Abstract
Meningoencephalomyelitis of unknown origin (MUO) is a common, naturally-occurring, clinical disease of pet dogs. It is an immune-mediated condition that has many similarities with experimental autoimmune encephalitis (EAE) in rodents and so investigation of its pathogenesis may aid in understanding factors that contribute to development of multiple sclerosis in people. Gut microbiota are known to modulate immune responses that influence susceptibility to immune-mediated brain disease. In this study we aimed to compare abundance of specific constituents of the fecal microbiota, namely Faecalibacterium prausnitzii and Prevotellaceae, between dogs diagnosed with MUO and matched controls. Fecal samples were obtained from 20 dogs diagnosed with MUO and 20 control dogs matched for breed, age and gender. Bacterial abundance was measured using qPCR and 16S rRNA sequencing. We found that Prevotellaceae were significantly less abundant in cases compared with controls (p = 0.003) but there was no difference in abundance of F.prausnitzii. There was no evidence of other differences in gut microbiota between groups. These data, derived from this naturally-occurring canine clinical model, provide strong corroborative evidence that high abundance of Prevotellaceae in the gut is associated with reduced risk for developing immune-mediated brain disease.
Introduction
Disease in pet dogs treated by veterinarians forms a unique, frequently-overlooked, resource of biomedical translational data [1]. There are many advantages of these diseases as models of their human equivalents. First, they arise spontaneously, often through multifactorial etiologies similar to those that induce the parallel diseases in humans. Second, both pet dogs and humans share a common environment that may be a source of disease-causing risks. Third, diagnostic procedures and therapeutic interventions are broadly similar between humans and dogs with similar diseases. Within the field of neurology these features of similarity have been most extensively discussed in regard to spinal cord injury [2,3]. In this study we investigate possible etiologies for another canine disease: meningoencephalomyelitis of unknown origin (MUO), which models many aspects of multiple sclerosis in people.
MUO is an umbrella term used to summarize clinically-diagnosed immune-mediated inflammatory disease of the meninges, spinal cord and, especially, the brain of dogs [4]. On histopathologic examination a variety of sub-types of MUO are recognized, differentiated mainly by the predominance of white or grey matter involvement, the extent of necrosis and whether meninges are also inflamed [5,6], but all sub-types show the common feature of inflammatory cell infiltrate. In common with multiple sclerosis (MS) [7], much effort has been directed toward searching for infectious causes for MUO in dogs [8], and has proved similarly unsuccessful.
Traditionally, experimental autoimmune encephalomyelitis (EAE) in rodents has been widely used as a model for investigating the pathogenesis of multiple sclerosis [9]. The self-directed immune response in EAE can be initiated by injection of central nervous system (CNS)-derived antigens into susceptible animals or by passive transfer of antibodies from affected animals [10,11]. Recent work has suggested that the self-directed immune response, which is dependent upon a suitable systemic immune environment, can in turn be modulated by components of the gut microbiome [12]. Thus, germ-free mice are resistant to development of EAE, whereas those with wild-type gut microbiota remain susceptible [12,13]. Further support for this pathogenetic mechanism is provided by the changes in susceptibility to EAE that can be elicited by using antibiotics [14], oral immunization with vaccine strain of Salmonella [15], or probiotic mixtures [16].
Subsequent comparisons of the gut microbiome between MS patients and unaffected individuals have provided evidence that it may also play a role in the etiopathogenesis of MS [17,18]. Notably, an association has been made between development of MS and depleted populations of Faecalibacterium prausnitzii, Prevotella spp, or both [18], which also supports a more widely held belief that these two bacterial types may be protective against development of self-directed immune responses [19–21].
MUO in dogs shows many histological and immunological similarities to both EAE in rodents and MS in humans [22,23], suggesting a common etiology and implying its value in interpretation of the potential importance of the gut microbiome in development of immune-mediated encephalomyelitis in humans. Of further benefit is the low level of genetic diversity within defined dog breeds [24], implying that genetic influences on susceptibility to this disease [25–28] can be controlled by experimental design. In this study, we exploited the well-known breed, age and gender susceptibility [29] to design a case-control study in which to dissect the effects of gut microbial populations and other possible environmental triggers on development of MUO in dogs. We hypothesized that, in fecal samples obtained from dogs with MUO, there would be a decreased abundance of F.prausnitzii and, or, Prevotellacea, compared with that found in unaffected dogs.
Methods
Materials and methods
Fecal samples were collected from dogs (‘Cases’) diagnosed with MUO at the veterinary hospitals of Iowa State University (ISU) and Texas A&M University (TAMU) and stored in a -80°C freezer for analysis at completion of case recruitment. We also collected fecal samples from matched control dogs (‘Controls’) that were presented for diagnosis and treatment through the same veterinary school hospitals for other conditions.
Cases
Typical cases of MUO present with signs of brain dysfunction such as seizures or brainstem dysfunction (often disorders of vestibular function), although signs of disease anywhere in the CNS can be caused by MUO. Diagnostic tests include magnetic resonance imaging (MRI) scans and cerebrospinal fluid (CSF) analysis. Important rule-outs include infectious causes of brain inflammation such as Toxoplasma, canine distemper virus infection, Neospora and, depending on geographical location, tick-borne diseases. Final confirmation of the diagnosis is achieved through post mortem examination but cases can usually be securely diagnosed through clinical tests and long-term response to therapy alone.
