric forms of the protein [11–13]. This is the case for the enzyme from S. mutans, which has been recombinantly produced in the GlgC (having low activity), the GlgD (inactive) and the GlgC/GlgD (fully active) forms. GlgC and GlgC/GlgD were found distinctively regulated by metabolites, being the latter (supposedly the functional enzyme found in S. mutans) inhibited by phosphoenolpyruvate (PEP) and orthophosphate (Pi), in a way that is overcome by fructose-1,6-bis-phosphate (Fru-1,6-bisP) [11].
Several compounds have been assayed to control the cariogenic process, including many fluoride agents [14]. Among them, sodium monofluorophosphate (MFP) was found to be effective and is thus included in the formulation of toothpastes. Nevertheless, the use of MFP is empirical, as a particular mechanism for its action has never been described. Recently [15], the importance of classical compounds used to treat the caries process has been reviewed and has enumerated several molecules with specific enzymatic targets. Generally, the target enzymes are glycosyltransferases involved in the biosynthesis of exo-polysaccharides for bacterial adhesion and biofilm formation. In this work, we report the inhibitory effect of MFP on the ADP-Glc PPase of S. mutans, which induces a reduction in IPS biosynthesis and correlates with changes in the physiological features of the microorganism. Because as above detailed, glycogen synthesis takes place by different pathways in mammals and bacteria; we conclude that ADP-Glc PPase represents a key target for MFP to control S. mutans virulence.
Methods
Chemicals
All protein standards, antibiotics, isopropyl—thiogalactoside (IPTG), nalidixic acid and other chemicals were of the highest quality available obtained from Sigma-Aldrich or similar.
Cultures and in vivo assays
S. mutans ATCC 25175 planktonic cultures were incubated at 37°C in LAPTg medium, (10 g/l yeast extract, 10 g/l trypteine, 15 g/l meat peptone, 10 g/l glucose, 1% v/v Tween 80, pH 6.5) in a 3% CO2 atmosphere without stirring. The inoculum consisted of a 12 h culture adjusted to OD600 0.10. The factor for correlating OD600 and cellular dry mass (CDW) was determined. All cultures were conducted in triplicate. Acidification was measured using a pH-meter.
The minimal inhibitory concentration (MIC, the lowest compound concentration analyzed that prevents visible growth) for MFP and sodium fluoride (NaF) was determined following the broth and agar dilution method, according to reported protocols [16]. Briefly, serial twofold dilutions of MFP or NaF (in a 0.5–64 mM range) were assayed in planktonic S. mutans ATCC 25175 cultures. After 24 h, the culture turbidity at OD600 was determined to check the growth, which was further confirmed by plating in LAPTg-AGAR (LAPTg medium plus 2% agar).
Protein methods
The hetero-tetrameric S. mutans ADP-Glc PPase (the GlgC/GlgD conformation) was recombinantly produced and purified as previously described [11]. The protein concentration was determined by the modified Bradford assay [17] using BSA as a standard.
Enzyme assays
ADP-Glc PPase was measured following the synthesis of ADP-[14C]Glc from [14C]Glc1P and ATP, according to reported protocols [18]. The standard reaction mixture contained 100 mM MOPS buffer (pH 8.0), 10 mM MgCl2, 1 mM [14C]Glc-1P (100–1000 cpm/nmol), 3 mM ATP, 0.5 mU/μl inorganic pyrophosphatase, and 0.2 mg/ml bovine serum albumin plus enzyme in a total volume of 0.2 ml. Reactions were incubated for 10 min at 37°C and terminated by heating in a boiling-water bath for 1 min. The ADP-[14C]Glc formed during the reaction was then converted to [14C]glycogen by E. coli glycogen synthase. Then, glycogen was precipitated with 0.1 M KCl (in methanol 75% v/v), washed with the same solution and resuspended in distilled water. Radioactivity was measured by a scintillation counter. One unit (U) of enzyme activity is equal to 1 μmol of product formed per minute under the conditions specified above.
