Abstract
Background
Bicuspid aortic valve (BAV) is a heritable condition that has been linked by an unknown mechanism to a predisposition for ascending aortic aneurysm. Matrix metalloproteinases have been implicated in this predisposition. Metallothionein is a poorly characterized, metal-binding protein that regulates matrix metalloproteinases and is an antioxidant known to be upregulated under oxidative stress.
Methods and Results
To determine putative factors involved in the pathogenesis of aortic aneurysm in BAV patients, our first goal was to identify genes that are dysregulated in ascending aortic aneurysms of BAV patients compared with tricuspid aortic valve patients and nondiseased (control) donors. By microarray analysis (22 000 probe sets), 110 dysregulated genes were identified in BAV compared with tricuspid aortic valve patients and control donors; 8 were genes of the metallothionein family. Metallothionein gene expression and protein expression were significantly lower in aortic tissue and cultured aortic smooth muscle cells from BAV patients compared with control subjects. Matrix metalloproteinase-9 expression was increased in BAV aortic samples relative to controls. BAV aorta was more susceptible to oxidative stress, and induction of metallothionein under oxidative stress was reduced in BAV patients compared with control subjects.
Conclusions
These results demonstrate dysregulated metallothionein expression in ascending aortic smooth muscle cells of BAV patients that may contribute to an inadequate response to oxidative stress and provoke aneurysm formation. We hypothesize that metallothionein plays a pivotal role in the response of ascending aortic smooth muscle cells to oxidative stress cues normally involved in the maintenance of the extracellular matrix, including the regulation of matrix metalloproteinase expression.
Keywords: aorta, aneurysm, antioxidants, cells, genes, metalloproteinases, oxidative stress
Bicuspid aortic valve (BAV) is the most common congenital heart malformation, occurring in 1% to 2% of the population.1,2 BAV patients are at increased risk for aortic dilatation, aneurysm, and dissection.3–5 Its cause remains unknown, but the defect may arise during development of the aortic valvular cusps and aortic media from neural crest cells.1,6,7 There appears to be a genetic basis for BAV with some proven linkages to chromosomal regions 18q, 5q, and 13q.8 To date, no single gene has been identified; however, mutations in Notch 1 and 2 were shown to be associated with calcification of the aortic valve and aortic stenosis and suggest a potential association with BAV.9
The molecular biological basis for aneurysm formation in BAV patients is unknown. We postulate that there is a marked reduction in the integrity of the extracellular matrix (ECM) in the aortic wall. Changes in gene expression of ECM proteins are known in other aortopathies. One specific example is mutation of the fibrillin-1 gene, the basis of the Marfan syndrome.10 The integrity of the aortic ECM may be compromised by an increase in matrix-degrading enzymes such as the matrix metalloproteinase (MMP) family and/or a decrease in tissue inhibitors of MMPs.11
MMPs control degradation of ECM proteins, including elastin and collagen, and play an important role in vascular biology.11 MMPs are overexpressed in some types of aortic aneurysms.1,4,12,13 Specifically, MMP-2 and MMP-9 have been implicated in thoracic aortic disease14–16 as a result of overproduction by smooth muscle cells (SMCs) of the aorta and macrophages.17,18 MMP activity also is influenced by expression of their antagonists, the tissue inhibitors of MMPs, which are often downregulated.13,15,16 However, the MMP/tissue inhibitor of MMP expression patterns reported for different types of thoracic aortic aneurysms vary significantly and can be influenced by other factors such as hypertension.19 MMP production also is upregulated under conditions of cellular oxidative stress in vitro.20 Reactive oxygen species (ROS) scavengers (ie, antioxidants) decreased the expression of MMP-9 by macrophage-derived foam cells in arterial plaques.21 Inherent expression of other factors, including antioxidants, likely plays a role in the regulation of MMP production and ECM integrity of the aorta and may be dysregulated in patients with aortic pathologies.
Initially, the goal of the present study was to identify genes that are dysregulated in BAV-associated ascending aortic aneurysms (TAAs) compared with aneurysms in tricuspid aortic valve (TAV) patients and nondiseased aortas. Strikingly, we found by microarray analysis that genes of the metallothionein family represented a high percentage (8 of 110) of the dysregulated genes, demonstrating significantly lower expression compared with controls. Subsequent primary cultures of SMCs isolated from BAV aortas demonstrated reduced induction of metallothionein under oxidative stress in vitro and increased expression of MMP-9. Our studies suggest that metallothionein plays a role in the response of aortic SMCs to oxidative stress and that its diminished expression may adversely affect the integrity of the ECM of the aortic wall.
Methods
Patient Enrollment and Tissue Collection
To investigate the genetic basis for BAV-TAA, we maintain a tissue bank of aortic specimens from patients who undergo ascending aorta replacement and normal aortic tissue from heart transplant donors (all with TAVs). All tissues used for this study were from male patients with no history of coarctation and minimal to no comorbidities. Samples used for the various assays to compare TAV and BAV groups were matched for age and diameter of the ascending aorta. Donor control samples were generally younger because of typical donor ages, but samples used were male and as closely matched in age as possible within experiments. Age was determined not to be a covariant for gene expression studies by regression analyses. Demographics and valvular pathophysiologies of all of the study samples used are outlined in Table 1.
Table 1.
Patient Demographics for Aortic Tissue Included in Study: Age; Aortic Diameter; Percentage of Patients With Hypertension, Aortic Insufficiency, and Aortic Stenosis
| n | Age, y (Mean) | Aortic Diameter (Mean), mm | HTN, % | AI, %
|
AS, %
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–1+ | 2–3+ | 4+ | Mild | Moderate | Severe | |||||
| Control | 24 | 43.4±2.8 | 30±1.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TAV | 35 | 57.4±1.9 | 55.4±1.9 | 68 | 28 | 53 | 19 | 55 | 3 | 0 |
| BAV | 66 | 50.0±1.6 | 52.5±0.9 | 24 | 29 | 52 | 19 | 41 | 22 | 4 |
HTN indicates hypertension; AI, aortic insufficiency (mild, 0 to 1+; moderate, 2 to 3+; severe, 4+); and AS, aortic stenosis.