Potential Cases presented with various neurological signs relating to dysfunction of the brain (we specifically excluded dogs that were suspected of having MUO but had signs affecting the spinal cord or optic nerves alone), underwent routine MRI scanning and CSF analysis. For inclusion as a Case a dog had to show typical areas of diffuse hyperintensity on T2-weighted MRI scans (Fig 1) and increased cell counts (>5 white cells per μL) on CSF analysis. We also included any dogs that were definitively diagnosed at post mortem whether they fulfilled other clinical criteria or not. Dogs with evidence of gastrointestinal disease were specifically excluded, as were those that tested positive for any infectious disease. Any dog that had received oral antibiotics within 4 weeks of diagnosis, or that was diagnosed with cancer or immune-mediated disease affecting any part of the body, was also excluded.
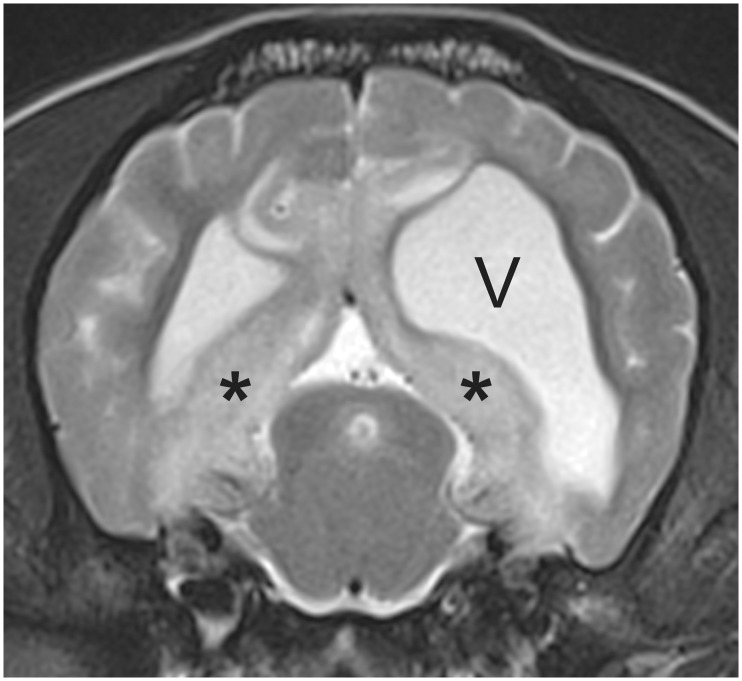
Fig 1. 3T T2W axial MR image at the level of the caudal colliculus illustrating the typical hyperintensity (*) that is often prominent adjacent to the ventricles in cases of MUO.
This dog also exhibits ventricular asymmetry (‘V’ indicates left ventricle), which is a common incidental finding in small breed dogs (this was a pug).
After MRI and CSF had been obtained owners were asked for permission to take a fecal sample and for their assistance in completing the study record form to complete a short questionnaire (see below). This protocol was specifically reviewed and approved by the Iowa State University Institutional Animal Care and Use Committee (log number 4-12-7347-K).
Controls
These were dogs that presented to ISU or TAMU for investigation or treatment of other conditions but were of the same breed, gender and matched age. In line with recommendations for case-control studies [30], we specifically sought Control animals from those presented to our veterinary university clinics because they were derived from the same population as the Cases. Control dogs were subject to the same exclusions as Cases, therefore dogs diagnosed with any gastro-intestinal disease, proven or suspected immune-mediated disease, cancer, or that had received systemic antibiotic therapy within 4 weeks were excluded. Owners of dogs that fulfilled these requirements (identified through daily appointment records) or that were of an age and breed likely to match in future with a Case were approached to seek consent to obtain a fecal sample and complete the Study Record Form, whether or not a matched Case had previously been recruited.
Fecal samples
Feces (at least 30g) were obtained from the rectum using a gloved finger and then stored in sterile containers in a -80°C freezer until the end of the sample acquisition phase.
Study Record Form
A specifically-designed Study Record Form recorded demographic data plus whether the dog was a Case or Control and the date of the most recent antecedent vaccination (of any type). Vaccination of both Cases and Controls within the previous calendar month was recorded as a dichotomous variable. The home environment in which the dog lived was categorized as rural, semi-rural (small town) or urban. We also asked questions about diet in general terms, such as whether dry (kibble), wet (canned) or home-cooked food was provided but did not request details. Any further follow-up information (e.g. post-mortem report) was added as free text.
Gut microbiota analysis
DNA isolation: DNA was extracted from the swabs with a MoBio Power soil DNA isolation kit (MoBio Laboratories, USA) following the manufacturer’s instructions.
Sequencing of 16S rRNA genes: The V4 region of the 16S rRNA gene was amplified with primers 515F (5’-GTGCCAGCMGCCGCGGTAA-3’) and 806R (5’-GGACTACVSGGGTATCTAAT-3’) at the MR DNA Laboratory (Shallowater, TX, USA) as previously described [31]. The Nextera® DNA sample Preparation kit including sequencing adapters and sample specific barcodes was used to prepare a DNA library and sequenced at MR DNA on an Illumina MiSeq instrument.
The raw sequence data was screened, trimmed, filtered, denoised and barcodes and chimera sequences were depleted from the dataset using QIIME v1.8 pipeline and UCHIME. Operational Taxonomic Units (OTUs) were assigned based on at least 97% sequence similarity against the Greengenes reference database. Sequences were rarefied to an even depth of 6,900 sequences per sample to account for unequal sequencing depth across samples. Observed species richness, Chao 1, and Shannon indexes were determined using QIIME. The sequences were deposited in SRA under accession number PRJNA319388.