Calculation of kinetic constants
MFP curves were performed by assaying enzymatic activity at saturating levels of substrates. The experimental data were plotted as relative enzyme activity versus MFP concentration, and the kinetic constants were determined by fitting the data to the Hill equation, as described elsewhere [19]. Fitting was performed with the Levenberg-Marquardt nonlinear least-squares algorithm provided by the computer program Origin™. The kinetic constant I0.5 corresponds to the concentration that gives 50% maximal inhibition. All kinetic constants are the mean of at least three sets of reproducible data within ± 10%.
Extraction and determination of intracellular polysaccharides
Polysaccharide extraction was achieved by a previously described alkali treatment protocol [20–22]. Briefly, samples from S. mutans cultures were collected by centrifugation, washed with ice-cold water and centrifuged again. Cells were resuspended to OD600 5.0 and boiled for 5 min. Then, 0.3 ml 30% w/v KOH was added per ml of cell suspension, and samples were boiled for 90 min. After cooling, solutions were neutralized with acetic acid, and polysaccharides were precipitated at 0°C with 3 volumes of 97% v/v ethanol. After centrifugation, polysaccharides were dissolved in 0.1 ml water, and a 30 μl aliquot was digested with 2 U of amyloglucosidase from Aspergillus niger in 100 mM acetate buffer pH 4.5 for 2 h at 55°C in a final volume of 100 μl. The released glucose was determined by the specific glucose oxidase method [23], and the amount of monosaccharide was taken as a measure of the glycogen content.
Results
In vivo studies
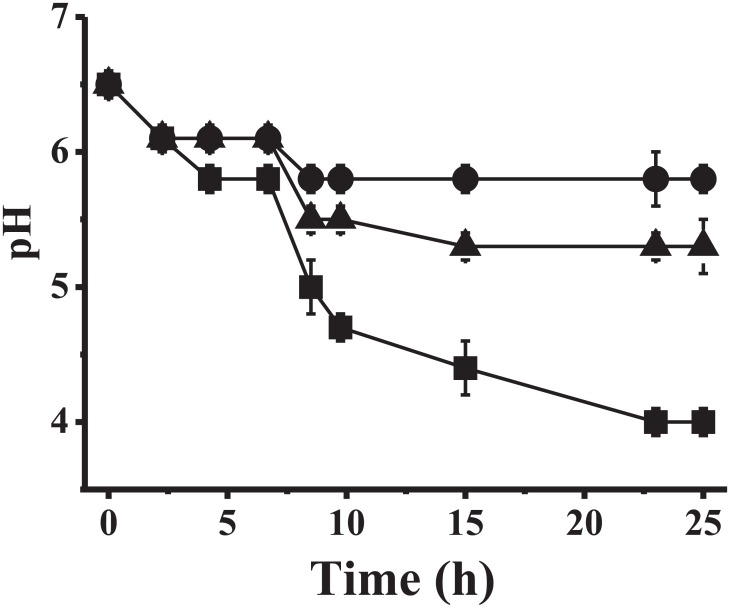
First, we analyzed the effect of NaF and MFP on the growth of S. mutans ATCC 25175, determining MIC values of 2 mM and 4 mM, respectively. Taking into account the values of MICs, we decided to analyze the MFP effect by supplementing LAPTg medium with 2 mM MFP as well as by adding 1 mM NaF to establish a comparative analysis, as described below. As shown in Fig 1, a culture of S. mutans ATCC 25175 lowered the pH of the medium from 6.50 to 4.00 after 24 h (control). On the other hand, when S. mutans was grown in identical conditions, but with the addition of MFP (2 mM), it lowered the pH of the medium only to 5.29. When 1 mM NaF was added to the control, the pH lowered to 5.80. For this reason, this experiment indicated that both NaF and MFP reduced the acidogenesis ability of the bacterium. In agreement with previous reports, when S. mutans was grown in the presence of 2 mM Pi, the cells behaved identically to control cultures (data not shown) [24].
Fig 1. pH reduction from S. mutans ATCC 25175 planktonic cultures in the presence of 2 mM MFP (circles) or 1 mM NaF (triangles).
Cultures with no MFP or NaF are shown in squares.