Samples were harvested by the surgeon during elective surgery for replacement of the ascending aorta with informed patient consent and Institutional Review Board approval. A portion of each sample was placed in RNAlater solution (Ambion, Austin, Tex) and stored at 4°C for 24 hours before long-term storage at −20°C until further processing for RNA isolation. Additional portions of aorta were placed in DMEM/F12 containing 1% penicillin/streptomycin and Fungizone (Invitrogen, Carlsbad, Calif) to create primary cultures of SMCs. The remaining samples were promptly snap-frozen in liquid nitrogen and stored at −80°C until further analysis.
Primary SMC Culture
Under sterile conditions, the aortic media was dissected away from the adventitia, and the tunica media was dissected into ≈1-mm pieces. A primary explant method was used to establish the SMC cultures from BAV, TAV, and control aortas. After washing in PBS, the sample was transferred into 25-cm2 culture flasks containing SMC growth medium (Cell Applications, San Diego, Calif). The SMCs from BAV were isolated by enzymatic digestion with 0.5 mg/mL elastase and 1 mg/mL collagenase in DMEM/F12 for 2 hours at 37°C. After brief digestion with trypsin for 1 minute, samples were washed and transferred to fresh 25-cm2 culture flasks containing SMC growth medium and incubated at 37°C with 5% CO2 and humidity, and the media was changed every other day. At confluence, cells were transferred to 75-cm2 culture flasks. The purity of the SMC was confirmed by homogeneous positive staining with a smooth muscle α-actin monoclonal antibody (Sigma, St Louis, Mo; data not shown).
RNA Extraction
For extraction of total RNA, tissue samples stored in RNAlater solution were pulverized under liquid nitrogen. Then, 25 to 30 mg tissue was homogenized by passing through in QIAshredder column (Qiagen, Valencia, Calif). RNA was extracted by Trizol Reagent (Invitrogen), followed by purification with the RNeasy Mini kit (Qiagen) according to the manufacturer’s instructions. Total RNA was isolated from primary SMCs with the Versagene RNA kit (Gentra Systems Inc, Minneapolis, Minn). Integrity of RNA was evaluated on Bioanalyzer 2100 (Agilent, Santa Clara, Calif). Only RNA with an integrity number >7 for tissue samples and >9 for cultured cells was used for quantitative real-time polymerase chain reaction (qPCR) analysis.
Microarray Analysis
Microarray analysis of 50 aortic samples was performed with the Affymetrix HG-U133A GeneChip (Santa Clara, Calif) and analyzed with the Affymetrix Microarray Suite version 5, Significance Analysis of Microarrays software (Stanford University, Palo Alto, Calif), and GeneSpring version 6.1 (Silicon Genetics, Redwood City, Calif).
Signal values and detection flags were calculated using default settings. Data for all arrays were imported into GeneSpring; principal-components analysis revealed 10 samples to be significantly different from the other 40. These samples were excluded from further analysis. Probe sets were filtered on the basis of the detection flags, with those probe sets flagged as present in at least 5 of the remaining 40 samples retained. Significance Analysis of Microarrays software was applied to this list to discover differentially expressed genes in a series of pairwise comparisons (TAV versus BAV, BAV versus control, TAV versus control).
Protein Isolation
Frozen aortic tissue samples were pulverized under liquid nitrogen. Pulverized tissue (25 to 30 mg) was homogenized in ice-cold Lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 0.5 Triton-X 100, 1 mmol/L phenylmethanesulfonylfluoride) in a Dounce grinder and then passed through a 21-gauge needle. Cultured primary SMCs were lifted with 0.05% trypsin, 0.53 mmol/L EDTA (Invitrogen), pelleted (5 minutes at 1000g), and homogenized in lysis buffer by passing through a 21-gauge needle. Tissue and cell samples were then incubated at 4°C for 30 minutes, and the supernatant was collected after centrifugation at 14 000g for 10 minutes at 4°C. Total protein concentration was determined with a Bradford assay kit (Bio-Rad Laboratories, Hercules, Calif).
Detection of Metallothionein by Western Blot
We used methods based on the chemical modification of the cysteine residues of metallothionein with monobromobimane adapted from Meloni et al22 and an improved Western blotting protocol adapted from Mizzen et al23 to detect metallothionein protein expression. Briefly, 25 μg total protein extract was incubated in Tris [2-carboxyethyl]phosphine hydrochloride (TCEP) buffer (100 mmol/L Tris, 10 mmol/L EDTA, 10 mmol/L TCEP) with 6.25 mmol/L monobromobimane for 5 minutes in the dark. The reaction was terminated by adding protein loading buffer (Bio-Rad) and boiling. A protein sample (10 μg) was subjected to SDS-PAGE on 15% gels. After SDS-PAGE, gels were incubated at real time for 20 minutes in transfer buffer (10 mmol/L 3-[cyclohexylamino]-1 propane sulfonic acid, pH 10.8, 2 mmol/L CaCl2 in 10% methanol) and transferred to polyvinyl difluoride membranes. Membranes were then fixed in 2.5% (vol/vol) glutaraldehyde for 1 hour and washed in PBS (3 times for 5 minutes, with 50 mmol/L monoethanolamine added to the final wash). Membranes were probed with mouse IgG anti-metallothionein antibodies (Dako Cytomation, Carpinteria, Calif), 1:50 dilution for tissue samples and 1:300 for cultured SMCs. Bands were detected with horseradish peroxidase– conjugated secondary goat anti-mouse antibodies (1:5000) and visualized with an ECL Western Detection Kit (Amersham Biosciences, Little Chalfont, UK). Blots were then stripped and reprobed for β-actin (Pierce Biotechnology Inc, Rockford, Ill; 1:20 000) to normalize protein loading. Autoradiographs were scanned and band intensity was determined with ImageJ software. Relative amounts of metallothionein were quantified and normalized to β-actin controls.
qPCR Analysis
Primers for amplification of the 8 metallothionein isoforms, designed from the divergent 5′ and 3′ untranslated regions of each metallothionein isoform, were adopted from Mididoddi et al.24 Primers for amplification of MMP-2 and MMP-9 were adopted from Choi et al.25 Total RNA (0.75 μg for RNA isolated from tissue, 1 μg for RNA isolated from primary SMCs) was reverse transcribed with the iScript cDNA Synthesis kit (Bio-Rad). qPCR analysis was performed with 1 μL cDNA as template and SYBR Green PCR Mastermix (Applied Biosystems, Foster City, Calif), with 200 nmol/L forward and reverse primers for target genes in a total reaction volume of 20 μL. PCR was performed with a sequence detector Prism 7000 (Applied Biosystems). The thermocycling conditions were as follows: 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. A final heating step of 65°C to 95°C was performed to obtain melting curves of the final PCR products. Changes in metallothionein gene expression levels were calculated as fold differences using the comparative ΔΔCT method as described in Applied Biosystems Bulletin No. 2 and as described previously26 using patient 1003 as the calibrator to compare all groups with normal aorta. When noted, total expression levels in arbitrary units were calculated using the ΔCT method.
