Abstract
Methylphenidate (MPH) is one of the few psychotropic agents approved for use in pediatric populations, underscoring the importance of elucidating any long-term consequences following exposure to this agent. Here, we examined the influence of several variables (i.e. age of assessment, age of exposure, sex, route of administration) on the effect of chronic low-dose MPH (2 mg/kg, twice daily) exposure on place conditioning to cocaine. Juvenile exposure to MPH, but not later exposure, resulted in aversions to cocaine-paired environments when assessed in young adult male rats, but not those entering adolescence. Juvenile MPH enhanced place preferences for cocaine-paired environments in female adolescent rats. The route of administration (i.p. injection or oral ingestion) did not produce enduring differential effects on behavior, and d-MPH was confirmed as the active enantiomer. These observations add to the growing literature on the enduring effects of MPH exposure, and highlight the need for more research in females.
Keywords: Place conditioning, Methylphenidate, Imprinting, Stimulant
Introduction
The risk of developing a significant drug addiction to psychostimulants is 4 times higher if use is initiated between 11 and 14 years of age than if initiated at any other age [1]. This startling statistic implies that drug addiction is less likely to occur if use is initiated before this period. Indeed, while stimulant use is reported by approximately 2% of the population in the 8th grade, large-scale epidemiological studies have not focused on earlier ages of onset [2]. Low abuse rates [1] are arguably due to low availability and minimal euphoric effects [3]. Additionally, research suggests that exposure to stimulants in children with attention-deficit hyperactivity disorder (ADHD) may actually reduce the risk of substance disorders later in life [4]. However, little is known about the mechanism underlying this effect or the factors that modulate this reduced risk.
Understanding the factors that reduce the risk of substance abuse following juvenile exposure to stimulants may have widespread implications for the treatment of drug addiction as well as ADHD. Moreover, the effects of stimulant exposure during postnatal development can provide valuable information about the development of the reward system. To this end, preclinical research on juvenile exposure to the stimulant methylphenidate (MPH) has been extensive in recent years [for a review, see 5]. These studies involved multiple drug-exposure paradigms, different ages of exposure, and assessment of short- and long-term effects, and have yielded a number of seemingly conflicting results and interpretations [5, 6]. For example, juvenile exposure to MPH produces an aversion to cocaine-associated environments in adulthood [7, 8], whereas exposure during adolescence [9] or adulthood [10] increases self-administration of cocaine. Other studies report no significant behavioral effect [11].
Drug-seeking, craving, and relapse play prominent roles in the addiction process [12]. These processes are initiated by the Pavlovian conditioning of drug-related cues [13]. Repeated pairing of the environment with a rewarding drug allows the environment to develop conditioned incentive properties as a drug-related cue [14]. Multiple, but consistent, measures of drug effects in animals enable extrapolation to the human condition about conditioned incentive. For example, conditioned drug effects are examined through self-administration [15], locomotor sensitization [5], and place conditioning [16]. Each of these paradigms has strengths and weaknesses for studying addiction. Self-administration assesses multiple aspects of drug addiction, including drug-taking, drug-seeking, and reinstatement [15]. However, the self-administration paradigm, while considered one of the most ideal paradigms for drug assessment, is not ideal for developmental studies due to technical issues such as catheterization, patency issues, and long training sessions. Alternatively, behavioral plasticity is assessed by repeatedly exposing animals to a drug in a given environment and testing for increased locomotor activity to drug challenge over time (sensitization) [17, 18]. Here, exposure to MPH in juvenile [19] or adolescent (41 days) rats produces either no effect or a slight diminution of the locomotor sensitizing effects of cocaine or methamphetamine [17], respectively. This paradigm also examines conditioned drug effects [14], and is better suited for developmental studies than self-administration [20, 21]. The limitation of this paradigm is the unclear relationship between locomotor activity and drug craving, taking, and seeking [14]. Comparisons across age are also difficult due to 7- to 14-day withdrawal periods often used for subsequent drug challenge [e.g. 22]. Place conditioning is a third paradigm wherein repeated pairing of a given environment with a drug yields a conditioned preference or aversion to that environment. Two place conditioning procedures are used: biased and unbiased. The biased paradigm associates the drug-associated environment with the initially least-preferred side, whereas, in the unbiased paradigm, subject assignment of drug-paired chambers eliminates side preferences by equally distributing any bias. In both paradigms, animals are subsequently tested for drug-seeking behavior in a drug-free state. More time spent in the drug-associated environment is interpreted as a drug preference [23–26]. However, the assessment of a drug aversion (i.e. time spent away from the drug-associated environment) is more clearly interpretable in the unbiased paradigm [26]. By testing in a drug-free state, one of the main advantages of place conditioning for across-age comparisons is that pharmacokinetics do not interfere with the assessment of the conditioned incentive.
In these studies, we systematically analyzed a number of factors that are known to influence drug-seeking behavior following developmental exposure. These factors include age of drug exposure [5, 7, 17, 19, 27, 28], age of testing [7, 19, 29], sex of the subject [19, 30, 31], route of drug administration [17, 27, 32, 33], and pharmacokinetics associated with MPH [34, 35]. To facilitate direct comparison of how MPH exposure interacts with these factors, we used an unbiased place conditioning procedure to model drug-seeking behavior in animals. The unbiased procedure allows the assessment of both aversions and preferences and the transitions in these behaviors as they occur. A similar analysis has been performed recently using locomotor sensitization as the metric, with the aim of explaining how MPH exposure can lead to sensitized drug responding [5].
General Methods
Subjects
Lactating female Sprague-Dawley rats obtained from Charles River (Worcester, Mass., USA) were housed with their litters on a 12-hour/12-hour light/dark cycle, with lights on at 07: 00 h with food and water provided ad libitum. Litters were culled to 10 pups of equal numbers of males and females on postnatal day (P) 1 and weaned at P 21. Animals were housed with same-sex littermates. A total of 214 rats were used in these experiments. Only 1 subject/litter was assigned to any individual condition, and the remaining littermates used in other studies. An average number of 7–8 per group were used, and no rats were used for more than a single study. Estrous state was not determined in females, as previous studies suggest that vaginal lavage may influence place conditioning [36]. All animals were treated in accordance with the policies established by NIH and the McLean Hospital Institutional Animal Care and Use Committee.
Drugs
MPH hydrochloride (d,l-MPH, unless noted otherwise), l-MPH, d-MPH, and cocaine hydrochloride were obtained from Sigma (St. Louis, Mo., USA). Drugs were dissolved in 0.9% saline vehicle (VEH) and administered in a volume of 1 ml/kg with a Hamilton syringe, except where otherwise stated (see Study 4).
Drug Treatment
Unless otherwise indicated (see Study 4), MPH (2 mg/kg) or VEH was administered by injection (intraperitoneally; i.p.) twice daily, with care taken to distribute each condition (drug) to 1 male and female from each litter. Subjects were weighed daily at 09:00, and given their first drug administration; the second was 4 h later at 13:00. The 2-mg/kg dose of MPH was initially selected on the basis of our previous studies [7, 8], and approximates a clinically relevant dose in humans [17, 37] based on clinical plasma levels. All drug exposure periods lasted 15 days, and animals received no further treatments until behavioral testing. Cocaine doses for the place conditioning experiments were chosen based on previous results that demonstrated place conditioning [7, 8].