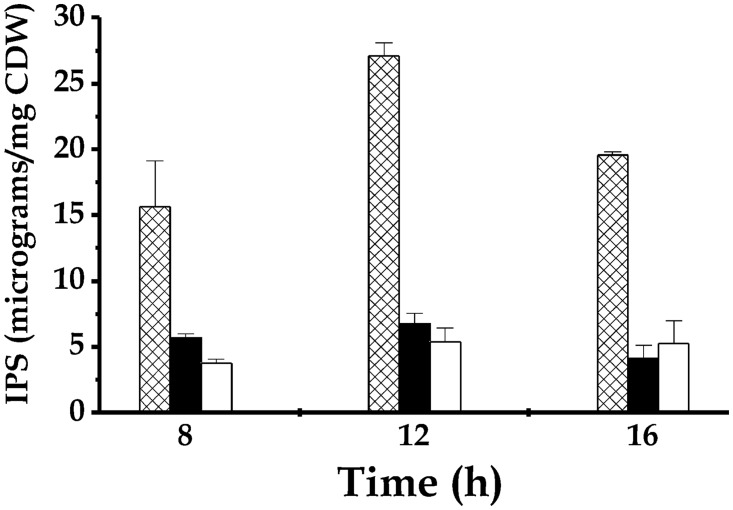
Cultures with and without MFP were analyzed with regard to their IPS content at three points of growth (8, 12 and 16 h). Cultures with NaF were included to compare with previously reported S. mutans behavior [25]. As shown in Fig 2, IPS accumulation was strongly affected in the presence of 2 mM MFP. Towards the end of the exponential phase (8 h samples), the IPS level was one-third that of the control. Entering the stationary phase (12 h), IPS accumulation was approximately 4-fold lower than control cultures. Additionally, less IPS accumulation was observed in control cultures when the advanced stationary phase was reached. Nevertheless, the ratio (4 to 1) between the IPS content in control and MFP cultures was sustained. In addition, the effect of MFP on IPS accumulation was similar to that exerted by NaF, which is in accordance with previous reports on the effect of NaF on S. mutans cultures [25]. It is noteworthy that the presence of 2 mM Pi [same concentration as MFP] affected neither growth of S. mutans cells nor IPS accumulation (data not shown).
Fig 2. Glycogen content from S. mutans ATCC 25175 planktonic cultures in the presence of 2 mM MFP (black bars) or 1 mM NaF (white bars).
Cultures with no MFP or NaF are shown in plaid bars.
Enzymatic studies
Previously [11], we characterized both active S. mutans ADP-Glc PPase conformations, GlgC and GlgC/GlgD, and demonstrated that this enzyme catalyzes the key step in IPS synthesis, known to be a major cariogenic factor [4,6,26–28]. In addition, we showed that S. mutans ADP-Glc PPase activity is allosterically regulated by metabolites from the glycolytic pathway, such as PEP, Fru-1,6-bisP and Pi. Additionally, GlgC/GlgD was sensitive to salt ions, although NaF (up to 25 mM) exhibited no effect [11].
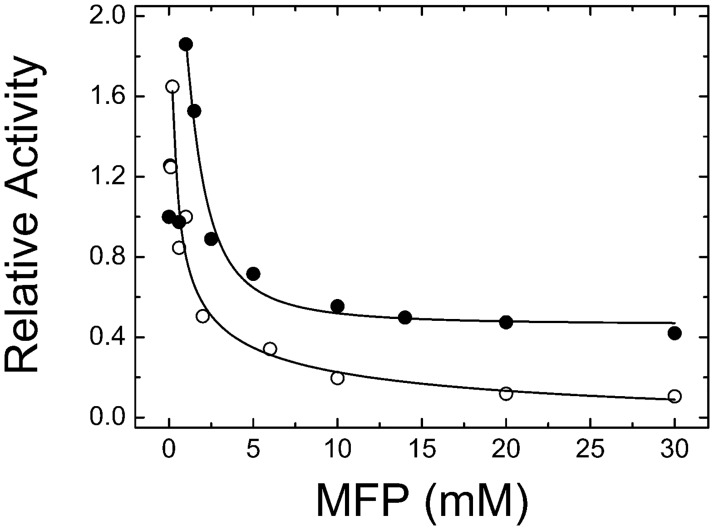
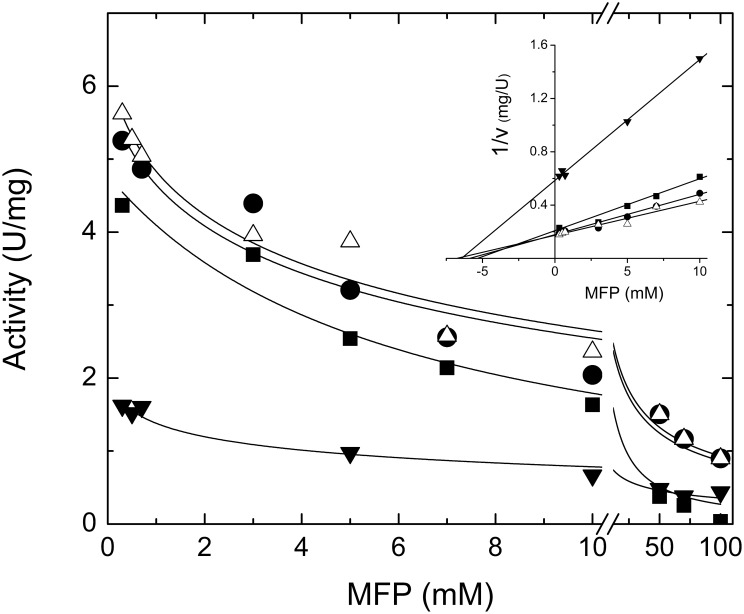