Induction of Metallothionein by Cadmium Treatment
SMCs were plated in 6-well plates at a density of 100 000 cells per well in duplicate in SMC growth medium and cultured to 100% confluence. The cell medium was then replaced with fresh medium with or without 5 μmol/L CdCl2 for 24 hours. Total RNA and protein were then extracted, and the level of metallothionein gene and protein expression was determined as described above.
Oxidative Stress Culture Conditions
SMCs were plated in 96-well plates at a density of 10 000 cells per well, cultured in SMC growth medium until 80% confluent, and then treated in triplicate with 0 to 9600 μmol/L t-butyl hydroperoxide for 3 hours. Cell viability was monitored by MTS CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, Wis).
Statistical Analysis
Microarray analysis was completed with the Significance Analysis of Microarrays software (Stanford University, Palo Alto, Calif). All subsequent experiments were repeated at least 3 times, and 1 representative experiment is shown. qPCR and Western blot densitometry readings are presented as mean±SEM with n values. One-way ANOVA, followed by Tukey’s highest-significant-difference posthoc test using SPSS software (SYSTAT Software, Inc, Chicago, Ill), was performed to determine significance among treatment groups and appropriate age matching among groups. A regression analysis was performed to ascertain potential age effects on gene expression studies, and no linearity was found between age and gene expression among the groups. A value of P<0.05 was considered statistically significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Microarray Analysis
Microarray analysis of 50 ascending aortic samples was performed with the Affymetrix HG-U133A GeneChip. Data were organized with Affymetrix Microarray Suite (version 5). Principal-components analysis of the samples was performed with GeneSpring (version 6.1), which identified 10 samples that were inexplicably different than the other 40. These 10 samples were discarded. The remaining 40 samples were then analyzed for differential gene expression using Significance Analysis of Microarrays software with the default settings with 2 class, unpaired data yielding a false discovery rate of 5.1%. One hundred eight dysregulated genes were identified in BAV compared with control aortic tissue. Genes of interest that showed the greatest change in BAV compared with control encode proteins involved in the immune response (FK506 binding protein 5, type II precursor of interleukin 1 receptor, CD163 antigen), matrix components (tissue inhibitor of metalloproteinase 4 precursor, heparin sulfate D-glucosaminyl 3-O-sulfotransferase 2, matrix metalloproteinase 3 preproprotein, tropomodulin 1), and stress response proteins (metallothionein isoforms) are shown in Table 2.
Table 2.
Microarray Analysis for BAV and Control Aorta
| Gene | Fold Change (BAV Versus Control)* | Function |
|---|---|---|
| MT-1L | −3.46 | Heavy metal binding |
| MT-1X | −3.04 | |
| MT-1H–like | −2.72 | |
| MT-1H | −2.52 | |
| MT-2A | −2.47 | |
| MT-1F | −2.37 | |
| MT-1E | −2.01 | |
| MT-1G | −2.00 | |
| Tissue inhibitor of MMP-4 precursor | −4.20 | Binds to MMP-1, -2, -3, -7, and -9 |
| Heparin sulfate D-glucosaminyl 3-O-sulfotransferase 2 | −4.08 | Sulfation and epimerization of heparin sulfate polysaccharides |
| MMP-3 preproprotein | −3.75 | ECM degradation |
| Tropomoldulin 1 | −1.52 | Cell-cell and cell-ECM adhesion |
| FK506 binding protein 5 | −16.11 | Protein folding |
| CD163 antigen | −4.72 | Scavenger receptor activity |
| Interleukin 1 receptor, type II precursor | −9.03 | Immune response |
False discovery rate, 5.1%.
Metallothionein Expression in Ascending Aortic Tissue
Microarray analysis showed that the expression levels of many genes differed significantly in patients with aneurysm compared with nondiseased (control) aorta. We observed that several genes were differentially expressed in BAV compared with TAV patients. Among these, we focused on the metallothionein family genes, which constituted 8 of 110 genes with altered expression. Metallothionein isoforms are known to participate in the oxidative stress response,27–30 and we suspected that they play a role in the pathogenesis of aortic aneurysms. To further analyze the gene expression of known functional metallothionein-1 (MT-1) isoforms (A, B, E, F, H, G, X) and MT-2A, conventional real-time PCR was performed on tissue samples from 6 patients according to established protocols.24 MT-1B and MT-1H were omitted from further analysis on the basis of results of real-time PCR (data not shown).
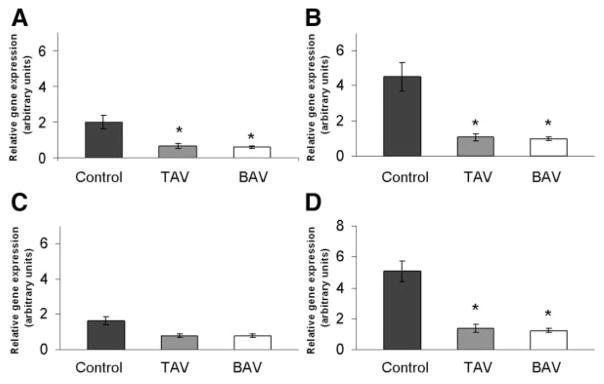
Using qPCR analysis, we observed consistent expression of MT-1A, MT-1E, MT-1X, and MT-2A in aortas of both TAV and BAV patients (Figure 1) and control donors. Metallothionein gene expression was reduced in diseased aorta patients compared with control; however, this effect was of varying extent for each isoform examined. The MT-1X and MT-2A isoforms exhibited the highest expression levels relative to the other isoforms. MT-1G was omitted from further analysis because of extremely low basal expression (data not shown). MT-1A, MT-1X, and MT-2A each exhibited significantly less expression for BAV and TAV diseased aortas (P<0.001) compared with control aorta (Figure 1). There was no significant correlation of metallothionein expression with either age or size of aneurysm for any isoform examined.
Figure 1.