Place Conditioning
Place conditioning occurred in a 3-chamber apparatus according to methods established by Carlezon et al. [8, 38]. The conditioning chamber consisted of 2 large (24 × 18 × 33 cm) side compartments separated by a small (12 × 18 × 33 cm) middle compartment. The 2 compartments differed in floor texture, lighting, and wall coloring (black or white), and these drug-associated environments were counterbalanced within a condition. On day 1, rats freely explored the apparatus to initially screen for baseline preferences, which was defined a priori by spending greater than 18 min of the 30-min session on 1 side. If preferences were detected, these subjects were eliminated from further testing. Less than 10% of rats displayed initial preferences to 1 chamber, which occurred regardless of exposure condition. This frequency of elimination was consistent with previous studies using the unbiased conditioning procedure in this laboratory. Conditioning to cocaine and saline occurred on days 2 and 3. During the first conditioning session of each day, rats received a 1 ml/kg i.p. injection of saline, and were confined to 1 side for 60 min. They were then returned to the home cage. Four hours later, rats received 10 mg/kg i.p. of cocaine (except where noted), and were confined to the other side. This dose was chosen because it consistently produces a place aversion in juvenile-MPH-exposed adults [7, 8]. On day 4, subjects were permitted to freely explore the entire apparatus for 30 min in a drug-free state. Time spent in each compartment was analyzed to reflect conditioning to the environmental cues associated with each compartment. Relative to time spent on the saline-associated side of the chamber, time spent in the drug-conditioned side was considered a drug preference, whereas time spent on the saline-conditioned side was considered an aversion.
Experimental Design
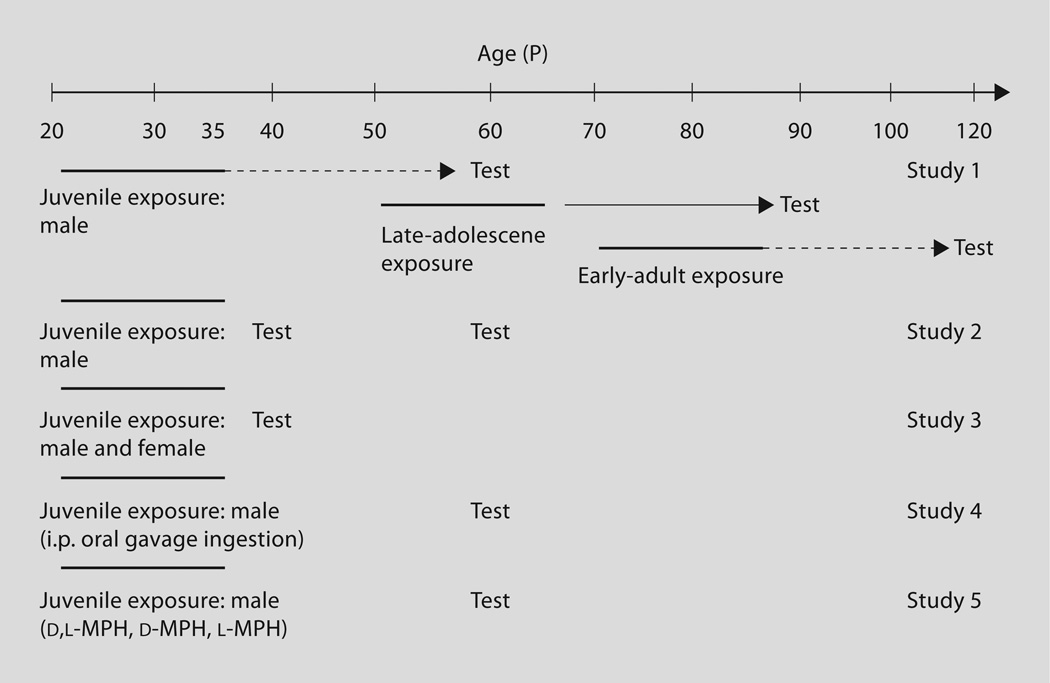
Five studies were conducted to determine the impact of age of exposure, age of testing, sex, route of administration, and MPH enantiomers on subsequent cocaine place conditioning (fig. 1).
Fig. 1.
Schematic of ages of drug administration and test periods. Rats were administered VEH or MPH (2 mg/kg, twice daily) during a 15-day period.
Study 1
Male rats were chronically treated with MPH (2 mg/kg, twice daily) for 15 days beginning either at P 20 (juvenile; n = 8), P 50 (late adolescence; n = 8), or P 70 (adult; n = 8). Place conditioning was assessed 25 days following treatment cessation.
Study 2
This experiment investigated the effects of juvenile MPH exposure on adolescent versus adult place conditioning. Male rats were chronically treated with MPH (2 mg/kg, twice daily) for 15 days beginning at P 20. Place conditioning commenced either at P 40 (5 days following treatment cessation; n = 7) or P 60 (25 days following treatment cessation; n = 8).
Study 3
Sex was examined as a factor in the effects of juvenile MPH on adolescent place conditioning. Males and females were treated with MPH as described in study 2. Place conditioning commenced on P 40, using 3 doses of cocaine (5, 10, and 20 mg/kg; n = 6–8) in order to gauge sensitivity to the conditioning effects of cocaine between sexes. We have previously found dose-dependent effects of this cocaine dose range at P 60 [7].
Study 4
Route of administration was by i.p. injection (n = 8), oral gavage (n = 12), or ingestion (n = 5). For the ingestion paradigm, 2 mg/kg of MPH (dissolved in saline) was added to a palliative food substance (Fruit Loops™ cereal). Subjects were pre-exposed to the cereal for 3 days prior to drug administration to reduce any effects of novelty. By the second day of exposure, subjects readily consumed the food within 2 minutes of presentation, and continued to do so once it contained the MPH. An additional VEH group (Fruit Loops alone; n = 8) was run for the ingestion condition. MPH (2 mg/kg) dissolved in saline or saline alone was used in the gavage condition, administered through a balled-end gavage needle in order to prevent damage to the esophagus.
Study 5
Role of enantiomers was investigated by exposing juvenile rats to MPH (d,l-MPH), or equimolar amounts of d-MPH or l-MPH (n = 10, 11, and 8, respectively) between P 20 and 35. The dose of the individual enantiomers was 1 mg/kg to account for their equimolar amounts in the mixed racemic form. Therefore, the groups were VEH, d,l-MPH (2 mg/kg), d-MPH (1 mg/kg), or l-MPH (1 mg/kg). The 2 mg/kg dose of d,l-MPH was selected on the basis of our previous studies [7, 8]. Rats were tested for place conditioning to cocaine at P 60.
Data Analysis
Data were analyzed using a mixed ANOVA with repeated measures [experimental factor × drug pre-exposure × conditioning (time spent in drug side: before vs. after)]. Significant effects were further analyzed with simple main effect (F) tests or Fisher’s honestly significant difference tests using SPSS (SPSS, Chicago, Ill., USA). For all post hoc drug comparisons, statistical significance was set at p < 0.05 following Bonferroni correction. Under all testing conditions, preconditioning baseline values did not differ across any of the comparisons (p > 0.5).
Results
Study 1: The Age of Drug Exposure
This study was conducted to determine whether exposure to MPH later in adulthood, i.e. P 70–85, will produce a differential effect on place conditioning to a modest dose of cocaine (10 mg/kg) as compared to exposure at P 20–35 and P 50–65.