Because of the structural analogy it exhibits with Pi, we analyzed whether the S. mutans ADP-Glc PPase is sensitive to MFP. We found that MFP inhibits the hetero-tetrameric GlgC/GlgD (the form with physiologic relevance) enzyme. As it has been reported for the effect of Pi [11], MFP produced somehow complex effect, with a near 2-fold activation at low concentrations followed by a marked inhibitory effect, where enzymatic activity decreased more than 10-fold with an I0.5 of 1.5 mM (Fig 3). The curves were also conducted at four different ATP concentrations (1, 3, 5 and 10 mM; see Fig 4). As shown, in all the cases the pattern complexity of the effect was similar. Dixon plots [29] of the data obtained in the inhibitory range indicate that the major effect of MFP is not competitive with ATP. Line intercepts suggest that the effect is non-competitive or slightly mixed with an apparent KI in the range of 5–7 mM (Fig 4, inset). Fig 4 shows that I0.5 values for MFP do not strongly depend on ATP concentration. In general, MFP inhibition resembles the effect reported for Pi [11] (see also Fig 3), although the latter has a slightly smaller I0.5 values. This similarity may be due to the geometry shared by both compounds. However, a difference between the inhibition caused by MFP or Pi is that the latter is slightly reverted by Fru-1,6-bisP, while that of MFP is not (Table 1). In addition, PEP, a highly sensitive inhibitor of GlgC/GlgD (I0.5 0.08 mM), is also reverted by Fru-1,6-bisP [11].
Fig 3. S. mutans ADP-Glc PPase inhibition when Pi (empty circles) or MFP (filled circles) are present in the reaction mixture.
Curves were obtained from reactions conducted at 1 mM Glc-1P, 2 mM ATP and 10 mM Mg2+.
Fig 4. S. mutans ADP-Glc PPase inhibition curves by MFP at different concentrations (▼-1 mM, ■-3 mM, ●-5 mM and △-10mM) of ATP.
In the inset, the data represented according Dixon plots (1/v vs MFP concentration) are shown to estimate the inhibition constant.