Metallothionein gene expression was reduced in TAV and BAV patients vs control. Total RNA was harvested from aortic tissue and analyzed by qPCR for metallothionein isoforms. A, MT-1A; B, MT-1X; C, MT-1E; and D, MT-2A. Bars represent relative metallothionein expression in TAAs of BAV and TAV patients and normal donor patients normalized to the housekeeping gene, 18S rRNA (mean±SEM; n=12, 19, and 24 for control, TAV, and BAV, respectively). *Significantly different from control, P<0.001.
To further explore the potential relationship among BAV, aneurysm formation, and metallothionein, we quantified metallothionein protein expression by Western blot analysis. There was a marked decrease in metallothionein protein expression in BAV patients compared with donor control aorta (27.5±12.7 versus 123.6±20.5, respectively; (P<0.01; Figure 2A and 2B). The mean values for aortic metallothionein content in TAV patients (101.0±19.6) were not statistically different from donor controls (P=0.673). There was no significant correlation between metallothionein content and patient age for both diseased groups (TAV, r=0.36; BAV, r=0.30) and some correlation for control patients (normal, r=0.55; Figure 2C). Interestingly, although there was no correlation between metallothionein expression and aortic diameter for BAV (r=0.13, P=0.43), there was a statistically significant correlation for TAV (r=0.61, P<0.002; Figure 2D).
Figure 2.
Metallothionein protein expression was reduced in aortic tissue from BAV patients vs control. A, Western blot analyses for 6-kDa MT-1/2 protein and the loading control, β-actin. B, Band intensities from A were quantified by densitometry and displayed as a ratio of metallothionein to β-actin. Bars represent mean±SEM metallothionein protein expression (n=12, 19, and 24 for control, TAV, and BAV, respectively). C, Metallothionein protein expression vs age. D, Metallothionein protein expression vs diameter (dia.) of the ascending aorta. Bars represent normalized metallothionein protein expression. *Significantly different from control and TAV, P<0.01.
Metallothionein Expression in SMCs
We isolated SMCs derived from TAA and healthy donors to investigate the cell response to oxidative stress in vitro. Cadmium is a known inducer of metallothionein gene transcription, and it can bind and stabilize MT-1/2 protein in most mammalian cell systems.31 We designed studies to determine the effect of cadmium exposure on metallothionein mRNA and protein expression in SMCs. SMCs were exposed to CdCl2 (0.1 to 100 μmol/L) for 19 hours. There was no significant cell death as detected by MTS assay (data not shown); however, BAV-derived SMCs displayed a rounded morphology, indicative of poor cell attachment within 19 hours of CdCl2 treatment.
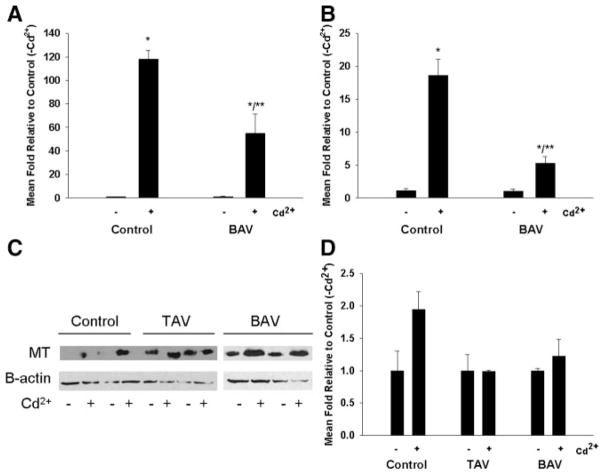
Next, we compared mRNA levels of metallothionein isoforms in SMCs cultured in the presence and absence of CdCl2. Analysis of mRNA by qPCR demonstrated that all analyzed SMCs displayed the same expression pattern as aortic tissue, with MT-1E, MT-1X, and MT-2A being the most abundant isoforms. There was no apparent difference in the basal mRNA levels among BAV, TAV, or control patients. Exposure to CdCl2 at concentrations >2 μmol/L resulted in significant induction of all analyzed metallothionein isoforms. The expression of metallothionein mRNA in the nontreated cells was normalized to a control value of 1.0, and metallothionein induction in cadmium-treated cells was calculated relative to metallothionein expression levels in nontreated cells. Relative induction of MT-1E, MT-1F, and MT-2A was similar among all analyzed SMCs (data not shown). Induction was significantly lower in SMCs from BAV patients compared with controls for MT-1A (55±16.6 versus 118±7.5; P<0.005; Figure 3A). MT-1X was significantly induced in control cells (18±2.6-fold; P<0.002) but not in BAV cells (P=0.973; Figure 3B). Induction of MT-1A and MT-1X was 880-fold and 40-fold, respectively, for TAV in the absence of cadmium treatment relative to control cells (data not shown). This trend was reflected at the protein level; cadmium exposure increased the expression of metallothionein protein in SMCs derived from control and BAV cells (Figure 3C), but induction of metallothionein expression was less in BAV cells than in control SMCs (Figure 3D).
Figure 3.
Induction of metallothionein gene expression by Cd2+ is reduced in BAV patients vs control. SMCs were isolated from aortic tissue and cultured in the presence or absence of 5 μmol/L CdCl2 for 24 hours. Total RNA was isolated and subjected to qPCR analysis for gene expression of metallothionein isoforms. A, MT-1A. B, MT-1X. Bars represent mean±SEM of 3 assay replicates. C, Western blot analyses of MT-1/2 protein expression and β-actin loading controls in SMCs as pooled triplicates cultured in the presence and absence of 5 μmol/L Cd2+ for 24 hours. D, Quantification of band intensities in C. MT-1/2 protein expression is normalized to β-actin loading controls.
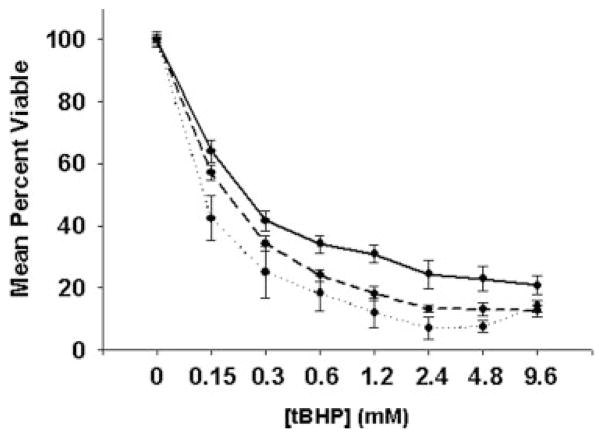
SMCs derived from BAV, TAV, and control aortas were analyzed for response to oxidative stress in vitro. BAV cells conveyed the poorest resistance to oxidative stress. Control SMCs demonstrated the highest cell viability under oxidative stress, and TAV cells displayed intermediate cell viability relative to control and BAV (Figure 4).
