Abstract
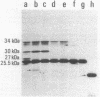
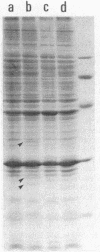
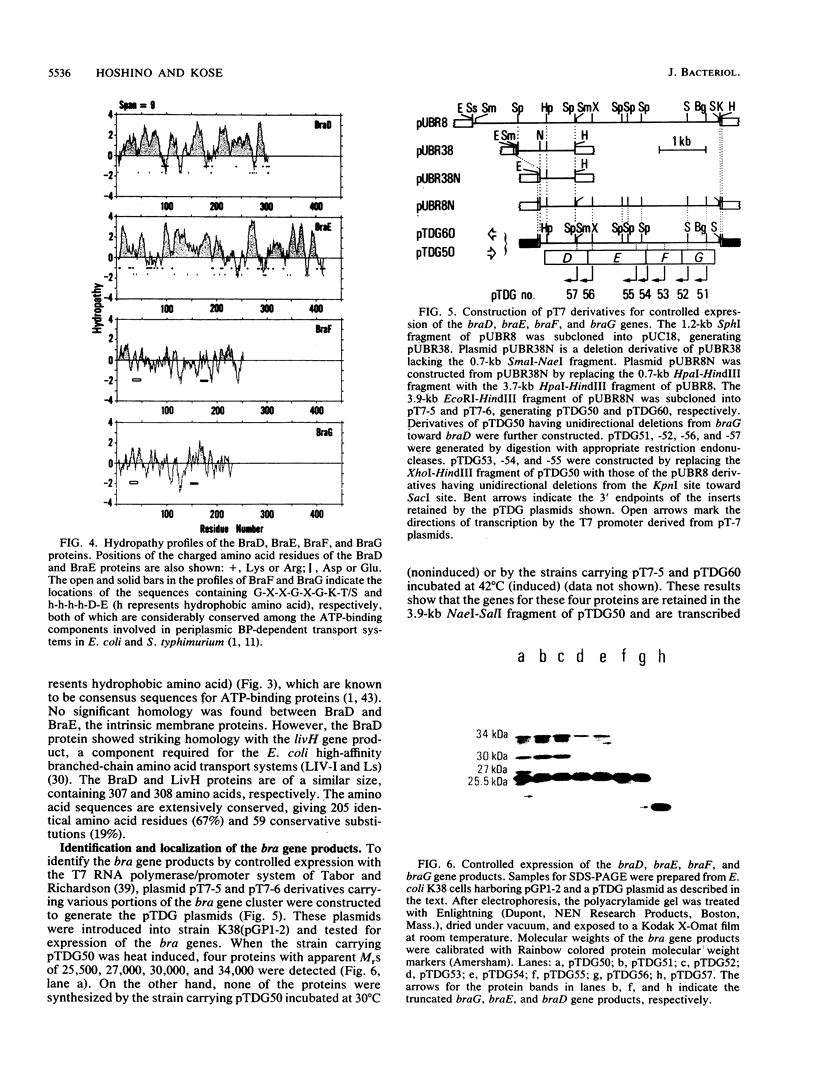
A DNA fragment of Pseudomonas aeruginosa PAO containing genes specifying the high-affinity branched-chain amino acid transport system (LIV-I) was isolated. The fragment contained the braC gene, encoding the binding protein for branched-chain amino acids, and the 4-kilobase DNA segment adjacent to 3' of braC. The nucleotide sequence of the 4-kilobase DNA fragment was determined and found to contain four open reading frames, designated braD, braE, braF, and braG. The braD and braE genes specify very hydrophobic proteins of 307 and 417 amino acid residues, respectively. The braD gene product showed extensive homology (67% identical) to the livH gene product, a component required for the Escherichia coli high-affinity branched-chain amino acid transport systems. The braF and braG genes encode proteins of 255 and 233 amino acids, respectively, both containing amino acid sequences typical of proteins with ATP-binding sites. By using a T7 RNA polymerase/promoter system together with plasmids having various deletions in the braDEFG region, the braD, braE, braF, and braG gene products were identified as proteins with apparent Mrs of 25,500, 34,000, 30,000, and 27,000, respectively. These proteins were found among cell membrane proteins on a sodium dodecyl sulfate-polyacrylamide gel stained with Coomassie blue.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M. M., Amann E., Lurz R., Rückert B., Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983 Dec;26(2-3):273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- Bell A. W., Buckel S. D., Groarke J. M., Hope J. N., Kingsley D. H., Hermodson M. A. The nucleotide sequences of the rbsD, rbsA, and rbsC genes of Escherichia coli K12. J Biol Chem. 1986 Jun 15;261(17):7652–7658. [PubMed] [Google Scholar]
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Frantz B., Ngai K. L., Chatterjee D. K., Ornston L. N., Chakrabarty A. M. Nucleotide sequence and expression of clcD, a plasmid-borne dienelactone hydrolase gene from Pseudomonas sp. strain B13. J Bacteriol. 1987 Feb;169(2):704–709. doi: 10.1128/jb.169.2.704-709.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Haag P. D., Nikaido K., Ardeshir F., Garcia G., Ames G. F. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature. 1982 Aug 19;298(5876):723–727. doi: 10.1038/298723a0. [DOI] [PubMed] [Google Scholar]
- Hiles I. D., Gallagher M. P., Jamieson D. J., Higgins C. F. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J Mol Biol. 1987 May 5;195(1):125–142. doi: 10.1016/0022-2836(87)90332-9. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1980 Mar;141(3):1055–1063. doi: 10.1128/jb.141.3.1055-1063.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Sodium-dependent transport of L-leucine in membrane vesicles prepared from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):73–81. doi: 10.1128/jb.137.1.73-81.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kose K. Cloning and nucleotide sequence of braC, the structural gene for the leucine-, isoleucine-, and valine-binding protein of Pseudomonas aeruginosa PAO. J Bacteriol. 1989 Nov;171(11):6300–6306. doi: 10.1128/jb.171.11.6300-6306.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kose K. Genetic analysis of the Pseudomonas aeruginosa PAO high-affinity branched-chain amino acid transport system by use of plasmids carrying the bra genes. J Bacteriol. 