Table 1. Inhibition of the S. mutans ADP-Glc PPase activity by Pi or MFP in the absence or presence of Fru-1,6-bisP.
| 10 mM Fru-1,6-bisP | 1 mM Fru-1,6-bisP | No Fru-1,6-bisP | ||
|---|---|---|---|---|
| Inhibitor | Activity (% remaining activity) | |||
| Pi | 0 mM | 100 ± 8 | 99 ± 7 | 100 ± 8 |
| 0.5 mM | 67 ± 5 | 61 ± 7 | 51 ± 6 | |
| 2.5 mM | 38 ± 6 | 31 ± 4 | 15 ± 2 | |
| MFP | 0 mM | 99 ± 9 | 99 ± 8 | 100 ± 9 |
| 5 mM | 61 ± 7 | 65 ± 6 | 67± 8 | |
| 10 mM | 42 ± 5 | 39 ± 5 | 41 ± 4 | |
In the above context, MFP arises as an inhibitor of IPS synthesis with ADP-Glc PPase as its target, which is the key step in bacterial glycogen synthesis, particularly the cariogenic IPS accumulation in S. mutans. MFP primarily behaves as an inhibitor of the enzyme because of its analogous structure to the physiological effector Pi, although for the former (contrarily to Pi), the inhibitory effect is not reverted by the regulatory metabolite Fru-1,6-bisP and thus might remain independent of changes in the cellular metabolism. Altogether, the results presented in this work constitute an important mechanistic link for the role of MFP, as S. mutans with reduced IPS accumulation are less cariogenic and are more quickly removed from the oral microflora [4], opposing those hypercariogenic strains that over-produce IPS [6].
Discussion
The use of fluoride as an anticariogenic agent has been widely established, and its efficacy involves a complex sum of factors affecting microbial physiology due to the effects of HF or fluoride ions (F-) [30]. The latter targets the enzyme enolase (EC 4.2.1.11) in S. mutans [31,32], decreasing the glycolytic rate and consequently acid production thus diminishing cariogenic effects [30,33]. MFP has been used in toothpaste formulations, but the precise anti-caries mechanism, i.e., whether MFP itself is an anti-caries agent or whether it is a source of fluoride ion, is not known with certainty [34–36]. Moreover, a specific molecular target for MFP action has never been described and its proven cariostatic effect in clinical trials is comparable to that of NaF. Although there is some uncertainty, it has been suggested that its mode of action stems from MFP being a source of fluoride ions [36].
Results reported herein support the action of MFP through its inhibitory effect on the key enzyme in the biosynthesis of bacterial glycogen. Under comparable conditions, NaF does not exert such an effect, whereby it is shown that the MFP molecule is responsible for inhibition, rather than F- ions that could be generated by hydrolysis. We found that MFP decreases the IPS content of S. mutans, even in conditions (late exponential phase) where the supply of ATP and Glc-1P (ADP-Glc PPase substrates) are guaranteed. In addition, as we discussed elsewhere, the amount of IPS and acidification capacity correlate with levels of ADP-Glc PPase activity during the exponential growth of S. mutans [11]. Interestingly we found that in vitro, MFP inhibits the key enzyme in polyglucan biosynthesis, a particular feature that distinguishes it from other anticariogenic agents tested, such as NaF. Moreover, the inhibition by MFP exhibits as a critical characteristic that is not reversed by Fru-1,6-bisP, a metabolite indicating sugar utilization; which contrasts with inhibition caused by the physiological inhibitors Pi and PEP (both reversed by Fru-1,6-bisP [11]). In addition, the effect of MFP takes at a metabolic step that is not inhibited by NaF. Many glycosyltransferases were identified as targets for the action of the specific molecules utilized in classical anti-caries compounds [15], although the respective interfering mechanisms (i.e., ligand-protein interaction, effect on kinetics, or others) remain largely unknown. The identification of ADP-Glc PPase as a specific molecular point of inhibition by MFP represents a contribution towards a better understanding of the action of active compounds on the carbohydrate metabolism of oral bacteria. Importantly, illuminating these interactions and specific molecular targets is critical for optimizing and/or designing new treatments for the control of caries.
Concluding Remarks
MFP affects the capacity of S. mutans to acidify the medium as well as to accumulate IPS. We found a correlation between in vivo and in vitro studies, as we determined an enzymatic MFP target (ADP-Glc PPase), thus providing a useful tool for caries control.
Acknowledgments
This work was supported by Colgate Research Funds (CN) and by grants to AAI from UNL [CAI+D], ANPCyT [PICT’14 3256] and to MDAD [PICT’14 3362] and to MAB from the National Science Foundation [MCB 1024945]. MDAD and AAI are members of the Research Career from CONICET. The authors declare no conflict of interest.