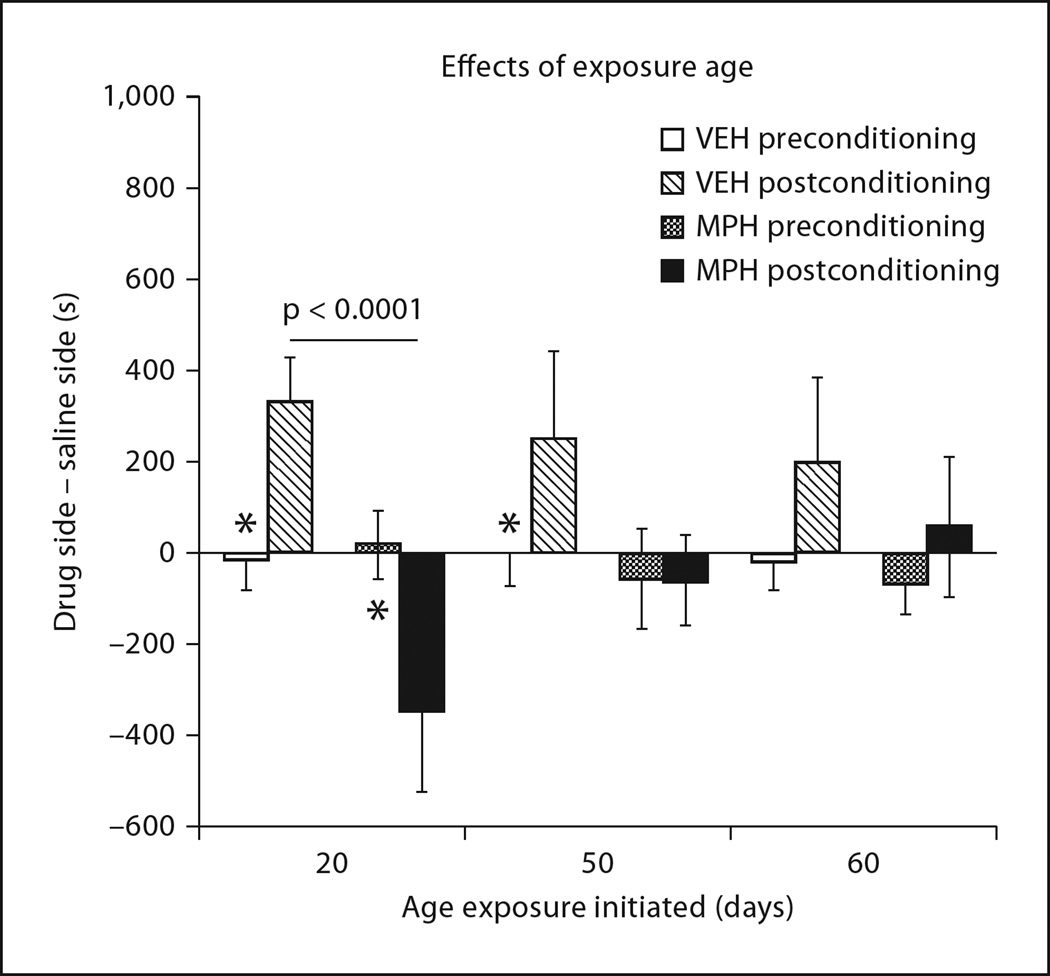
Exposure to MPH significantly interacted with age of exposure to differentially affect conditioning to a 10 mg/kg dose of cocaine (exposure × age × conditioning interaction: F2, 46 = 3.05, p = 0.05). As shown in figure 2, this interaction manifested in decreased conditioned preferences for cocaine-paired environments in VEH-exposed animals across age, contrasted with decreased conditioned aversions in MPH-exposed animals across age. VEH-treated rats produced significant preferences for cocaine-paired environments when tested at P 60 compared to a significant aversion displayed by MPH-treated rats (p < 0.001). However, no preferences or aversions were observable in rats tested at P 90 or 110 after VEH (p = 0.27 and 0.58) or MPH (p = 0.7) injections during late adolescence or young adulthood, respectively. Therefore, both sets of differences diminished with later exposure ages.
Fig. 2.
Age of exposure influenced the effects of MPH on cocaine-induced place conditioning, when assessed 25 days following treatment (F2, 46 = 3.05, p = 0.05). Age also influenced conditioned preferences for cocaine-paired environments in general, as displayed by the VEH-exposed groups. *p<0.001, t tests comparing postconditioning values between VEH- and MPH-exposed groups.
Study 2: The Age of Testing
In this experiment, subjects were tested with place conditioning either 5 or 25 days after juvenile MPH exposure to determine whether the age of testing influences the expression of aversion to cocaine-associated environments described previously [7]. Animals were tested during adolescence (P 40) or late adolescence/young adulthood (P 60).
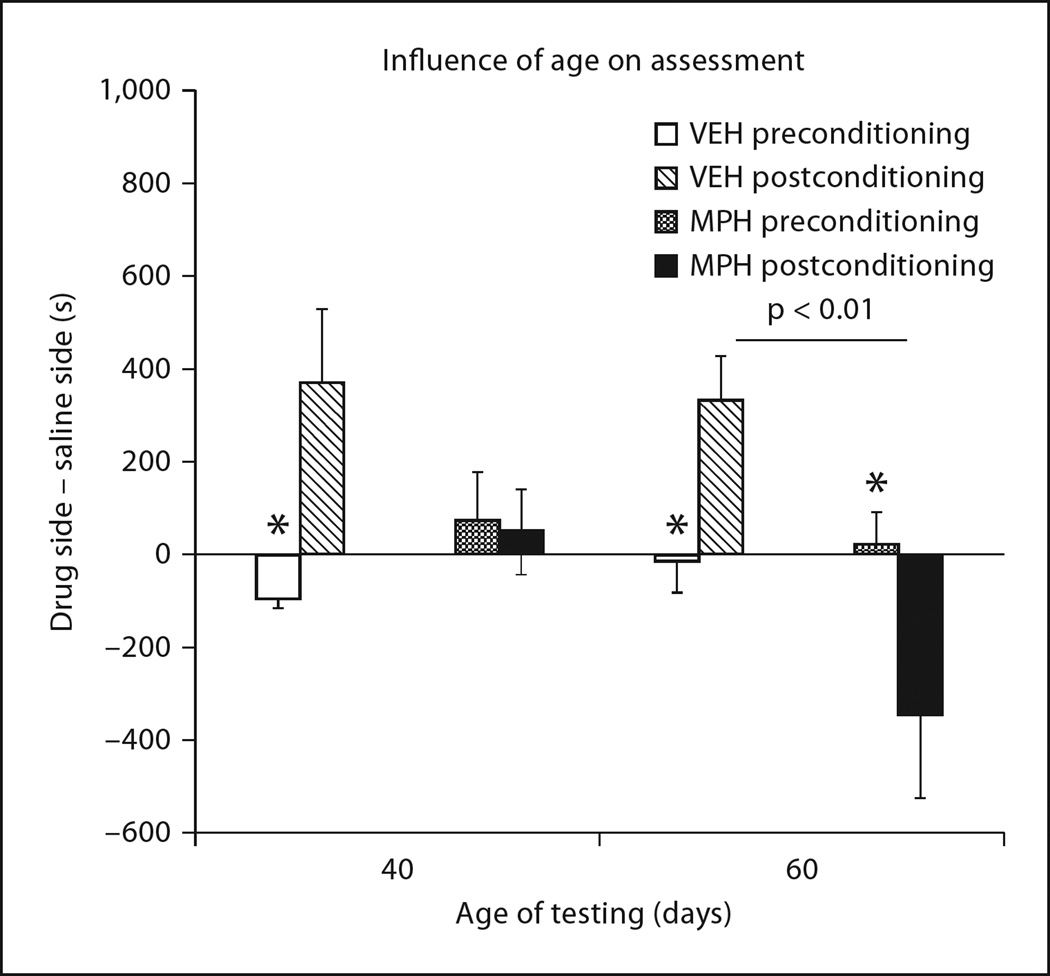
Data illustrated in figure 3 suggests that the postexposure interval is important in determining the enduring effects of MPH exposure. However, the exposure × age × conditioning interaction was not significant (p = 0.2). A significant exposure × conditioning interaction (F1, 33 = 24.96, p < 0.0001) and an overall exposure effect (F1, 33 = 5.42, p < 0.05) were observed, suggesting that MPH exposure did alter place conditioning to cocaine-associated environments. When each exposure group was analyzed for postconditioning effects separately, VEH subjects displayed significant postconditioning preferences for the cocaine-paired environment (p < 0.05) regardless of post-exposure interval. In contrast, MPH subjects displayed a significant age × conditioning interaction (F1, 14 = 5.69, p < 0.05); the preferences displayed by VEH subjects were attenuated by MPH exposure when tested in adolescence, but significant aversions were observed (p < 0.05) when MPH subjects were tested in early adulthood. This suggests that the long-term effects of MPH on conditioned aversions to cocaine-paired cues manifest over time.