Figure 4.
Cell viability under oxidative stress conditions in vitro. SMCs isolated from BAV patients (dotted line) exhibit the least cell viability under oxidative stress relative to TAV (dashed line) and control (solid line) cells. SMCs from control, TAV, and BAV patients were cultured in the presence or absence of t-butyl hydroperoxide (tBHP; 0 to 9.6 mmol/L) for 3 hours. Cell viability was assessed by MTS assay. Lines represent mean±SEM; n=3, 3, and 2, respectively.
MMP Expression in Aorta
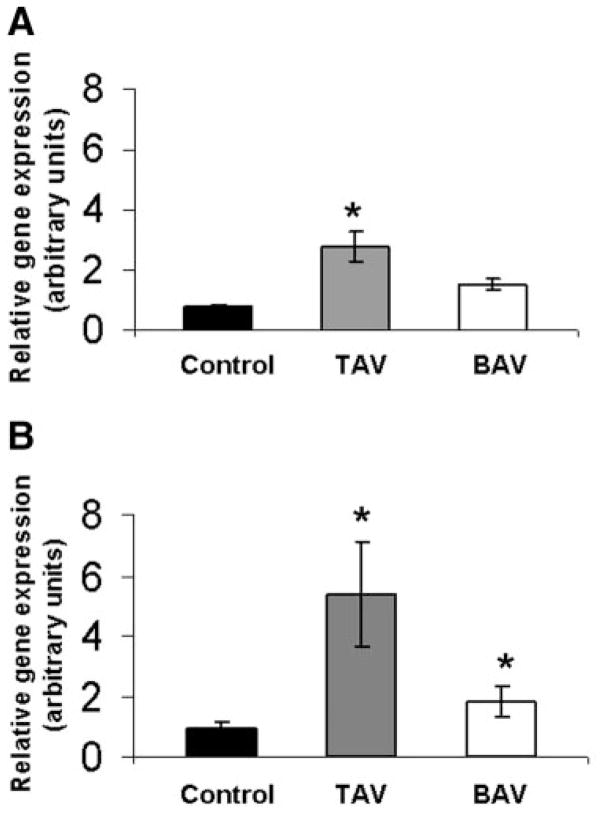
We analyzed expression levels of MMP-2 and MMP-9 in aortas of 54 patients and 11 controls using qPCR. MMP-2 and MMP-9 gene expression was significantly elevated in TAV aortas compared with control aorta (P<0.002 and P<0.05, respectively; Figure 5A and 5B). Interestingly, although MMP-2 expression levels in BAV aortas were not significantly different from controls (P=0.341), MMP-9 gene expression was significantly higher compared with control (P<0.05). Elevated MMP expression in aneurysmal tissue is in agreement with reports from other groups.14–16 An increase in MMP-2 or MMP-9 expression did not correlate with aneurysm size or age for BAV. However, there was a trend for higher MMP-2 and MMP-9 expression in older patients with TAV (data not shown).
Figure 5.
MMP gene expression was elevated in TAV and BAV patients vs control. RNA was harvested from aortic tissue and analyzed by qPCR for MMP-2 (A) and MMP-9 (B) gene expression. Bars represent mean±SEM MMP-2 or -9 gene expression for TAV and BAV relative to control; n=11, 19, and 20 for control, TAV, and BAV, respectively. *Significantly from control, P<0.05.
Discussion
A definitive cause for the formation of ascending aortic aneurysms in BAV patients has not yet been elucidated. Our results demonstrate decreased expression of metallothionein in tissue isolated from the aorta of BAV patients compared with that of nondiseased aorta. Metallothionein deficiency in the aortic microenvironment of BAV patients may contribute to decreased ECM integrity of the vessel. The present study is consistent with the hypothesis that a defect in the cellular microenvironment, resulting from inherent, genetically determined abnormalities of the aortic wall, causes or contributes to the aortic pathology associated with BAV.
The biological function of metallothionein has been perplexing since its discovery. Metallothionein appears to be primarily a stress response protein and is transcriptionally activated via phosphorylation and binding of metal-regulatory transcription factor-1 to the metal response elements in the metallothionein promoter. This occurs under conditions of ROS, hypoxia, ultraviolet irradiation, and heavy metal exposure, except for cadmium treatment, which has little to no effect on metal-regulatory transcription factor-1 binding to metallothionein.32,33 The mechanism of action for metallothionein has not been delineated. Many studies have suggested that metallothionein plays a role in the homeostasis of essential metals such as zinc34 and cadmium,35 detoxification of metals, and protection against oxidative stress.27–30 Metallothionein has been implicated in the pathogenesis of multiple diseases, including oxidative stress–induced cardiac dysfunction.36 However, to the best of our knowledge, this is the first report that suggests a role for metallothionein in TAA pathogenesis in patients with BAV. Although cultures of aortic SMCs did not precisely mimic the results in tissue samples of lower basal metallothionein gene expression, we found that BAV TAA SMCs are clearly more sensitive to oxidative stress than cells from nondiseased tissue and TAV TAA SMCs. This supports our working hypothesis that metallothionein is involved in the oxidative stress response of aortic SMCs. Furthermore, the trend observed of reduced aortic metallothionein gene expression was reflected in vitro at the protein level in cultures of primary aortic SMCs. It has been well described that SMC apoptosis is an inherent aspect of the pathology of TAA, resulting in cystic media degeneration.37,38 It is reasonable to postulate that low metallothionein expression and reduced capacity for induction contribute to SMC apoptosis in BAV aorta.