Statistical analysis
Our study was designed to be analyzed using conditional logistic regression so as to take advantage of the case-control design, in which each Case was matched with a single Control of the same breed, gender and approximate age. MUO status (yes or no) was the dependent outcome variable. The primary, pre-defined outcome measure—based on the previous data on association in multiple sclerosis [18]—was to test whether reduced abundance of F.prausnitzii and Prevotellaceae was significantly associated with diagnosis of MUO. We also examined the association with two other putative risk factors for MUO that might also have relevance for development of MS in people: vaccination status (vaccinated within the previous month or not) and environment (in three graded categories). Analysis was conducted using Stata 11.0 (Stata Corp, College Station, TX) with P<0.05 taken to indicate significance.
We also planned further exploratory comparison of the microbial populations between Cases and Controls using analysis of beta-diversity by unweighted Unifrac distance metrics, similarly to a previously published report [31]. Statistical significance of the resulting distance metric was tested by analysis of similarities (ANOSIM) using the QIIME software.
There was no formal power calculation for this study, because we had little previous available information on which to base a sample size calculation, although the study by Miyake et al (2014) [18] examined 20 human MS patients. Instead, we aimed to accumulate as many matched pairs as possible during the 2-year period of study sponsorship (American Kennel Club Health Foundation, grant # 01731).
Results
Samples with adequate documentation of all variables were collected from a total of 70 dogs, of which 39 were Cases and 31 were Controls. As expected from previous studies [29], the majority were small breed dogs (<20kg bodyweight). There were 20 matched pairs of dogs (40 dogs in total); 5 pairs were male and 15 pairs were female; this gender predominance is also similar to that reported in previous studies [29].
The full breed list is: Chihuahua (n = 8), Maltese (n = 6), Labrador (n = 4), pug (n = 4), shih-tzu (n = 4), then golden retriever, Chesapeake bay retriever, Weimeraner, dachshund, beagle, miniature pinscher and bichon frise (each n = 2). Cases were presented at the following periods in the year: 8 dogs in winter (Dec-Feb); 6 dogs in spring (Mar-May); 3 dogs in summer (Jun-Aug) and 3 dogs in fall (Sep-Nov).
Pre-specified comparisons between Cases and Controls
Univariable analysis
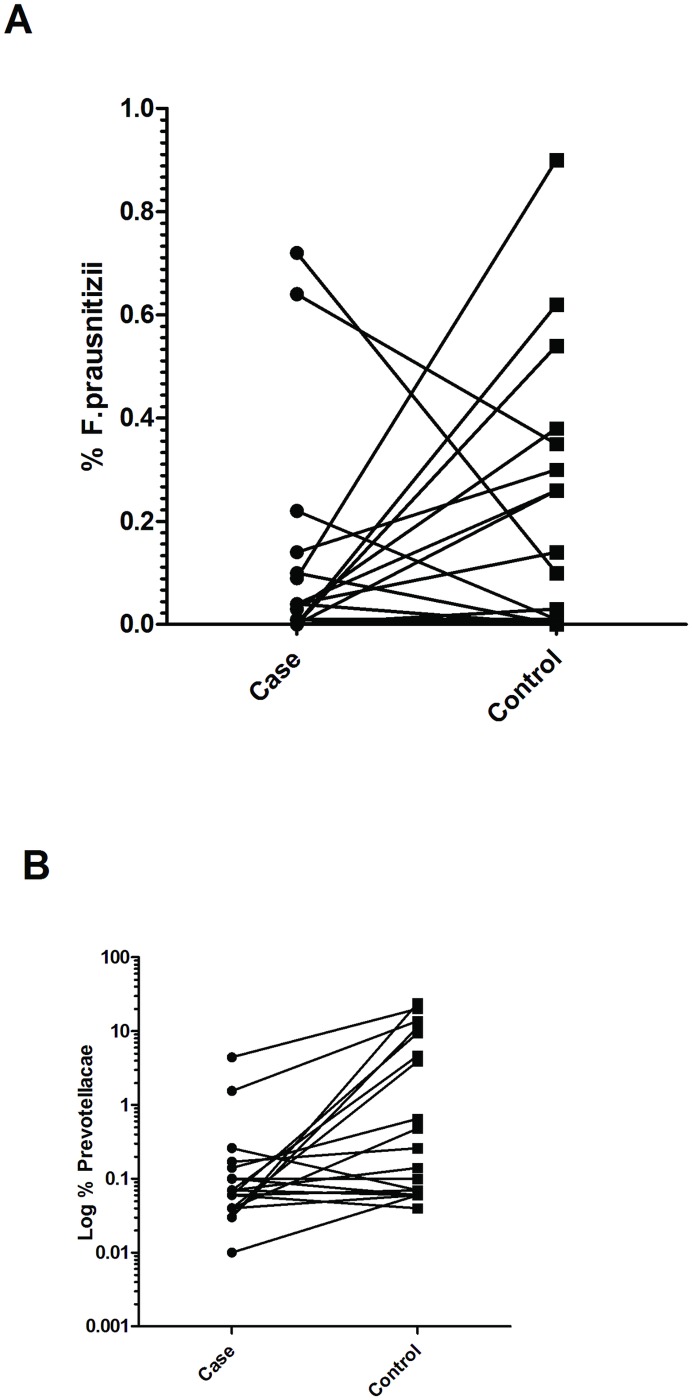
Initially we examined the abundance of F.prausnitzii and Prevotellaceae in Cases and their matched Controls, neither of which constituted normally-distributed data. Amongst all sampled dogs F. prausnitzii abundance ranged from 0–0.9% of the total bacterial population and differed little between Cases and Controls (Wilcoxon paired signed ranks test, P = 0.198). In 9 of 21 Cases and 5 of 21 Controls no bacteria of this species were detected at all. Abundance of Prevotellaceae ranged from 0.01–23.91% across all sampled dogs but was significantly different between Cases and Controls (Wilcoxon paired signed ranks test, P = 0.003) (Fig 2). The data we recorded on diet proved to be insufficiently detailed to allow further analysis. Owners in both groups reported feeding similar diets in general terms: 17/21 Cases and 19/21 Controls were fed various brands of kibble food as their main, or only, diet. However, most owners also indicated that there was variation in the daily diet and many dogs received variable amounts of table scraps and rewards.