Fig. 3.
Age of assessment influenced the apparent effect of juvenile MPH exposure on subsequent place conditioning to cocaine. Compared to VEH-treated subjects, which formed preferences for environments associated with 10 mg/kg cocaine regardless of age (* p < 0.05), juvenile MPH-exposed rats displayed neither preferences nor aversions to cocaine-paired environments when tested 5 days following exposure, at P 40 (adolescence). However, when tested 25 days after exposure at P 60 (young adulthood), rats displayed significant aversions to the cocaine-paired environment (* p < 0.05).
Study 3: The Sex of the Subject
The purpose of this study was to determine whether sex differences would be observed in response to a cocaine challenge following low-dose exposure to MPH (2 mg/kg; P 20–35). Dose responsiveness to cocaine-paired environments (5, 10, and 20 mg/kg) was assessed at P 40 in males and female rats, and a conditioning × cocaine interaction was significant (F2, 79 = 3.70, p < 0.05), independent of the other factors.
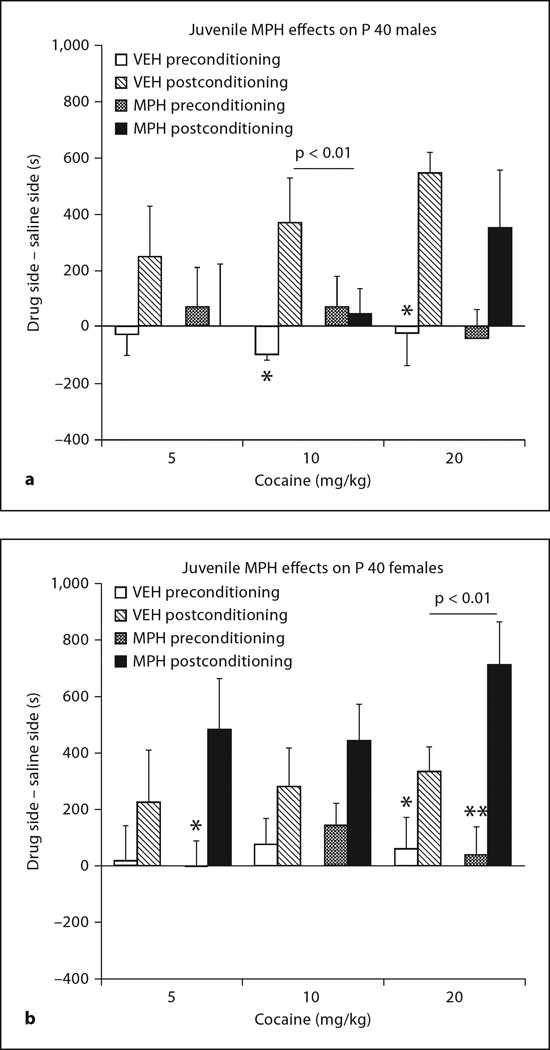
Figure 4 shows that the effects of juvenile MPH exposure on conditioning to cocaine-associated environments depend on the sex of the subject. An exposure × sex × conditioning interaction was observed (F1, 79 = 10.27, p < 0.002). To better understand the sex-dependent effects of MPH exposure, each sex was analyzed independently. For males, the effects of MPH were still emerging, as compared to the effects at P 60 (fig. 3). MPH animals demonstrated reduced preferences for cocaine-associated environments relative to VEH animals, regardless of cocaine dose (exposure × conditioning, F1, 40 = 5.85, p < 0.05). Males exposed to VEH spent significantly more time in environments associated with 10 and 20 mg/kg cocaine after conditioning (p < 0.05; fig. 4a), whereas males exposed to MPH did not demonstrate any preferences (or aversions) to cocaine environments at any dose. While 20 mg/kg cocaine appeared to provoke stronger preferences in MPH-exposed males than lower doses, this difference was not significant, nor was there a significant effect of conditioning at this dose. In contrast, MPH exposure in females enhanced the sensitivity to the conditioning effects of cocaine (exposure × conditioning: F1, 39 = 4.43, p < 0.05; fig. 4b). This effect also did not significantly interact with the dose of cocaine, suggesting an overall increase in sensitivity to the conditioning effects of cocaine across all doses examined.
Fig. 4.
Juvenile exposure to MPH produces sex-dependent effects on conditioning to cocaine-associated environments (F1, 79 = 10.27, p < 0.002). a Prior to place conditioning, P 40 males spent equal amounts of time in the saline- or drug-paired environment (p > 0.5). After place conditioning, VEH rats demonstrated significant place preferences for environments associated with 10 and 20 mg/kg cocaine (* p < 0.05, t tests before and after conditioning). MPH male rats, however, formed neither a preference nor aversion to cocaine-associated environments at the 10 mg/kg dose. b In contrast, females exposed to MPH preferred the environment conditioned to 5 and 20 mg/kg cocaine (* p < 0.05, ** p < 0.01; 10 mg/kg: p = 0.08), whereas VEH-exposed females significantly preferred only the 20 mg/kg cocaine-associated environment relative to preconditioning baselines (t tests).
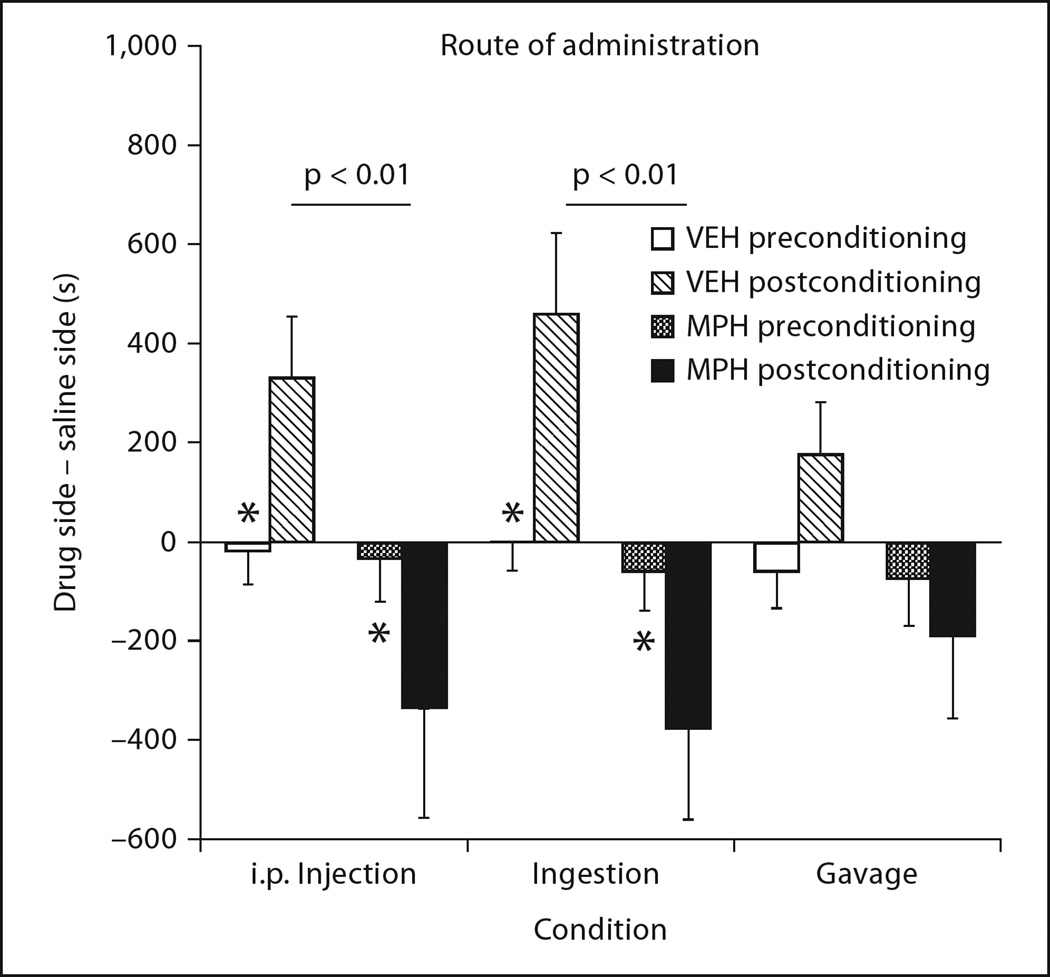
Study 4: Route of Administration
This study compared i.p. versus oral administration (ingestion and gavage) of juvenile MPH exposure on place conditioning to 10 mg/kg cocaine at P 60.