We also found that MMP-9 is upregulated in BAV TAA compared with nondiseased aorta, which is in accordance with previous studies.15 Our results are consistent with those of Göbel et al,39 who proposed that both metallothionein and MMP-9 were regulated by ROS in atherosclerotic plaques. Metallothionein and MMP-9 were shown to be expressed in adjacent SMCs of the synthetic phenotype (low expression of smooth muscle α-actin). Conversely, our results demonstrate that metallothionein is expressed in aortic SMCs that were positive for smooth muscle α-actin, indicative of the contractile SMC phenotype. Although a direct regulatory effect of ROS on metallothionein or MMP expression has not been conclusively demonstrated, the close proximity of their expression shown by Gobel et al, taken together with our current data, supports the notion that neighboring cells may lack a sufficient stress response via induction of metallothionein to protect matrix-producing SMCs of the synthetic phenotype from ROS. ROS has been shown to upregulate MMPs, potentially destabilizing atherosclerotic plaques and increasing risk for plaque rupture.40 Galis et al21 showed that N-acetyl-cysteine, an ROS scavenger, decreased MMP-9 expression in macrophage-derived foam cells isolated from plaque tissue in rabbits. We suggest that under conditions of oxidative stress, ROS may increase MMP expression/activity and that the lack of a metallothionein response under such an environment limits cell survival and the ability to rebuild matrix to maintain aortic integrity. Furthermore, because metallothionein is known to bind zinc ions, lack of adequate metallothionein could affect the availability of free zinc for MMP-related functions.
A relationship between oxidative stress and TAA has not been previously studied; however, oxidative stress has been shown to play a role in the formation of abdominal aneurysms.41 Oxidative stress also affects the pathogenesis of myocardial remodeling and congestive heart failure by regulating the quantity and quality of the ECM.42–44 In the present study, we have shown that BAV TAA SMCs have reduced capacity to upregulate metallothionein in response to oxidative stress in vitro. Thus, there is an increasing pool of data demonstrating that oxidative stress and heavy metal exposure play a role in regulating the quantity and quality of the ECM. We suggest that a difference in metallothionein induction among BAV, TAV, and control patients could be responsible for altered responses to oxidative stress and affect the integrity of the ECM. Increased expression and activity of matrix degrading proteins (eg, MMPs) within BAV TAA have previously been implicated in the disarray of the system,1,4,12,45,46 and we surmise that metallothionein affects MMP expression in this milieu.
Clinically, BAV is clearly associated with aortic dilatation and TAA formation.4,5,47,48 Given this association and the proven heritability of BAV by linkage analysis,49 a genetic basis for BAV TAA is putative. Our data add significantly to our understanding of the changes present in the ascending aortic walls of BAV patients, which differ from nonpathological aortas and TAV-degenerative aneurysms. The characteristic ascending aortic dilatation that occurs in BAV patients may be due in part to increased wall stress at the right anterolateral aspect of the ascending aorta,50 yielding a localized, somewhat asymmetric pattern of medial degeneration in the aorta.51 The notion that aneurysm occurs in BAV patients because of a single genetic alteration or by a multifactorial process remains unresolved. However, the data presented provide insight into the potential role of metallothionein in regulating ECM homeostasis within the media of the ascending aorta as an antioxidant that regulates cellular responses to oxidative stress.
Study Limitations
We have specifically tried to limit the discrepancies of age and comorbid factors, but because of the availability of donor tissue, there were often not sufficient numbers to precisely age match to TAV and BAV patients. Moreover, degenerative TAAs inherently occur in older TAV patients relative to the age of presentation of TAA in BAV patients. Despite these clinical limitations, we made every attempt to compare BAV TAAs only to the youngest of the TAV TAAs and the oldest donor aortas housed in our bank. Prestudy and posthoc statistical analyses of the cohorts included in the microarray studies do not demonstrate any statistically significant difference in age among the groups. From that point forward, we matched samples for sex, age, and the lack of comorbidities for each of the subsequent experiments. Despite the more limited number of donor control samples, they appear to be adequately age matched for the TAV and BAV samples for the subsequent experiments.
Conclusions
Our data suggest a role for metallothionein in the local microenvironment of BAV TAAs. Metallothionein may function to regulate response of aortic SMCs to oxidative stress, which could include regulating MMP expression and tissue homeostasis of the aortic ECM. Ongoing studies in our group are focused on identifying the potential cellular and molecular mechanisms that govern oxidative stress responses of SMCs and aortic ECM integrity as they pertain to the formation of BAV TAAs.
CLINICAL PERSPECTIVE.
Bicuspid aortic valve (BAV) is the most common cardiac anomaly, occurring in 1% to 2% of the population. There is no known genetic mutation or cause of BAV despite the fact that it has been proven to be hereditary. Patients with BAV are predisposed to developing aneurysms of the ascending aorta. At least 40% of patients undergoing elective replacement of the ascending aorta for aneurysmal disease have BAV in most thoracic aortic practices. The pathology of ascending aorta aneurysms involves noninflammatory smooth muscle cell loss and degeneration of the elastin- and collagen-rich extracellular matrix. Here, we report that patients with BAV have significantly decreased levels of metallothionein within the ascending aorta. The role of metallothionein in the ascending aortic wall has never been characterized, yet it has been shown to be an antioxidant involved in oxidative stress responses in other systems. Coincident with altered metallothionein expression, we demonstrate that there is a diminished tolerance of oxidative stress apparent among BAV aortic smooth muscle cells compared with control, nonaneurysmal aortic smooth muscle cells. We propose a putative role for metallothionein in the regulation of smooth muscle cell viability that contributes to extracellular matrix homeostasis and consequent aortic wall integrity. Understanding the mechanisms by which the extracellular matrix homeostasis is disrupted, leading to aortic medial degeneration in BAV patients, may allow the prediction of one’s potential for aneurysm formation based on metallothionein gene or protein expression and may facilitate pharmacological manipulation of the extracellular matrix homeostasis in BAV patients to prevent aneurysm formation.
Acknowledgments
We thank Phil Campbell (Carnegie Mellon University) for assistance with statistical analyses.
Sources of Funding
We gratefully acknowledge the intramural funding provided by the University of Pennsylvania’s Department of Surgery, the Northwestern Memorial Hospital and Faculty Foundation, and the University of Pittsburgh Medical Center to help complete these studies.
Footnotes
Disclosures
None.