Fig 2. Dot plots illustrating relative abundance of F.prausnitzii (A) and Prevotellaceae (x103) (B) in Cases and matched Controls.
Prevotellaceae abundance is shown on a log scale to improve detail; this is not possible for F.prausnitzii because many animals had a zero score.
Conditional logistic regression (which takes advantage of pair-matching) was used to examine the relationships between Prevotellaceae and F.prausnitzii abundances and development of MUO in more detail. Because the data populations were not normally-distributed and because we could not assume that relationships between bacterial abundance and development of MUO were linear we divided the populations into tertiles (i.e. low, medium and high abundance) using an automatic function in Stata. This analysis revealed a tendency toward lower prevalence of MUO in animals with higher abundance of F.prausnitzii, although this was not statistically significant in this population (Odds ratio [OR] = 0.575; P = 0.192). In contrast there was a stronger, statistically-significant relationship of reduced likelihood of MUO in dogs with higher abundance of Prevotellaceae (OR = 0.303; P = 0.038) (Table 1). This was also reflected in the significant trend of decreasing odds of developing MUO with increasing abundance of Prevotellaceae (χ2 = 6.89; P = 0.010) (Table 2).
Table 1. Results of univariable conditional logistic regression analyzing association of various putative risk factors (expressed as categories) with the development of MUO.
| Odds ratio | SE | Z | P | 95% CI | |
|---|---|---|---|---|---|
| F.prausnitizii | 0.574 | 0.244 | -1.30 | 0.192 | 0.250–1.322 |
| Prevotellaceae | 0.303 | 0.175 | -2.07 | 0.038 | 0.098–0.937 |
| Environment | 2.809 | 1.584 | 1.83 | 0.067 | 0.930–8.481 |
Table 2. Analysis of odds of development of MUO associated with various abundances of Prevotellaceae.
| Prevotellaceae abundance | Cases | Controls | Odds | 95% CI |
|---|---|---|---|---|
| Low | 7 | 1 | 7.000 | 0.861–56.895 |
| Medium | 9 | 9 | 1.000 | 0.397–2.519 |
| High | 4 | 10 | 0.400 | 0.125–1.275 |
Test of trend of odds: χ2 = 6.65; P = 0.010
We next examined the possible effects of two other possible environmental factors: recent vaccination and home environment. None of the unaffected Control dogs had been vaccinated within the past month, whereas 5/20 Cases had been, resulting in a statistically significant association (Fisher’s exact test, p = 0.047). Living environment appeared to be weakly associated with development of MUO; overall the odds of developing MUO were lower for dogs living in a rural environment that for those living in urban areas although not significant at the P<0.05 level (OR = 2.810; P = 0.067; Table 1) and this was also reflected in the significant trend of odds associated with increasingly more populated environments (χ2 = 4.53; P = 0.033; Table 3).
Table 3. Analysis of odds of development of MUO associated with various environmental conditions.
| Environment | Cases | Controls | Odds | 95% CI |
|---|---|---|---|---|
| Rural | 1 | 6 | 0.167 | 0.020–1.384 |
| Small town / edge of city | 8 | 8 | 1.000 | 0.375–2.664 |
| Urban | 11 | 6 | 1.833 | 0.678–4.957 |
Test of trend of odds: χ2 = 4.53; P = 0.033
Multivariable analysis
We intended to include all the relevant variables in multivariable conditional logistic regression but, because of the lack of recent vaccination in any Control dogs, it was not possible to include this variable. When all three other variables were included together in multivariable conditional regression analysis there was considerable change in the association of diagnosis of MUO with the dog’s living environment (OR = 10.936), and moderate changes for association with F.prausnitzii (OR = 0.255) and with Prevotellaceae (OR = 0.446) (Table 4). A series of subsequent exploratory analyses using stratification of exposure to both Prevotellaceae and environmental categories, plus χ2 analysis of association between these variables, were impaired by null values in some categories. Nevertheless, inspection of the odds ratios (and the instability of the figures for environment) strongly suggests interaction amongst these variables.
Table 4. Multivariable conditional logistic regression analyzing association of various putative risk factors with development of MUO.
| Odds ratio | SE | Z | P | 95% CI | |
|---|---|---|---|---|---|
| F.prausnitizii | 0.255 | 0.227 | -1.54 | 0.125 | 0.044–1.460 |
| Prevotellaceae | 0.446 | 0.291 | -1.24 | 0.216 | 0.124–1.603 |
| Environment | 10.936 | 16.051 | 1.63 | 0.103 | 0.616–194.142 |
Exploratory analysis of bacterial phyla populations
One of the difficulties in analyzing gut microbiota is that there is an almost infinite number of possible hypotheses to test. Nevertheless, our next step was preliminary exploratory analysis of the bacterial phyla abundances that were available from the sequencing studies. Similar studies have been conducted for human patients with MS [17,18] and we used a parallel methodology.