An exposure × conditioning interaction was observed (F1, 50 = 28.43, p < 0.0001), and occurred independently of the route of administration. The conditioned aversions displayed by MPH-treated subjects were consistent with the aversions displayed in study 2. Figure 5, however, shows that the administration of MPH or VEH by i.p, injection, or ingestion via Fruit Loops produced nearly identical results (conditioned preferences in VEH subjects, p < 0.05; conditioned aversions in MPH subjects, p < 0.05). Exposure by oral gavage attenuated conditioning to cocaine-associated environments in both MPH and VEH groups relative to the other routes of administration, although this difference was not significant.
Fig. 5.
The route of MPH administration did not significantly influence the effect of MPH exposure on subsequent place conditioning. Intraperitoneal injection and ingestion both resulted in similar aversions (* p < 0.05) to cocaine-paired environments when tested in young adulthood, compared to preferences displayed in VEH-treated subjects (* p < 0.05). Oral gavage, however, resulted in diminished effects in both VEH- and MPH-treated subjects.
Study 5: Pharmacokinetics of Juvenile MPH Exposure
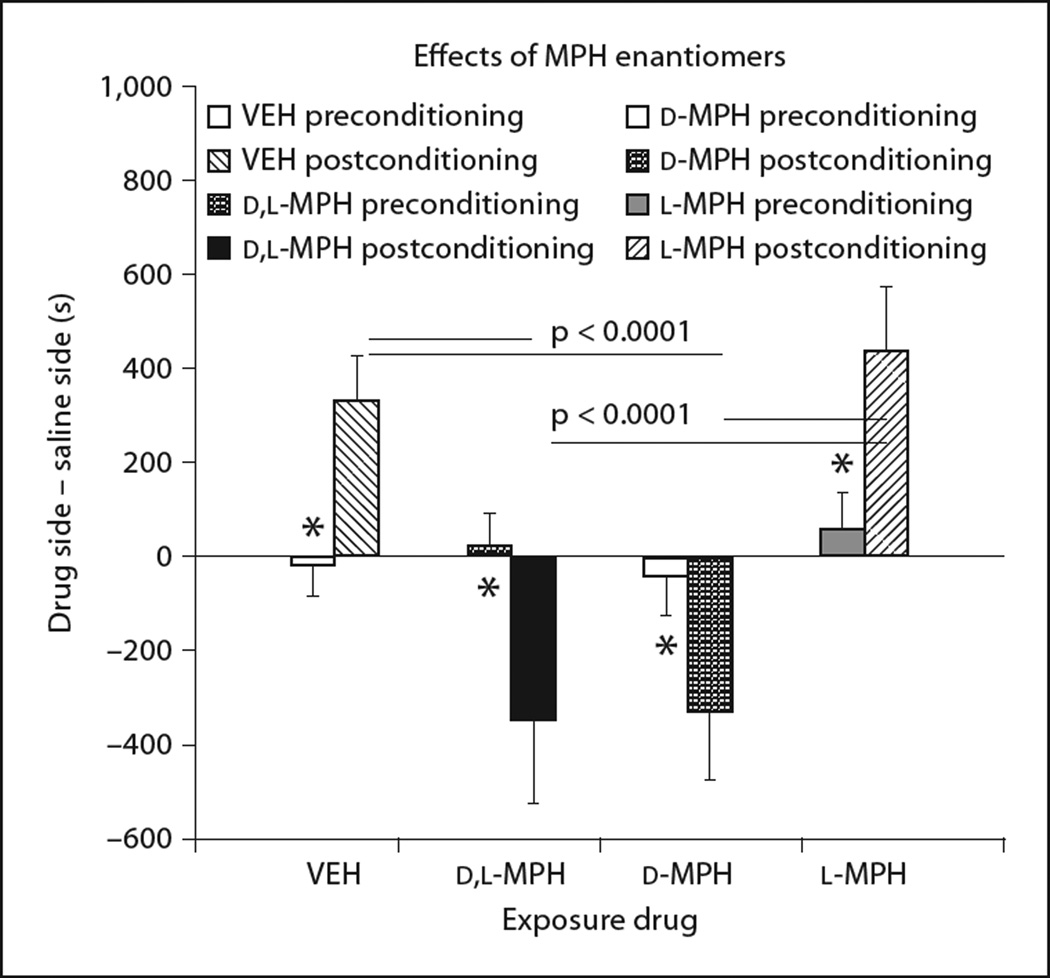
Study 5 was performed to determine which, if any, metabolic differences could mediate the age-dependent effects of MPH. While most, but not all, clinical preparations of MPH are composed of both the d and the l enantiomers [35], it is believed that the d-MPH is the active compound. However, l-MPH might produce subtle antagonistic effects [34].
The enantiomer of MPH used significantly influenced the effect of exposure on subsequent cocaine-induced place conditioning (exposure × conditioning interaction: F3, 39 = 9.99, p < 0.0001). Moreover, a significant main effect for drug type (without VEH) was found (F2, 26 = 6.04, p < 0.008). Exposure to d,l-MPH and d-MPH produced a significant aversion to cocaine-associated environments, whereas l-MPH produced a significant place preference (fig. 6). Postconditioning effects of cocaine were significantly different between l-MPH and both the d-MPH and d,l-MPH forms (all p < 0.01, with Bonferroni correction).
Fig. 6.
The enduring effects of d,l-MPH are mediated by the d enantiomer. A significant drug × conditioning interaction (F2, 26 = 6.04, p < 0.008) indicated that the enantiomers (l-MPH, d,l-MPH, and d-MPH) had differential effects on place conditioning to cocaine-associated environments. Place preferences for cocaine-associated environments were comparable for the VEH-and l-MPH-exposed groups when tested at P 60 (postconditioning comparison: p > 0.8). Conversely, place aversions were similar between the d,l-MPH- and d-MPH-exposed groups (p > 0.8). * p < 0.05, t tests for pre- versus postconditioning to 10 mg/kg cocaine within each exposure group.
Discussion
Systematic analysis of various factors that influence drug-seeking behavior yielded largely consistent effects when compared with other addiction-relevant measures. It is important to examine the effects of these factors in a paradigm that pinpoints drug-cue conditioning, which is governed by overlapping, but separate, neurobiological substrates [39,40]. Age of MPH exposure [also examined by 5, 7, 17, 19, 27, 41 in locomotor tests], age of testing [also examined by 19, 29, 42], and the sex of the subject [also examined by 19, 30, 31] significantly influence the enduring effects of MPH exposure on place conditioning to cocaine. In contrast, the route of drug administration [also examined by 17, 27, 32, 33] exerts no significant difference on the long-term effects of juvenile drug exposure. These findings should serve to reduce well-warranted concerns regarding the applicability of a number of pre-clinical findings to clinical populations [17, 43].