References
- 1.Nataatmadja M, West M, West J, Summers K, Walker P, Nagata M, Watanabe T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(suppl II):II-329–II-334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 2.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–85. doi: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleason TG. Heritable disorders predisposing to aortic dissection. Semin Thorac Cardiovasc Surg. 2005;17:274–281. doi: 10.1053/j.semtcvs.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Fedak PW, de Sa MP, Verma S, Nili N, Kazemian P, Butany J, Strauss BH, Weisel RD, David TE. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg. 2003;126:797–806. doi: 10.1016/s0022-5223(03)00398-2. [DOI] [PubMed] [Google Scholar]
- 5.Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900–904. doi: 10.1161/01.cir.0000027905.26586.e8. [DOI] [PubMed] [Google Scholar]
- 6.Bonderman D, Gharehbaghi-Schnell E, Wollenek G, Maurer G, Baumgartner H, Lang IM. Mechanisms underlying aortic dilatation in congenital aortic valve malformation. Circulation. 1999;99:2138–2143. doi: 10.1161/01.cir.99.16.2138. [DOI] [PubMed] [Google Scholar]
- 7.Schievink WI, Mokri B. Familial aorto-cervicocephalic arterial dissections and congenitally bicuspid aortic valve. Stroke. 1995;26:1935–1940. doi: 10.1161/01.str.26.10.1935. [DOI] [PubMed] [Google Scholar]
- 8.Martin LJ, Ramachandran V, Cripe LH, Hinton RB, Andelfinger G, Tabangin M, Shooner K, Keddache M, Benson DW. Evidence in favor of linkage to human chromosomal regions 18q, 5q and 13q for bicuspid aortic valve and associated cardiovascular malformations. Hum Genet. 2007;121:275–284. doi: 10.1007/s00439-006-0316-9. [DOI] [PubMed] [Google Scholar]
- 9.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 10.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 11.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195–202. doi: 10.1016/s0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 12.Boyum J, Fellinger EK, Schmoker JD, Trombley L, McPartland K, Ittleman FP, Howard AB. Matrix metalloproteinase activity in thoracic aortic aneurysms associated with bicuspid and tricuspid aortic valves. J Thorac Cardiovasc Surg. 2004;127:686–691. doi: 10.1016/j.jtcvs.2003.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, 3rd, Spinale FG, Gorman RC. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with bicuspid or tricuspid aortic valves. J Thorac Cardiovasc Surg. 2007;133:1028–1036. doi: 10.1016/j.jtcvs.2006.10.083. [DOI] [PubMed] [Google Scholar]
- 14.Lesauskaite V, Tanganelli P, Sassi C, Neri E, Diciolla F, Ivanoviene L, Epistolato MC, Lalinga AV, Alessandrini C, Spina D. Smooth muscle cells of the media in the dilatative pathology of ascending thoracic aorta: morphology, immunoreactivity for osteopontin, matrix metalloproteinases, and their inhibitors. Hum Pathol. 2001;32:1003–1011. doi: 10.1053/hupa.2001.27107. [DOI] [PubMed] [Google Scholar]
- 15.Segura AM, Luna RE, Horiba K, Stetler-Stevenson WG, McAllister HA, Jr, Willerson JT, Ferrans VJ. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan’s syndrome. Circulation. 1998;98(suppl):II-331–II-337. [PubMed] [Google Scholar]
- 16.Tamarina NA, McMillan WD, Shively VP, Pearce WH. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery. 1997;122:264–271. doi: 10.1016/s0039-6060(97)90017-9. [DOI] [PubMed] [Google Scholar]
- 17.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms: an elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laviades C, Varo N, Fernandez J, Mayor G, Gil MJ, Monreal I, Diez J. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98:535–540. doi: 10.1161/01.cir.98.6.535. [DOI] [PubMed] [Google Scholar]
- 20.Brenneisen P, Briviba K, Wlaschek M, Wenk J, Scharffetter-Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic Biol Med. 1997;22:515–524. doi: 10.1016/s0891-5849(96)00404-2. [DOI] [PubMed] [Google Scholar]
- 21.Galis ZS, Asanuma K, Godin D, Meng X. N-acetyl-cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells: new target for antioxidant therapy? Circulation. 1998;97:2445–2453. doi: 10.1161/01.cir.97.24.2445. [DOI] [PubMed] [Google Scholar]
- 22.Meloni G, Knipp M, Vasak M. Detection of neuronal growth inhibitory factor (metallothionein-3) in polyacrylamide gels and by Western blot analysis. J Biochem Biophys Methods. 2005;64:76–81. doi: 10.1016/j.jbbm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Mizzen CA, Cartel NJ, Yu WH, Fraser PE, McLachlan DR. Sensitive detection of metallothioneins-1, -2 and -3 in tissue homogenates by immunoblotting: a method for enhanced membrane transfer and retention. J Biochem Biophys Methods. 1996;32:77–83. doi: 10.1016/0165-022x(95)00044-r. [DOI] [PubMed] [Google Scholar]
- 24.Mididoddi S, McGuirt JP, Sens MA, Todd JH, Sens DA. Isoform-specific expression of metallothionein mRNA in the developing and adult human kidney. Toxicol Lett. 1996;85:17–27. doi: 10.1016/0378-4274(96)03632-6. [DOI] [PubMed] [Google Scholar]
- 25.Choi JH, Choi KC, Auersperg N, Leung PC. Gonadotropins activate proteolysis and increase invasion through protein kinase A and phosphatidylinositol 3-kinase pathways in human epithelial ovarian cancer cells. Cancer Res. 2006;66:3912–3920. doi: 10.1158/0008-5472.CAN-05-1785. [DOI] [PubMed] [Google Scholar]
- 26.Jadlowiec J, Koch H, Zhang X, Campbell PG, Seyedain M, Sfeir C. Phosphophoryn regulates the gene expression and differentiation of NIH3T3, MC3T3-E1, and human mesenchymal stem cells via the integrin/MAPK signaling pathway. J Biol Chem. 2004;279:53323–53330. doi: 10.1074/jbc.M404934200. [DOI] [PubMed] [Google Scholar]
- 27.Pitt BR, Schwarz M, Woo ES, Yee E, Wasserloos K, Tran S, Weng W, Mannix RJ, Watkins SA, Tyurina YY, Tyurin VA, Kagan VE, Lazo JS. Overexpression of metallothionein decreases sensitivity of pulmonary endothelial cells to oxidant injury. Am J Physiol. 1997;273:L856–L865. doi: 10.1152/ajplung.1997.273.4.L856. [DOI] [PubMed] [Google Scholar]
