Abstract
Adolescence as highlighted in this special issue is a period of tremendous growth, synaptic exuberance, and plasticity, but also a period for the emergence of mental illness and addiction. This commentary aims to stimulate research on prevention science to reduce the impact of early life events that often manifest during adolescence. By promoting a better understanding of what creates a normal and abnormal trajectory, the reviews by van Duijvenvoorde et al., Kilford et al., Lichenstein et al., and Tottenham and Galvan in this special issue comprehensively describe how the adolescent brain develops under typical conditions and how this process can go awry in humans. Preclinical reviews also within this issue describe how adolescents have prolonged extinction periods to maximize learning about their environment (Baker et al.), whereas Schulz and Sisk focus on the importance of puberty and how it interacts with stress (Romeo). Caballero and Tseng then set the stage of describing the neural circuitry that is often central to these changes and psychopathology. Factors that affect the mis-wiring of the brain for illness, including prenatal exposure to anti-mitotic agents (Gomes et al.) and early life stress and inflammation (Schwarz and Brenhouse), are included as examples of how exposure to early adversity manifests. These reviews are synthesized and show how information from the maturational stages that precede or occur during adolescence is likely to hold the key towards optimizing development to produce an adolescent and adult that is resilient and well adapted to their environment.
Keywords: Adolescence, Adversity, Amygdala, Anxiety, Inflammation, Prefrontal cortex, Puberty, Salience, Stress
1. Introduction
Adolescence is an important transitional period of social development, risk-taking, and preparation for the independence of adulthood (reviewed by van Duijvenvoorde et al., 2016). Adolescence is also a period when mental illness and addiction appear (Paus et al., 2008; Kuhn, 2015). Often, these challenges emerge from a latent past of early traumatic events, drug exposure, or both (De Bellis, 2002; Andersen and Teicher, 2009). The reviews in this special issue comprehensively describe how the adolescent brain develops under typical conditions (Kilford et al., 2016; Caballero and Tseng, 2016), but also how early antecedent events may already have mis-wired the brain for illness long before it arrives at this stage. Factors that influence the trajectory of brain development are discussed in this issue, including stress (Romeo, 2016), sex hormones (Schulz and Sisk, 2016), and inflammation (Schwarz and Brenhouse, 2016). This commentary discusses the importance of understanding typical developmental processes to encourage research on prevention to reduce the impact of early life events on adolescence (Stanis and Andersen, 2014).
2. The shape of the trajectory matters
The process of maturation leading to adulthood requires that a number of processes, including cognitive, reward, social, and hormonal, come together at the right time. Events that occur during critical periods lay a foundation and later influences of the environment during a sensitive period shape the final trajectory. Derailing this process at either the critical or the sensitive period stage will have a significant impact to a yield a different trajectory of development (Paus et al., 2008; Andersen and Teicher, 2008). As a result, the emergence of depression, addiction, and other serious mental illnesses (e.g., schizophrenia and bipolar illness) often occurs during adolescence.
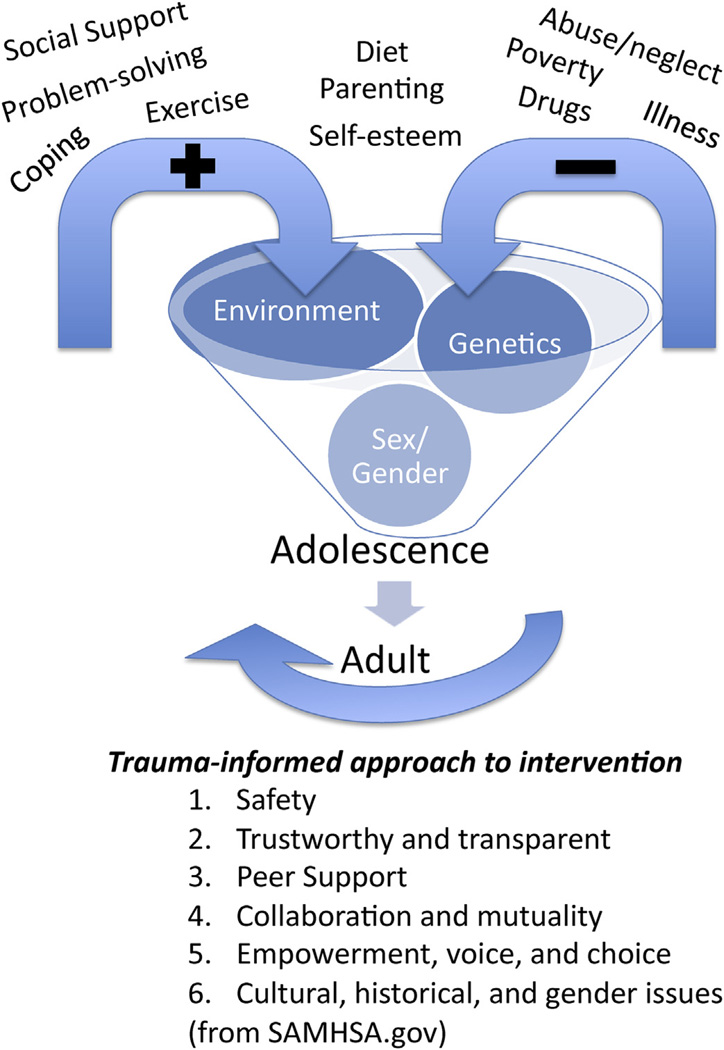
The interactions of many different factors culminate during the periadolescent period to shape the brain (Spear, 2000; Andersen, 2003). The timing of this peak in development depends on the measure (e.g., receptors, synapses, functional activity), the region of the brain, and the sex of the subject (reviewed by Brenhouse and Andersen, 2011a). Since its initial introduction in human post-mortem studies (Huttenlocher, 1979), the phenomenon of overproduction and later elimination is now a widely accepted process of brain development. However, less attention has been paid to the progressive increase in the underlying factors that support the transitions between prenatal life, childhood, and adolescence. By examining the trajectory of development, we know that some disorders can be characterized by differences in the rate to reach a final synaptic density. For example, the prefrontal cortex (PFC) shows delayed development (e.g., pruning) in attention deficit hyperactivity disorder relative to normal, age-matched controls (Shaw et al., 2007). Exposure to early adversity (poor parenting, neglect, abuse, poverty, drugs) also alters development, but in this case the trajectory reflects accelerated development (Andersen and Teicher, 2008) to serve an adaptive function to survive in a malevolent world (Teicher, 2002). Regardless of the impetus for the change (genetic, environmental, or their interaction; Fig. 1), clues lie within these altered trajectories that can inform improved outcomes of at-risk or affected teenagers.