Long-Term Effects of MPH Treatment on Drug-Seeking
Juvenile exposure to MPH alters conditioned responses to cocaine-paired cues in adulthood, when examined in a place conditioning paradigm [7]. While VEH-exposed or unadulterated animals display conditioned preferences to cocaine-paired environments in young adulthood, subjects that are exposed to MPH early in life display conditioned aversions to these same environments. In study 2, we observed that these effects develop over time: 5 days following exposure, the ability to form conditioned preferences had diminished, but aversions were not yet apparent. These data, however, should be superimposed on the natural peak in place preferences for cocaine observed in adolescence [29,44]. Adolescent rats are highly sensitive to cocaine-associated environments [29, 44] due, in part, to maturational changes in the prelimbic prefrontal cortex D1 dopamine receptor expression [29]. While juvenile exposure to MPH increases prefrontal dopamine function [33, 45, 46], normal adolescent changes in D1 receptors may be relatively unaffected by juvenile exposure to MPH. In other words, these 2 mechanisms may compete to modulate place-conditioned behavior at this age. For example, we recently found a reduction in prefrontal D3 dopamine receptors in MPH-exposed animals that is associated with increased blood flow in this region [46]. Moreover, age differences in the behavior itself continue to manifest, and may reduce the ability to detect a difference between treatment groups. This is demonstrated in figure 2, where place preferences from 10 mg/kg cocaine decrease with age [29]. Thus, the age of assessment is important, and the full effects of drug manipulations early in life manifest completely in adulthood, as observed with other stimulants [42].
The amount of time lapsed after MPH exposure is also likely to be important, independent of the ontogeny of drug-seeking. Studies in adult animals clearly show that locomotor sensitization is maximal after a delay following withdrawal [47]. While locomotor sensitization and place conditioning involve overlapping, yet different, sets of neuronal substrates, prior data from locomotor effects suggest that this interval likely reflects long-term changes in gene expression and neuroanatomy associated with drug processing [41, 48]. Thus, reduced aversion after the 5-day withdrawal may be expected as time is needed for these effects to occur.
It may be fair to say that MPH sensitizes conditioned incentive properties in a dose-dependent manner, again noting that comparisons across studies are difficult. At a low dose, juvenile exposure sensitizes the aversive properties (fig. 2). At higher doses, MPH sensitizes the appetitive properties [19]. Behavioral assessment at these high doses also becomes more complex due to the appearance of more stereotypical behavior [20, 49].
MPH Exposure at Different Developmental Stages
Using a 25-day delay to obviate the lack of short-term effects described above, we found that exposure to MPH during late adolescence or during early adulthood had less of an effect than exposure early in life. We replicated previous observations that MPH exposure during late adolescence/early adulthood (P 50–65) blunts subsequent place preferences to 10 mg/kg cocaine, while juvenile exposure (P 20–35) results in conditioned aversions in adulthood [7]. Exposure to MPH later in adulthood (P 70–85) had similar effects as late adolescent exposure, suggesting that the effects of juvenile exposure to MPH are unique to that developmental stage. Moreover, these data suggest that conditioning to cocaine-associated cues diminishes across age in general, further reducing the likelihood of observing a difference between the treated groups. This observation is consistent with recent characterizations of age differences in place preferences for cocaine [29, 44]. These results are also consistent with reduced conditioned preferences exhibited by Swiss-Webster mice exposed to MPH between P 28–39 and tested after a 14-day delay [6]. Other MPH treatment regimens may cause different effects. For example, a more brief exposure to MPH during early adolescence (P 35–42) may increase sensitivity to the rewarding effects of cocaine during adulthood [9]. Still others have reported that pre-pubertal exposure to MPH, followed by repeated adolescent exposure, failed to sensitize locomotor response to cocaine in adult mice [50]. In this case, the protective effects of prepubertal exposure to MPH may have offset the sensitizing effects of adolescent exposure. Adolescent exposure (P 41–57) to MPH (0.5–3.0 mg/kg, given orally) produces only a slight decrease in locomotor sensitization to methamphetamine when tested at P 78 [17]. In contrast, daily MPH exposure between P 40 and 45 (at 10 and 20 mg/kg) produced a significant difference in locomotor behavior in males tested as adults [19]. Similarly, adolescent exposure to MPH given in escalating doses [28] or adult exposure to 20 mg/kg [10] increases sensitization to cocaine. Taken together, it appears that the reduced responsiveness to cocaine-paired cues resulting from low-dose MPH exposure is only apparent with pre-pubertal treatment. Place conditioning further characterizes this response as an aversion to cocaine-paired cues.
MPH Dose and Kinetics
Exposure to low doses of MPH (2 mg/kg) was originally developed to model clinical exposures to this agent in the ADHD child population [7, 17, 51, 52], and not to investigate the effect of its abuse [5, 19, 28]. Interest in both the clinical and abused use of MPH in pediatric populations has exploded in the past years, and results across laboratories need to be reconciled to potentially guide clinical use in the end. A recent review by Dafny and Yang [5] synthesized the effects of multiple doses of MPH and provides additional factor analysis on the locomotor sensitizing effects of this psychostimulant. Here, we specifically examined a number of these same parameters directly with place conditioning, using a single exposure dose of MPH to ease comparison. Multiple studies have been performed that examine different doses of MPH, ranging from 0.05 to 5 mg/kg, with the aim of modeling clinical levels. Differences in plasma levels of MPH have been reported as a function of time sampling, route of administration, and species [5, 17, 33, 37, 51, 53]. The results from studies 4 and 5 suggest that the long-term effects of juvenile MPH exposure occur independently of pharmacokinetic parameters in Sprague-Dawley rats. Rather, exposure to MPH within specific windows of development (i.e. between P 20 and 35) is important for the development of reduced preferences for cocaine-associated environments. The rate of drug uptake and its metabolism may be more important for addiction [17, 54], but may not exert a significant influence upon the long-term effects on development, as oral, i.p., and subcutaneous administration all enter the bloodstream within the first 15 min [53], but reach peak levels at different rates. Together, these results are consistent with previous reports of prenatal [50] and postnatal exposure to the stimulants MPH [e.g. 7, 19, 50, 51] and cocaine [55, 56]. MPH and cocaine have similar affinities for the dopamine transporter, although MPH is twice as potent [54]. We believe that it is unlikely that age differences in blood and brain levels of MPH influenced the enduring effects of MPH at different exposure ages. Brain levels of cocaine following 3 successive doses of 15 mg/kg cocaine in juveniles, adolescents, and young adults did not differ, and plasma levels were comparable between juveniles and adolescents [49].
A number of preclinical studies on MPH differ from standard clinical exposure by route of administration. Humans ingest MPH orally, whereas MPH is often administered via i.p. injection in a number of studies. Differences in the rate of absorption are known to play a role in drug sensitization [27, 32] and raise the question of whether the route of administration is an important factor for the enduring effects of MPH. To best model clinical exposure, MPH should be delivered in a comparable manner, i.e. orally [43]. Oral administration would allow any first-pass kinetics by the stomach [57] to potentially modulate drug levels. For example, higher cocaine levels were observed following subcutaneous administration when compared with oral administration [58]. Here, we observed that oral ingestion and i.p. injection of d,l-MPH had similar effects on later cocaine conditioning, although oral gavage may not be a preferable means of oral administration. While the animals did not appear to be in discomfort, the stress of the exposure may have reduced the conditioned effects of cocaine-associated environments in both VEH- and MPH-exposed subjects [59].
Exposure to d-MPH by i.p. injection produced a comparable aversion to cocaine-associated environments following d,l-MPH exposure. In contrast, l-MPH was without effect, and produced place preferences similar to those in the VEH group. When given by i.p. injection, we did not observe differences that would be consistent with the suggestion that l-MPH, which may work antagonistically, blunts the effects of d,l-MPH racemic mixture [34, 35]. Microdialysis studies show that d-MPH increased dopamine levels 2.2-fold higher in the striatum than l-MPH, and was accompanied by a relative increase in locomotor activity [60]. While our results with l-MPH alone demonstrate no significant behavioral difference when compared with the VEH group, the effects of l-MPH pre-exposure may not be completely without enduring drug action. Clinical pharmacokinetic studies show that l-MPH increases the bioavailability of ethanol [35]. The adverse risk for alcohol-associated disorders following juvenile exposure to the d,l-MPH formulation should be investigated with this enantiomer in mind. Taken together, the results of this study suggest that pre-pubertal exposure to either d,l-MPH or the d-MPH enantiomer is associated with a place aversion to cocaine-associated environments in adulthood. This effect of d-MPH is independent of the route of administration, where the effects of i.p. injection, oral gavage, or ingestion of the mixed enantiomer on the enduring behavioral effects did not statistically differ. We also observed that juvenile exposure to d-MPH by oral gavage also produced an aversion to cocaine-associated environments (data not shown). Together, these data support the suggestion that the d enantiomer is the active component in MPH effectiveness [34, 61].