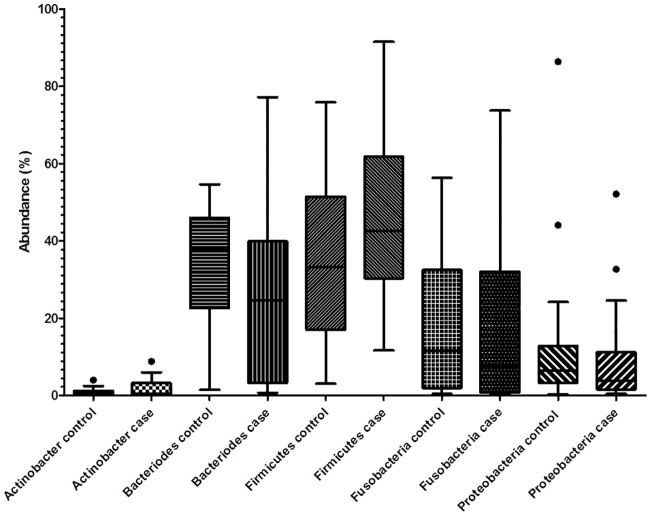
Abundance of bacteria was recorded for each of 8 major phyla (Euryarchaeota, Actinobacteria, Bacteroidetes, Deferribacteres, Firmicutes, Fusobacteria, Proteobacteria, Tenericutes). Abundance was recorded as zero in a substantial proportion (>50%) of all dogs in some categories (Euryarchaeota, Deferribacteres, Tenericutes) leaving a total of 5 phyla for analysis of the relationship between abundance and diagnosis of MUO. For none of these phyla was there evidence of a statistical difference in abundance between Cases and Controls (Fig 3) and conditional logistic regression analysis of the matched-pair data using tertiles (as described above for Prevotellaceae and F.prausnitzii) did not support an association between abundance of any of these bacterial phyla and development of MUO (Table 5).
Fig 3. Tukey box-and-whisker plots illustrating relative abundance of the major bacterial phyla in fecal samples from Case and Control dogs.
Table 5. Results of exploratory multivariable conditional logistic regression on association of various bacteria phyla with development of MUO.
| Odds ratio | SE | Z | P | 95% CI | |
|---|---|---|---|---|---|
| Actinobacteria | 1.168 | 0.514 | 0.35 | 0.724 | 0.493–2.768 |
| Bacterioidetes | 0.456 | 0.230 | -1.55 | 0.120 | 0.170–1.228 |
| Firmicutes | 0.736 | 0.411 | -0.55 | 0.583 | 0.247–2.197 |
| Fusobacteria | 0.705 | 0.371 | -0.66 | 0.508 | 0.251–1.980 |
| Proteobacteria | 0.582 | 0.293 | -1.07 | 0.283 | 0.217–1.563 |
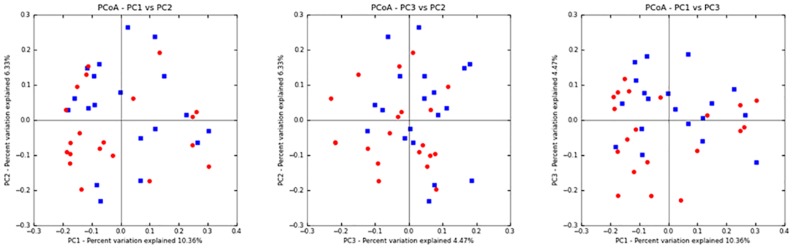
Finally, we used principal component analysis as a means to detect more global association between fecal microbial content and development of MUO. Alpha diversity as described by Chao 1, Observed species (species richness), and Shannon diversity index were not significantly different between paired samples. PCoA plots of unweighted Unifrac distances (Fig 4) confirmed that there was not significant clustering between samples from Case or Control groups (R-statistic = 0.054; ANOSIM p = 0.15).
Fig 4. 2-dimensional representations of the principal component analysis illustrating the lack of clustering of microbiota constituents in Cases (blue squares) or Controls (red dots).
Discussion
The analysis presented here supports an association between low Prevotellaceae abundance and a diagnosis of MUO in dogs, which also corroborates previous observations of an association between this bacterial group and diagnosis of MS in people [18]. Such corroboration, especially in a different species, is important because investigation of such a complicated system as the gut microbiome carries an inherently high risk of false discovery. In this study, we specifically targeted only two bacterial populations in our primary pre-specified analysis implying that the risk of false discovery is low. Therefore, this evidence carries high value as corroboration of the importance of Prevotellaceae in reducing risk of immune-mediated CNS disease. Interestingly, low abundance of Prevotellaceae has also been associated with Parkinson’s disease in people [32]. The rigid case-control design has enabled us to minimize the possible confounding factors of age, gender and genetic variability.
On the other hand, the possible association of low abundance of F.prausntizii with MS in people [17,18] has not been supported by our data. This could simply be because of the relatively low power of our study, especially since many of the dogs had zero counts of this bacterium that may have confounded our ability to make associations. Nevertheless, our observations on matched pairs of dogs (Fig 1) would suggest that any effects must be relatively small since in many pairs the Case had a higher abundance of F.prausnitzii than its matched Control. It is also possible that for some reason, such as diet, dogs in general, or dogs in this specific study, simply have low levels of F.prausnitzii and its association with immune-mediated disease in this species is not the same as in others. Lastly, in the study by Canterel at el (2015) [17] the abundance of various bacterial groups, including Faecalibacterium, was altered by vitamin D levels. Because dogs are not dependent on sunlight for production of vitamin D [33], levels of this vitamin are likely adequate in dogs receiving a reputable manufacturer’s diet (as all dogs in this study were) and so the relationship between levels of this vitamin and abundance of this specific fecal bacterium may differ between the two species.