- 28.Abel J, de Ruiter N. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol Lett. 1989;47:191–196. doi: 10.1016/0378-4274(89)90075-1. [DOI] [PubMed] [Google Scholar]
- 29.Thornally P, Vasak M. Possible role for metallothionein in protections against radiation-induced oxidative stress: kinetics and mechanism of its reaction with superoxide hydroxyl radicals. Biochem Biophys Acta. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 30.Oh SH, Deagen JT, Whanger PD, Weswig PH. Biological function of metallothionein, V: its induction in rats by various stresses. Am J Physiol. 1978;234:E282–E285. doi: 10.1152/ajpendo.1978.234.3.E282. [DOI] [PubMed] [Google Scholar]
- 31.Bourdineaud JP, Baudrimont M, Gonzalez P, Moreau JL. Challenging the model for induction of metallothionein gene expression. Biochimie. 2006;88:1787–1792. doi: 10.1016/j.biochi.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Smirnova IV, Bittel DC, Ravindra R, Jiang H, Andrews GK. Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J Biol Chem. 2000;275:9377–9384. doi: 10.1074/jbc.275.13.9377. [DOI] [PubMed] [Google Scholar]
- 33.LaRochelle O, Gagne V, Charron J, Soh JW, Seguin C. Phosphorylation is involved in the activation of metal-regulatory transcription factor 1 in response to metal ions. J Biol Chem. 2001;276:41879–41888. doi: 10.1074/jbc.M108313200. [DOI] [PubMed] [Google Scholar]
- 34.Robbins AH, McRee DE, Williamson M, Collett SA, Xuong NH, Furey WF, Wang BC, Stout CD. Refined crystal structure of Cd, Zn metallothionein at 2.0 A resolution. J Mol Biol. 1991;221:1269–1293. [PubMed] [Google Scholar]
- 35.Arseniev A, Schultze P, Worgotter E, Braun W, Wagner G, Vasak M, Kagi JH, Wuthrich K. Three-dimensional structure of rabbit liver [Cd7]metallothionein-2a in aqueous solution determined by nuclear magnetic resonance. J Mol Biol. 1988;201:637–657. doi: 10.1016/0022-2836(88)90644-4. [DOI] [PubMed] [Google Scholar]
- 36.Fang CX, Doser TA, Yang X, Sreejayan N, Ren J. Metallothionein antagonizes aging-induced cardiac contractile dysfunction: role of PTP1B, insulin receptor tyrosine phosphorylation and Akt. Aging Cell. 2006;5:177–185. doi: 10.1111/j.1474-9726.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 37.Schmid FX, Bielenberg K, Schneider A, Haussler A, Keyser A, Birnbaum D. Ascending aortic aneurysm associated with bicuspid and tricuspid aortic valve: involvement and clinical relevance of smooth muscle cell apoptosis and expression of cell death-initiating proteins. Eur J Cardiothorac Surg. 2003;23:537–543. doi: 10.1016/s1010-7940(02)00833-3. [DOI] [PubMed] [Google Scholar]
- 38.Niwa K, Perloff JK, Bhuta SM, Laks H, Drinkwater DC, Child JS, Miner PD. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. 2001;103:393–400. doi: 10.1161/01.cir.103.3.393. [DOI] [PubMed] [Google Scholar]
- 39.Göbel H, van der Wal AC, Teeling P, van der Loos CM, Becker AE. Metallothionein in human atherosclerotic lesions: a scavenger mechanism for reactive oxygen species in the plaque? Virchows Arch. 2000;437:528–533. doi: 10.1007/s004280000260. [DOI] [PubMed] [Google Scholar]
- 40.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro: implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalman RL. Oxidative stress and abdominal aneurysms: how aortic hemodynamic conditions may influence AAA disease. Cardiovasc Surg. 2003;11:417–419. doi: 10.1016/S0967-2109(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 42.Henderson BC, Tyagi SC. Oxidative mechanism and homeostasis of proteinase/antiproteinase in congestive heart failure. J Mol Cell Cardiol. 2006;41:959–962. doi: 10.1016/j.yjmcc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Tsutsui H, Ide T, Kinugawa S. Mitochondrial oxidative stress, DNA damage, and heart failure. Antioxid Redox Signal. 2006;8:1737–1744. doi: 10.1089/ars.2006.8.1737. [DOI] [PubMed] [Google Scholar]
- 44.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 45.LeMaire SA, Wang X, Wilks JA, Carter SA, Wen S, Won T, Leonardelli D, Anand G, Conklin LD, Wang XL, Thompson RW, Coselli JS. Matrix metalloproteinases in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res. 2005;123:40–48. doi: 10.1016/j.jss.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Koullias GJ, Korkolis DP, Ravichandran P, Psyrri A, Hatzaras I, Elefteriades JA. Tissue microarray detection of matrix metalloproteinases, in diseased tricuspid and bicuspid aortic valves with or without pathology of the ascending aorta. Eur J Cardiothorac Surg. 2004;26:1098–1103. doi: 10.1016/j.ejcts.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 47.Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MG. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation. 2000;102(suppl III):III-35–III-39. doi: 10.1161/01.cir.102.suppl_3.iii-35. [DOI] [PubMed] [Google Scholar]
- 48.Yasuda H, Nakatani S, Stugaard M, Tsujita-Kuroda Y, Bando K, Kobayashi J, Yamagishi M, Kitakaze M, Kitamura S, Miyatake K. Failure to prevent progressive dilation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: comparison with tricuspid aortic valve. Circulation. 2003;108(suppl II):II-291–II-294. doi: 10.1161/01.cir.0000087449.03964.fb. [DOI] [PubMed] [Google Scholar]
- 49.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–143. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 50.Robicsek F, Thubrikar MJ, Cook JW, Fowler B. The congenitally bicuspid aortic valve: how does it function? Why does it fail? Ann Thorac Surg. 2004;77:177–185. doi: 10.1016/s0003-4975(03)01249-9. [DOI] [PubMed] [Google Scholar]
- 51.Cotrufo M, Della Corte A, De Santo LS, Quarto C, De Feo M, Romano G, Amarelli C, Scardone M, Di Meglio F, Guerra G, Scarano M, Vitale S, Castaldo C, Montagnani S. Different patterns of extracellular matrix protein expression in the convexity and the concavity of the dilated aorta with bicuspid aortic valve: preliminary results. J Thorac Cardiovasc Surg. 2005;130:504–511. doi: 10.1016/j.jtcvs.2005.01.016. [DOI] [PubMed] [Google Scholar]