Our results are consistent with previous findings following oral dosing of d,l-MPH in approximately the same dose range (0.5–3.0 mg/kg) [17], but at different ages. Kuczenski and Segal [17] did not find evidence of enduring neuroadaptation (i.e. sensitization to methamphetamine challenge) following oral exposure in adolescent rats. Thus, the paradigms presented in our rodent model are in agreement with the preclinical observations that no locomotor sensitization occurs following juvenile exposure and clinical observations of reduced problems with drug abuse in a treated population [62–64]. The issue of whether treatment during the active part of the day (that is, during the dark cycle in rats) contributes further to the enduring changes observed in the clinic remains to be addressed in younger ages. Locomotor sensitization following MPH exposure in adults is actually heightened when measured during the light cycle [65], suggesting that baseline differences in activity may play a role.
Influence of Sex on MPH Effects
While not surprising given previous reports, the sex of the subject interacts with MPH exposure and conditioned incentive. Adult females consistently show greater locomotor sensitization to both the acute and chronic effects of cocaine in adulthood [66, 67] and following chronic exposure as juveniles and challenged in adulthood [30]. Locomotor sensitization to cocaine challenge later (P 90) in females following MPH exposure depends on age of exposure (greater following juvenile MPH than later ages) and on the dose (10 mg/kg MPH for 5 days sensitized locomotion, whereas 20 mg/kg did not [19]). Sex differences also exist in the reinforcing and rewarding properties of cocaine. For example, female rats demonstrate a more rapid acquisition to cocaine self-administration [68], and develop conditioned place preferences using lower doses of cocaine and with fewer conditioning days than males [69]. Here, we demonstrated that place preferences for cocaine are significantly different between VEH-exposed adolescent males and females; males were more sensitive to cocaine-associated environments at the 10 mg/kg dose than females. However, juvenile exposure to MPH increased preferences for cocaine environments at the 20 mg/kg dose in females and diminished preferences at the 10 mg/kg dose in males. It is possible that gonadal hormones interact with the developmental alterations set into motion by MPH, since estrogen and progesterone have been proposed to enhance the development of place preferences for cocaine [69]. Alternatively, sexually dimorphic dopamine receptor profiles may account for these differences [70]. Adult females are less sensitive to the blunting effects of D1 antagonists on place conditioning [71]. D1 receptor differences may emerge early, causing increased cortical dopamine to differentially effect maturation of cortical circuits. In addition, estrogen down-regulates D2 activity and reduces GABA activity [72]. Together, females are more primed to sensitize to stimulant exposure than males, although normal (VEH) sex differences in sensitivity are emerging during adolescence.
Conclusions
Clinically relevant MPH exposure paradigms were used here to examine various influential factors on the development of cocaine place-conditioning. The current studies aimed to elucidate factors that influence the effects of MPH on the immature brain. This information can help guide future research on the effects of MPH in individuals that receive psychostimulant treatment for ADHD. It is noted that no animal model for ADHD perfectly models all of the symptoms found in the clinic. However, the results from these cue-induced drug-seeking experiments and others are consistent with reduced substance use in adolescents and adults who were treated prepubertally with psychostimulants [for reviews, see 7, 73].
These results expand on previous reports of treatment effects on other measures of drug-induced neuroplasticity and reinforcement. Juvenile chronic exposure to a low dose of MPH has long-term effects on cocaine place conditioning, which are only fully apparent after a 25-day delay and are opposite in males and females. Exposure to MPH at later developmental stages did not have as robust long-term effects, although place conditioning was blunted in these experimental groups. These effects of MPH exposure were observed along with a natural diminution of the conditioning effects of cocaine with age, as seen in the VEH-treated subjects. Together, with a lack of influence of the route of administration, we can confirm that the d-MPH enantiomer is the active component of the d,l-MPH formulation when measuring these long-term effects of exposure. These data can further advance our understanding of the consequences of MPH treatment throughout development on the vulnerability to drug abuse and addiction later in life.
Acknowledgments
This work was funded by DA-015403 with additional support from the Simches family.
References
- 1.Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 2.Johnston LD, et al. Monitoring the future. National results on adolescent drug use. Overview of key findings. 2007 [Google Scholar]
- 3.Rapoport JL, Buchsbaum MS, Zahn TP, Weingartner H, Ludlow C, Mikkelsen EJ. Dextroamphetamine: cognitive and behavioral effects in normal prepubertal boys. Science. 1978;199:560–563. doi: 10.1126/science.341313. [DOI] [PubMed] [Google Scholar]
- 4.Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse?. A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- 5.Dafny N, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull. 2006;68:393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Achat-Mendes C, Anderson KL, Itzhak Y. Methylphenidate and MDMA adolescent exposure in mice: long-lasting consequences on cocaine-induced reward and psychomotor stimulation in adulthood. Neuropharmacology. 2003;45:106–115. doi: 10.1016/s0028-3908(03)00135-7. [DOI] [PubMed] [Google Scholar]
- 7.Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- 8. Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 10.Schenk S, Izenwasser S. Pretreatment with methylphenidate sensitizes rats to the reinforcing effects of cocaine. Pharmacol Biochem Behav. 2002;72:651–657. doi: 10.1016/s0091-3057(02)00735-9. [DOI] [PubMed] [Google Scholar]
- 11.McFadyen MP, Brown RE, Carrey N. Sub-chronic methylphenidate administration has no effect on locomotion, emotional behavior, or water maze learning in prepubertal mice. Dev Psychobiol. 2002;41:123–132. doi: 10.1002/dev.10059. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND. What do we know about drug addiction? Am J Psychiatry. 2005;162:1401–1402. doi: 10.1176/appi.ajp.162.8.1401. [DOI] [PubMed] [Google Scholar]
- 13.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 14.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 15.Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: uPate of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford CA, McDougall SA, Meier TL, Collins RL, Watson JB. Repeated methylphenidate treatment induces behavioral sensitization and decreases protein kinase A and dopamine-stimulated adenylyl cyclase activity in the dorsal striatum. Psychopharmacology (Berl) 1998;136:34–43. doi: 10.1007/s002130050536. [DOI] [PubMed] [Google Scholar]
- 19.Torres-Reveron A, Dow-Edwards DL. Repeated administration of methylphenidate in young, adolescent, and mature rats affects the response to cocaine later in adulthood. Psychopharmacology (Berl) 2005;181:38–47. doi: 10.1007/s00213-005-2221-7. [DOI] [PubMed] [Google Scholar]
- 20.McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp Clin Psychopharmacol. 1999;7:208–218. doi: 10.1037//1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- 21.McDougall SA, Duke MA, Bolanos CA, Crawford CA. Ontogeny of behavioral sensitization in the rat: effects of direct and indirect dopamine agonists. Psychopharmacology (Berl) 1994;116:483–490. doi: 10.1007/BF02247482. [DOI] [PubMed] [Google Scholar]
- 22.Brenhouse HC, Stellar JR. c-Fos and delta-FosB expression are differentially altered in distinct subregions of the nucleus accumbens shell in cocaine-sensitized rats. Neuroscience. 2006;137:773–780. doi: 10.1016/j.neuroscience.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 23.Carr GD, Phillips AG, Fibiger HC. Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology. 1988;94:221–226. doi: 10.1007/BF00176849. [DOI] [PubMed] [Google Scholar]