Two further findings in this study may be useful in research into etiology of MS in humans. First, we show the possibility that MUO may, in some cases, be triggered by vaccination. However, it is important that this finding be treated with caution: there were few dogs that had received vaccination and developed disease and so confidence in this result must be low. Nevertheless, the possibility of vaccine triggering MS or other CNS demyelinating conditions has been previously studied in MS patients; overall the conclusions have been that there is no evidence of increased risk [34,35], although there is a suggestion that some individuals that are in sub-clinical stage of other demyelinating diseases may be triggered into disease by vaccination [35]. Our results are consistent with this explanation.
Pet dogs can also have potential for helping to identify disease risk factors in the environment because they share living spaces with humans and so may have similar exposure patterns. In this study, there is evidence that an urban environment is associated with increased risk of MUO. Our study provides a little support for the notion that this risk is mediated via effects on the microbiome (because there was weak evidence of interaction between environment and Prevotellaceae abundance) and so other mechanisms could be evoked. In humans there is strong evidence that specific regions of the world carry risk for development of MS, in particular through linkage with vitamin D status [36]. This would be an unlikely association in dogs because, as noted above, they do not manufacture vitamin D in the skin [33]; instead, dogs may be able to provide evidence of more local environmental risk, such as the characteristics of a specific neighborhood that might play a role in susceptibility to immune-mediated CNS diseases. There is some previous evidence that urban living may be a risk factor for MS in people [37], and this has been suggested to be because of increased hygiene. It is possible that the same could apply to dogs living in urban environments (since they have less exposure to wildlife, for instance) but, alternatively, urban-living dogs may also have greater exposure to environmental toxins, such as organic solvents, which have been suggested as possible risks in human immune-mediated disease [38]. On the whole, dogs are excellent sentinel species for environmental toxins because of their well-developed scavenging behavior.
Finally, the association of high abundance of Prevotellaceae with reduced likelihood of developing MUO may have two general explanations. As a ‘fermenting’ group of bacteria, Prevotellaceae produce butyrate, which has been identified as a specific inducer of Treg cell differentiation [39]. It is possible that this butyrate-producing metabolic profile may thus constitute the uniting mechanism of resistance to immune-mediated disease thought to be mediated by both Prevotellaceae and F. prausnitzii [21]. Similarly, hormones produced by specific gut microbes could also directly influence the immune system [40]. Together these effects may modulate the changed intestinal permeability that has been detected in both MS patients [41] and rats with EAE [42]. On the other hand, it is also possible that high Prevotellaceae abundance might result as a bystander effect of another interaction between the host (immune system) and the gut microbiome. If this were true, a high population of Prevotellaceae might simply be a biomarker of an immune system that is in a state that moderates inflammatory responses. Support for this notion is provided by their relatively low overall abundance: Prevotellaceae were recorded in our study as a median of only ~0.2% of the detected bacterial microbiota; nevertheless, hormonal effects need not depend on high abundance.
What limitations might there be in the conclusions that can be reached from this study? First, because it was designed with the specific aim of exploring the possibility that reduced fecal bacterial population of one pre-specified species and one pre-specified family might be associated with development of this specific immune-mediated disease these results must be regarded as robust. There is little risk of a false positive finding with this experimental design and, furthermore, the outcome largely corroborates previous evidence. Nevertheless, there are an almost unlimited number of unrecorded variables, such as specific dietary components, dog activity levels and gut permeability that might possibly have some influence on the development of MUO and might have been overlooked in this study. On the other hand, there is little reason to suppose that these factors would be systematically differently distributed between our Case population and the Controls, especially since we specifically selected our Controls from animals presenting to our clinics for treatment. Further investigation in new sample populations is required to investigate all the possible interactions amongst gut microbiota, environment and other disease states.
Finding different abundances of specific bacterial species between affected and unaffected groups in this study directs a spotlight at diet as a possible cause of these differences. Here we recorded only coarse information that proved impossible to analyze in a meaningful way since there was insufficient detail to be able to analyze the proportions of specific nutrients in any particular animal’s diet. Unfortunately, pet dog diets are often difficult to analyze because there is a great deal of variability in the way that owners feed dogs, both between individuals and between days. Further exploration of these dietary effects will require collaboration with a specialist nutritionist.
There is an almost infinite variety of environmental factors that could be involved in the risk of developing MUO and it is not possible for all these to be investigated, or accounted for, in a single study. For instance, there is some evidence that ingestion of non-steroidal anti-inflammatory drugs (NSAIDs) may alter not only gut permeability, but also may be associated with changes in gut microbiota [43]. Unfortunately, investigation of such effects is complicated because different NSAIDs appear to be associated with different gut microbial constituents and may also be affected by duration of therapy. Therefore, the possible effect of NSAID therapy, which was accounted for in this study as an unknown that would ‘randomize out’, provides an example of a factor that requires further investigation in the context of CNS inflammatory disease. On the other hand, in this study we did collect simple data on a small number of some possible explanatory variables, with the aim of providing some preliminary information on possible risk factors for development of MUO in dogs and, by implication, possibly also for MS in humans. The study design implies that the results of analysis of the association of these other factors (apart from the targeted bacterial populations) with development of MUO must be regarded as tentative and will require further validation in repeated studies. For instance, the apparent association of recent vaccination with development of MUO is weak—few dogs had been vaccinated in temporal proximity to disease onset and so the confidence intervals associated with this possible risk are wide—implying low analytical power and low confidence in their repeatability. Furthermore, it is plausible that different vaccinations might differ in their effects. The association of urban living with development of MUO is more robust, especially since there is a strong trend of odds across the range of exposure (Table 4) and there is a previous suggestion of a link between urban living and development of MS in people [37]. Nevertheless, our environmental data are not highly detailed (for instance we did not link zip code to population density) and will therefore require verification in a new sample population.