1990 Oct;172(10):5540–5543. doi: 10.1128/jb.172.10.5540-5543.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kose K., Uratani Y. Cloning and nucleotide sequence of the gene braB coding for the sodium-coupled branched-chain amino acid carrier in Pseudomonas aeruginosa PAO. Mol Gen Genet. 1990 Feb;220(3):461–467. doi: 10.1007/BF00391754. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Nishio K. Isolation and characterization of a Pseudomonas aeruginosa PAO mutant defective in the structural gene for the LIVAT-binding protein. J Bacteriol. 1982 Aug;151(2):729–736. doi: 10.1128/jb.151.2.729-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T. Transport systems for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1979 Sep;139(3):705–712. doi: 10.1128/jb.139.3.705-712.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Tsuda M., Iino T., Nishio K., Kageyama M. Genetic mapping of bra genes affecting branched-chain amino acid transport in Pseudomonas aeruginosa. J Bacteriol. 1983 Mar;153(3):1272–1281. doi: 10.1128/jb.153.3.1272-1281.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landick R., Oxender D. L. The complete nucleotide sequences of the Escherichia coli LIV-BP and LS-BP genes. Implications for the mechanism of high-affinity branched-chain amino acid transport. J Biol Chem. 1985 Jul 15;260(14):8257–8261. [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T., Miyazawa S., Yasunaga T. Two types of amino acid substitutions in protein evolution. J Mol Evol. 1979 Mar 15;12(3):219–236. doi: 10.1007/BF01732340. [DOI] [PubMed] [Google Scholar]
- Nazos P. M., Antonucci T. K., Landick R., Oxender D. L. Cloning and characterization of livH, the structural gene encoding a component of the leucine transport system in Escherichia coli. J Bacteriol. 1986 May;166(2):565–573. doi: 10.1128/jb.166.2.565-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazos P. M., Mayo M. M., Su T. Z., Anderson J. J., Oxender D. L. Identification of livG, a membrane-associated component of the branched-chain amino acid transport in Escherichia coli. J Bacteriol. 1985 Sep;163(3):1196–1202. doi: 10.1128/jb.163.3.1196-1202.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Russel M., Model P. Replacement of the fip gene of Escherichia coli by an inactive gene cloned on a plasmid. J Bacteriol. 1984 Sep;159(3):1034–1039. doi: 10.1128/jb.159.3.1034-1039.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scripture J. B., Voelker C., Miller S., O'Donnell R. T., Polgar L., Rade J., Horazdovsky B. F., Hogg R. W. High-affinity L-arabinose transport operon. Nucleotide sequence and analysis of gene products. J Mol Biol. 1987 Sep 5;197(1):37–46. doi: 10.1016/0022-2836(87)90607-3. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Iino T. Ordering of the flagellar genes in Pseudomonas aeruginosa by insertions of mercury transposon Tn501. J Bacteriol. 1983 Feb;153(2):1008–1017. doi: 10.1128/jb.153.2.1008-1017.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato I., Anraku Y. Genetic and biochemical studies of transport systems for branched-chain amino acids in Escherichia coli K-12: isolation and properties of mutants defective in leucine-repressible transport activities. J Bacteriol. 1980 Oct;144(1):36–44. doi: 10.1128/jb.144.1.36-44.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]