Data Availability
All data are within the paper.
Funding Statement
This work was supported by Colgate Research Funds (CN) and by grants to AAI from UNL [CAI+D], ANPCyT [PICT’14 3256] and to MDAD [PICT’14 3362] and to MAB from the National Science Foundation [MCB 1024945]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. MDAD and AAI are members of the Research Career from CONICET. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klein MI, Hwang G, Santos PH, Campanella OH, Koo H (2015) Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Frontiers in cellular and infection microbiology 5: 10 10.3389/fcimb.2015.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krzysciak W, Jurczak A, Koscielniak D, Bystrowska B, Skalniak A (2014) The virulence of Streptococcus mutans and the ability to form biofilms. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology 33: 499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50: 353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busuioc M, Mackiewicz K, Buttaro BA, Piggot PJ (2009) Role of intracellular polysaccharide in persistence of Streptococcus mutans. J Bacteriol 191: 7315–7322. 10.1128/JB.00425-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA (2006) The role of sucrose in cariogenic dental biofilm formation—new insight. J Dent Res 85: 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spatafora G, Rohrer K, Barnard D, Michalek S (1995) A Streptococcus mutans mutant that synthesizes elevated levels of intracellular polysaccharide is hypercariogenic in vivo. Infect Immun 63: 2556–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonafonte MA, Solano C, Sesma B, Alvarez M, Montuenga L, et al. (2000) The relationship between glycogen synthesis, biofilm formation and virulence in salmonella enteritidis. FEMS Microbiol Lett 191: 31–36. [DOI] [PubMed] [Google Scholar]
- 8.Jackson DW, Suzuki K, Oakford L, Simecka JW, Hart ME, et al. (2002) Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J Bacteriol 184: 290–301. 10.1128/JB.184.1.290-301.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballicora MA, Iglesias AA, Preiss J (2003) ADP-glucose pyrophosphorylase, a regulatory enzyme for bacterial glycogen synthesis. Microbiology and molecular biology reviews: MMBR 67: 213–225, table of contents. 10.1128/MMBR.67.2.213-225.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballicora MA, Iglesias AA, Preiss J (2004) ADP-Glucose Pyrophosphorylase: A Regulatory Enzyme for Plant Starch Synthesis. Photosynth Res 79: 1–24. 10.1023/B:PRES.0000011916.67519.58 [DOI] [PubMed] [Google Scholar]
- 11.Asencion Diez MD, Demonte AM, Guerrero SA, Ballicora MA, Iglesias AA (2013) The ADP-glucose Pyrophosphorylase from Streptococcus mutans Provides Evidence for the Regulation of Polysaccharide Biosynthesis in Firmicutes. Mol Microbiol. [DOI] [PubMed] [Google Scholar]
- 12.Kiel JA, Boels JM, Beldman G, Venema G (1994) Glycogen in Bacillus subtilis: molecular characterization of an operon encoding enzymes involved in glycogen biosynthesis and degradation. Mol Microbiol 11: 203–218. [DOI] [PubMed] [Google Scholar]
- 13.Takata H, Takaha T, Okada S, Takagi M, Imanaka T (1997) Characterization of a gene cluster for glycogen biosynthesis and a heterotetrameric ADP-glucose pyrophosphorylase from Bacillus stearothermophilus. J Bacteriol 179: 4689–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold WH, Haase A, Hacklaender J, Gintner Z, Banoczy J, et al. (2007) Effect of pH of amine fluoride containing toothpastes on enamel remineralization in vitro. BMC Oral Health 7: 14 10.1186/1472-6831-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren Z, Chen L, Li J, Li Y (2016) Inhibition of Streptococcus mutans polysaccharide synthesis by molecules targeting glycosyltransferase activity. J Oral Microbiol 8: 31095 10.3402/jom.v8.31095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiegand A, Buchalla W, Attin T (2007) Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dental materials: official publication of the Academy of Dental Materials 23: 343–362. [DOI] [PubMed] [Google Scholar]