Supporting Information
https://dx.doi.org/10.6084/m9.figshare.4541227.v1. doi: 10.6084/m9.figshare.4541227.v1.
(XLSX)
https://dx.doi.org/10.6084/m9.figshare.4541233.v1. doi: 10.6084/m9.figshare.4541233.v1.
(XLSX)
Data Availability
All relevant summary data are within the paper and its Supporting Information files. The full datasets are available at Figshare.com. S1 Table. Signalment, Prevotellacae and F prausnitzii abundance. https://dx.doi.org/10.6084/m9.figshare.4541227.v1. doi: 10.6084/m9.figshare.4541227.v1. S2 Table. Summary of bacterial phyla data. https://dx.doi.org/10.6084/m9.figshare.4541233.v1. doi: 10.6084/m9.figshare.4541233.v1.
Funding Statement
This study was sponsored by the American Kennel Club, grant # 01731, awarded to NDJ, JS and AJ. http://www.akcchf.org/research/?referrer=https://www.google.com/. The sponsor provided the funding based on a grant proposal designed by the applicants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Knapp DW, Waters DJ. Naturally occurring cancer in pet dogs: important models for developing improved cancer therapy for humans. Mol Med Today 1997; 3: 8–11. [DOI] [PubMed] [Google Scholar]
- 2.Jeffery ND, Smith PM, Lakatos A, Ibanez C, Ito D, Franklin RJ. Clinical canine spinal cord injury provides an opportunity to examine the issues in translating laboratory techniques into practical therapy. Spinal Cord 2006; 44: 584–93. 10.1038/sj.sc.3101912 [DOI] [PubMed] [Google Scholar]
- 3.Levine JM, Levine GJ, Porter BF, Topp K, Noble-Haeusslein LJ. Naturally occurring disk herniation in dogs: an opportunity for pre-clinical spinal cord injury research. J Neurotrauma 2011; 28: 675–88. 10.1089/neu.2010.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coates JR, Jeffery ND. Perspectives on meningoencephalomyelitis of unknown origin. Vet Clin North Am Small Anim Pract 2014; 44: 1157–85. 10.1016/j.cvsm.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 5.Talarico LR, Schatzberg SJ. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: a review and future perspectives. J Small Anim Pract 2011; 51: 138–149 [DOI] [PubMed] [Google Scholar]
- 6.Tipold A, Vandevelde M, Schatzberg SJ. Necrotizing encephalitis In: Greene C.E. (ed.), Infectious diseases of the dog and cat. 4th ed St Louis, Mo: Elsevier, 2012, pp. 856–858 [Google Scholar]
- 7.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol 2007; 61: 288–99. 10.1002/ana.21117 [DOI] [PubMed] [Google Scholar]
- 8.Barber RM, Porter BF, Li Q, May M, Claiborne MK, Allison AB et al. Broadly reactive polymerase chain reaction for pathogen detection in canine granulomatous meningoencephalomyelitis and necrotizing meningoencephalitis. J Vet Intern Med 2012; 26: 962–8. 10.1111/j.1939-1676.2012.00954.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 2011; 164: 1079–106. 10.1111/j.1476-5381.2011.01302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc 2006; 1: 1810–1819. 10.1038/nprot.2006.285 [DOI] [PubMed] [Google Scholar]
- 11.Robinson AP, Harp CT, Noronha A, Miller SD. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb Clin Neurol 2014; 122: 173–89. 10.1016/B978-0-444-52001-2.00008-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2011; 108 Suppl 1: 4615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011; 479: 538–41. 10.1038/nature10554 [DOI] [PubMed] [Google Scholar]
- 14.Ochoa-Repáraz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes 2010; 1: 103–108. 10.4161/gmic.1.2.11515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun S, Gilmore W, Callis, Rynda A, Haddad A, Pascual DW. A live diarrheal vaccine imprints a Th2 cell bias and acts as an anti-inflammatory vaccine. J. Immunol 2005; 175: 6733–6740. [DOI] [PubMed] [Google Scholar]
- 16.Kwon HK, Kim GC, Kim Y, Hwang W, Jash A, Sahoo A, et al. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin Immunol 2013; 146: 217–227 10.1016/j.clim.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 17.Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, et al. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med 2015; 63: 729–34. 10.1097/JIM.0000000000000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One 2015; 10: e0137429 10.1371/journal.pone.0137429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One 2011; 6: e25792 10.1371/journal.pone.0025792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen AK, Hansen CH, Krych L, Nielsen DS. Impact of the gut microbiota on rodent models of human disease. World J Gastroenterol 2014; 20: 17727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velasquez-Manoff M. Gut Microbiome: The Peacekeepers. Nature 2015; 518: S3–S11 10.1038/518S3a [DOI] [PubMed] [Google Scholar]
- 22.Park ES, Uchida K, Nakayama H. Comprehensive immunohistochemical studies on canine necrotizing meningoencephalitis (NME), necrotizing leukoencephalitis (NLE) and granulomatous meningoencephalomyelitis (GME). Vet Pathol 2012; 49: 682–92. 10.1177/0300985811429311 [DOI] [PubMed] [Google Scholar]