- 24.Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 25.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 26.Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods Mol Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- 27.Izenwasser S, Coy AE, Ladenheim B, Loeloff RJ, Cadet JL, French D. Chronic methylphenidate alters locomotor activity and dopamine transporters differently from cocaine. Eur J Pharmacol. 1999;373:187–193. doi: 10.1016/s0014-2999(99)00274-5. [DOI] [PubMed] [Google Scholar]
- 28.Augustyniak PN, Kourrich S, Rezazadeh SM, Stewart J, Arvanitogiannis A. Differential behavioral and neurochemical effects of cocaine after early exposure to methylphenidate in an animal model of attention deficit hyperactivity disorder. Behav Brain Res. 2006;167:379–382. doi: 10.1016/j.bbr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Brenhouse H, Sonntag KC, Andersen SL. Transient D1 dopamine receptor over-expression on prefrontal cortex projection neurons: a mechanism for enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melnick SM, Dow-Edwards DL. Differential behavioral responses to chronic amphetamine in adult male and female rats exposed to postnatal cocaine treatment. Pharmacol Biochem Behav. 2001;69:219–224. doi: 10.1016/s0091-3057(01)00545-7. [DOI] [PubMed] [Google Scholar]
- 31.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Davids E, Zhang K, Tarazi FI, Baldessarini RJ. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacology (Berl) 2002;160:92–98. doi: 10.1007/s00213-001-0962-5. [DOI] [PubMed] [Google Scholar]
- 35.Patrick KS, González MA, Straughn AB, Markowitz JS. New methylphenidate formulations for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Drug Deliv. 2005;2:121–143. doi: 10.1517/17425247.2.1.121. [DOI] [PubMed] [Google Scholar]
- 36.Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 37.Wargin W, Patrick K, Kilts C, Gualtieri CT, Ellington K, Mueller RA, Kraemer G, Breese GR. Pharmacokinetics of methylphenidate in man, rat and monkey. J Pharmacol Exp Ther. 1983;226:382–386. [PubMed] [Google Scholar]
- 38.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 39.Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- 40.McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacolog y. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- 41.Kosofsky BE, Genova LM, Hyman SE. Postnatal age defines specificity of immediate early gene induction by cocaine in developing rat brain. J Comp Neurol. 1995;351:27–40. doi: 10.1002/cne.903510104. [DOI] [PubMed] [Google Scholar]
- 42.Kosofsky BE, Hyman SE. No time for complacency: the fetal brain on drugs. J Comp Neurol. 2001;435:259–262. doi: 10.1002/cne.1027. [DOI] [PubMed] [Google Scholar]
- 43.Hyman SE. Methylphenidate-induced plasticity: what should we be looking for? Biol Psychiatry. 2003;54:1310–1311. doi: 10.1016/j.biopsych.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 45.Jezierski G, Zehle S, Bock J, Braun K, Gruss M. Early stress and chronic methylphenidate cross-sensitize dopaminergic responses in the adolescent medial prefrontal cortex and nucleus accumbens. J Neurochem. 2007;103:2234–2244. doi: 10.1111/j.1471-4159.2007.04927.x. [DOI] [PubMed] [Google Scholar]
- 46.Andersen SL, Napierata L, Brenhouse HC, Sonntag KC. Juvenile methylphenidate modulates reward-related behaviors and cerebral blood flow by decreasing cortical D3 receptors. Eur J Neurosci. 2008;27:2962–2972. doi: 10.1111/j.1460-9568.2008.06254.x. [DOI] [PubMed] [Google Scholar]
- 47.Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- 50.Guerriero RM, Hayes MM, Dhaliwal SK, Ren JQ, Kosofsky BE. Preadolescent methyl-phenidate versus cocaine treatment differ in the expression of cocaine-induced locomotor sensitization during adolescence and adulthood. Biol Psychiatry. 2006;60:1171–1180. doi: 10.1016/j.biopsych.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 51.Chase T, Carrey N, Soo E, Wilkinson M. Methylphenidate regulates activity regulated cytoskeletal associated but not brain-derived neurotrophic factor gene expression in the developing rat striatum. Neuroscience. 2007;144:969–984. doi: 10.1016/j.neuroscience.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 52.Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- 53.Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intra-peritoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295:51–57. [PubMed] [Google Scholar]
- 54.Volkow ND, Wang GJ, Fowler JS, Fischman M, Foltin R, Abumrad NN, Gatley SJ, Logan J, Wong C, Gifford A, Ding YS, Hitzemann R, Pappas N. Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 1999;65:PL7–PL12. doi: 10.1016/s0024-3205(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 55.Dow-Edwards DL, Busidan Y. Behavioral responses to dopamine agonists in adult rats exposed to cocaine during the preweaning period. Pharmacol Biochem Behav. 2001;70:23–30. doi: 10.1016/s0091-3057(01)00582-2. [DOI] [PubMed] [Google Scholar]
- 56.Mague SD, Andersen SL, Carlezon WA., Jr Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry. 2005;57:120–125. doi: 10.1016/j.biopsych.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 57.Teicher MH, Polcari A, Foley M, Valente E, McGreenery CE, Chang WW, McKay G, Midha KK. Methylphenidate blood levels and therapeutic response in children with attention-deficit hyperactivity disorder: I. Effects of different dosing regimens. J Child Adolesc Psychopharmacol. 2006;16:416–431. doi: 10.1089/cap.2006.16.416. [DOI] [PubMed] [Google Scholar]
- 58.Dow-Edwards D, Fico TA, Osman M, Gamagaris Z, Hutchings DE. Comparison of oral and subcutaneous routes of cocaine administration on behavior, plasma drug concentration and toxicity in female rats. Pharmacol Biochem Behav. 1989;33:167–173. doi: 10.1016/0091-3057(89)90446-2. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Robinson TE, Bhatnagar S. Effects of maternal separation on behavioural sensitization produced by repeated cocaine administration in adulthood. Brain Res. 2003;960:42–47. doi: 10.1016/s0006-8993(02)03752-6. [DOI] [PubMed] [Google Scholar]
- 60.Aoyama T, Kotaki H, Sawada Y, Iga T. Pharmacokinetics and pharmacodynamics of methylphenidate enantiomers in rats. Psychopharmacology (Berl) 1996;127:117–122. doi: 10.1007/BF02805984. [DOI] [PubMed] [Google Scholar]
- 61.Srinivas NR, Hubbard JW, Quinn D, Midha KK. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52:561–568. doi: 10.1038/clpt.1992.185. [DOI] [PubMed] [Google Scholar]
- 62.Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104:e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- 63.Hechtman L, Weiss G, Perlman T. Hyperactives as young adults: past and current substance abuse and antisocial behavior. Am J Orthopsychiatry. 1984;54:415–425. doi: 10.1111/j.1939-0025.1984.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 64.Loney J, Kramer J, Salisbury H. Medicated versus unmedicated ADHD children-adult involvement with legal and illegal drugs. In: Jensen PS, Cooper JR, editors. Attention Deficit Hyperactivity Disorder - State of the Science, Best Practices. Kingston: Civic Research Institute; 2002. pp. 1–16. [Google Scholar]
- 65.Gaytan O, Yang P, Swann A, Dafny N. Diurnal differences in sensitization to methylphenidate. Brain Res. 2000;864:24–39. doi: 10.1016/s0006-8993(00)02117-x. [DOI] [PubMed] [Google Scholar]
- 66.Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Click SD, Hinds PA, Shapiro RM. Cocaine-induced rotation: sex-dependent differences between left- and right-sided rats. Science. 1983;221:775–777. doi: 10.1126/science.6879177. [DOI] [PubMed] [Google Scholar]
- 68.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 69.Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quiñones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 70.Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- 71.Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V. The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull. 2004;63:295–299. doi: 10.1016/j.brainresbull.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- 73.Andersen SL. Stimulants and the developing brain. TINS. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]