- 17.Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 18.Yep A, Bejar CM, Ballicora MA, Dubay JR, Iglesias AA, et al. (2004) An assay for adenosine 5'-diphosphate (ADP)-glucose pyrophosphorylase that measures the synthesis of radioactive ADP-glucose with glycogen synthase. Anal Biochem 324: 52–59. [DOI] [PubMed] [Google Scholar]
- 19.Ballicora MA, Erben ED, Yazaki T, Bertolo AL, Demonte AM, et al. (2007) Identification of regions critically affecting kinetics and allosteric regulation of the Escherichia coli ADP-glucose pyrophosphorylase by modeling and pentapeptide-scanning mutagenesis. J Bacteriol 189: 5325–5333. 10.1128/JB.00481-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiPersio JR, Mattingly SJ, Higgins ML, Shockman GD (1974) Measurement of intracellular iodophilic polysaccharide in two cariogenic strains of Streptococcus mutans by cytochemical and chemical methods. Infect Immun 10: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elbein AD, Mitchell M (1973) Levels of glycogen and trehalose in Mycobacterium smegmatis and the purification and properties of the glycogen synthetase. J Bacteriol 113: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez MA, Mohn WW, Martinez E, Rost E, Alvarez AF, et al. (2008) Biosynthesis of storage compounds by Rhodococcus jostii RHA1 and global identification of genes involved in their metabolism. BMC Genomics 9: 600 10.1186/1471-2164-9-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6: 24–27. [Google Scholar]
- 24.Handelman SL, Kreinces GH (1973) Effect of phosphate and pH on Streptococcus mutans acid production and growth. J Dent Res 52: 651–657. [DOI] [PubMed] [Google Scholar]
- 25.Wegman MR, Eisenberg AD, Curzon ME, Handelman SL (1984) Effects of fluoride, lithium, and strontium on intracellular polysaccharide accumulation in S. mutans and A. viscosus. J Dent Res 63: 1126–1129. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi N, Nyvad B (2011) The role of bacteria in the caries process: ecological perspectives. J Dent Res 90: 294–303. 10.1177/0022034510379602 [DOI] [PubMed] [Google Scholar]
- 27.van Houte J, de Moor CE, Jansen HM (1970) Synthesis of iodophilic polysaccharide by human oral streptococci. Arch Oral Biol 15: 263–266. [DOI] [PubMed] [Google Scholar]
- 28.van Houte J, Winkler KC, Jansen HM (1969) Iodophilic polysaccharide synthesis, acid production and growth in oral streptococci. Arch Oral Biol 14: 45–61. [DOI] [PubMed] [Google Scholar]
- 29.Butterworth PJ (1972) The use of Dixon plots to study enzyme inhibition. Biochim Biophys Acta 289: 251–253. [DOI] [PubMed] [Google Scholar]
- 30.Marquis RE, Clock SA, Mota-Meira M (2003) Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev 26: 493–510. [DOI] [PubMed] [Google Scholar]
- 31.Curran TM, Buckley DH, Marquis RE (1994) Quasi-irreversible inhibition of enolase of Streptococcus mutans by fluoride. FEMS Microbiol Lett 119: 283–288. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann M, Bartholmes P (1992) Purification, characterization and inhibition by fluoride of enolase from Streptococcus mutans DSM 320523. Caries Res 26: 110–116. [DOI] [PubMed] [Google Scholar]
- 33.Marquis RE (1995) Antimicrobial actions of fluoride for oral bacteria. Can J Microbiol 41: 955–964. [DOI] [PubMed] [Google Scholar]
- 34.Klimek J, Jung M, Jung S (1997) Interindividual differences in degradation of sodium monofluorophosphate by saliva in relation to oral health status. Arch Oral Biol 42: 181–184. [DOI] [PubMed] [Google Scholar]
- 35.Pearce Ei DGH (1995) The diffusion and enzymic hydrolysis of monofluorophosphate in dental plaque. Journal of dental research 74: 691–697. [DOI] [PubMed] [Google Scholar]
- 36.Saporito RA, Boneta AR, Feldman CA, Cinotti W, Sintes JL, et al. (2000) Comparative anticaries efficacy of sodium fluoride and sodium monofluorophosphate dentifrices. A two-year caries clinical trial on children in New Jersey and Puerto Rico. Am J Dent 13: 221–226. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are within the paper.