- 23.Lassmann H, Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol 2016; October 20. [Epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellanby RJ, Ogden R, Clements DN, French AT, Gow AG, Powell R, et al. Population structure and genetic heterogeneity in popular dog breeds in the UK. Vet J 2013; 196: 92–7. 10.1016/j.tvjl.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 25.Greer KA, Schatzberg SJ, Porter BF, Jones KA, Famula TR, Murphy KE. Heritability and transmission analysis of necrotizing meningoencephalitis in the Pug. Res Vet Sci 2009; 86: 438–42. 10.1016/j.rvsc.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 26.Barber RM, Schatzberg SJ, Corneveaux JJ, Allen AN, Porter BF, Pruzin JJ, et al. Identification of risk loci for necrotizing meningoencephalitis in Pug dogs. J Hered 2011; 102 Suppl 1: S40–6. [DOI] [PubMed] [Google Scholar]
- 27.Safra N, Pedersen NC, Wolf Z, Johnson EG, Liu HW, Hughes AM, et al. Expanded dog leukocyte antigen (DLA) single nucleotide polymorphism (SNP) genotyping reveals spurious class II associations. Vet J 2011; 189: 220–6. 10.1016/j.tvjl.2011.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrauwen I, Barber RM, Schatzberg SJ, Siniard AL, Corneveaux JJ, Porter BF, et al. Identification of novel genetic risk loci in Maltese dogs with necrotizing meningoencephalitis and evidence of a shared genetic risk across toy dog breeds. PLoS One 2014; 9: e112755 10.1371/journal.pone.0112755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granger N, Smith PM, Jeffery ND. Clinical findings and treatment of non-infectious meningoencephalomyelitis in dogs: a systematic review of 457 published cases from 1962 to 2008. Vet J 2010; 184: 290–7. 10.1016/j.tvjl.2009.03.031 [DOI] [PubMed] [Google Scholar]
- 30.Grimes DA, Schulz KF. Compared to what? Finding controls for case-control studies. Lancet. 2005; 365: 1429–33. 10.1016/S0140-6736(05)66379-9 [DOI] [PubMed] [Google Scholar]
- 31.Bell ET, Suchodolski JS, Isaiah A, Fleeman LM, Cook AK, Steiner JM, et al. Faecal microbiota of cats with insulin-treated diabetes mellitus. PLoS One 2014; 9: e108729 10.1371/journal.pone.0108729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord 2015; 30: 350–8. 10.1002/mds.26069 [DOI] [PubMed] [Google Scholar]
- 33.How KL, Hazewinkel HA, Mol JA. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. General and Comparative Endocrinology 1994; 96: 12–18 10.1006/gcen.1994.1154 [DOI] [PubMed] [Google Scholar]
- 34.Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S; Vaccines in Multiple Sclerosis Study Group. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. N Engl J Med 2001; 344: 319–26. 10.1056/NEJM200102013440501 [DOI] [PubMed] [Google Scholar]
- 35.Langer-Gould A, Qian L, Tartof SY, Brara SM, Jacobsen SJ, Beaber BE, et al. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol 2014; 71: 1506–13. 10.1001/jamaneurol.2014.2633 [DOI] [PubMed] [Google Scholar]
- 36.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol 2010; 9: 599–612 10.1016/S1474-4422(10)70086-7 [DOI] [PubMed] [Google Scholar]
- 37.Malli C, Pandit L, D'Cunha A, Mustafa S. Environmental factors related to multiple sclerosis in Indian population. PLoS One 2015; 10: e0124064 10.1371/journal.pone.0124064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barragán-Martínez C, Speck-Hernández CA, Montoya-Ortiz G, Mantilla RD, Anaya JM, Rojas-Villarraga A. Organic solvents as risk factor for autoimmune diseases: a systematic review and meta-analysis. PLoS One 2012; 7: e51506 10.1371/journal.pone.0051506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504: 446–50. 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- 40.Galland L. The gut microbiome and the brain. J Med Food 2014; 17: 1261–72. 10.1089/jmf.2014.7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buscarinu MC, Cerasoli B, Annibali V, Policano C, Lionetto L, Capi M, et al. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult Scler 2016; pii: 1352458516652498. [DOI] [PubMed] [Google Scholar]
- 42.Nouri M, Bredberg A, Weström B, Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One 2014; 9: e106335 10.1371/journal.pone.0106335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers MA, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect 2016; 22: 178.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
https://dx.doi.org/10.6084/m9.figshare.4541227.v1. doi: 10.6084/m9.figshare.4541227.v1.
(XLSX)
https://dx.doi.org/10.6084/m9.figshare.4541233.v1. doi: 10.6084/m9.figshare.4541233.v1.
(XLSX)
Data Availability Statement
All relevant summary data are within the paper and its Supporting Information files. The full datasets are available at Figshare.com. S1 Table. Signalment, Prevotellacae and F prausnitzii abundance. https://dx.doi.org/10.6084/m9.figshare.4541227.v1. doi: 10.6084/m9.figshare.4541227.v1. S2 Table. Summary of bacterial phyla data. https://dx.doi.org/10.6084/m9.figshare.4541233.v1. doi: 10.6084/m9.figshare.4541233.v1.