Fig. 1.
Protective and risk factors interact with foundational developmental processes to produce an adolescent leading into an adult. Trauma-informed interventions may bridge the gap between negative and positive influences.
3. Early developmental processes leading up to adolescence
The progressive rise of neural infrastructure (neurons, glia) shapes and guides development, “locks it in” during critical periods (Hensch, 2005), and provides a foundation for a lifetime (Gogolla et al., 2009). Critical periods occur in concert with active brain growth (requiring growth factors) where the balance between excitatory and inhibitory activity is important (Huang et al., 1999; Hanover et al., 1999). Most of what we know is based on the visual system (Webster et al., 2002; Lein et al., 2000) where critical periods are closed by excess glutamatergic activity or decreased GABA activity (Fagiolini and Hensch, 2000; Fagiolini et al., 2004). If a significant event occurs during this time – positive or negative – the individual will show a permanent predisposition towards a set of behaviors. Critical periods are also evident in other functions where the optimal period occurs prior to adolescence – before age 12 for more complex behaviors. Experts suggest that learning music (Trainor, 2005), a foreign language with the correct accent (Flege et al., 1995) or sports (Tonnessen et al., 2015) is optimal before this age. Anxiety, which has early seeded roots in early childhood (reviewed by Tottenham and Galvan, 2016), influences the development of depression. Factors that shape vulnerability to schizophrenia or addiction likely preceded their adolescent emergence by a more than a decade. Such factors include exposure to anti-mitotic agents prenatally (Gomes et al., 2016), stress (Romeo, 2016; Schwarz and Brenhouse, 2016; Tottenham and Galvan, 2016), inflammation, gonadal hormones (Romeo, 2016; Schulz and Sisk, 2016), which all contribute to mental illness and are discussed below and in this edition.
As critical periods are integral for the foundation of building a brain, sensitive periods occur when the brain is uniquely sensitive to environmental impacts. Such increased salience to environmental events is especially heightened during adolescence (Brenhouseet al., 2008; Sonntag et al., 2014; Lichenstein et al., 2016 [this edition]). Adolescents are more sensitive to peer relationships (Kilford et al., 2016 [this edition]), rewarding stimuli such as drugs of abuse and alcohol, seeking novelty in general, or sexual activity (Steinberg, 2008). Reduced levels of some of these behaviors may lead to a depressive phenotype, and too much of these behaviors can be lead to addiction, early pregnancy, or even premature death. An appropriate level of salience attributed to the environment will lead to independence, reproductive fitness, and other behaviors necessary for individuating during this stage (Spear, 2000; Steinberg, 2008; Luna et al., 2015). The anterior cingulate cortex and its inter-relationships with other brain regions play an important role in a number of these functions, and is described as the hubby Lichenstein et al. (2016). To demonstrate how salient memories are made during adolescence, think about what you remember from high school relative to childhood or later (more recent) stages in your life. Good or bad, high school was quite salient.
Mechanistically, we have determined that the over-expression of the D1 dopamine receptor in the prelimbic PFC projections plays a significant role in increased motivational salience during adolescence (Brenhouse et al., 2008). Briefly, animals with high-D1 expression had elevated novelty preferences, increased preferences for cocaine-, nicotine-, and alcohol-associated environments, increased self-administration of cocaine and the motivation to do so, and impulsivity (Sonntag et al., 2014). High-D1 receptors in the prelimbic PFC also increased sexual activity or lead to its reduction when D1 receptors were normalized (Freund et al., 2016). However, when D1 receptors were no longer elevated (and salience reduced), the animals demonstrated depressive behavior (Freund et al., 2016). These data show how motivational salience plays a vital role in heightening adolescent behaviors during this sensitive period.
Once an adolescent forms an association between the behavior and the environment/cue, a process that requires salience, the association is more difficult to change compared with other maturational stages. In this edition, Baker et al. (2016) reports on how extinction learning of fear-related stimuli is uniquely impaired during adolescence; prolonged extinction in adolescence relative to other stages is also observed to cocaine-associated environments (Brenhouse and Andersen, 2008), but see Li and Frantz (2009). This process is also related to motivational salience mediated by D1 receptors (Brenhouse et al., 2015). Taken together, these studies imply a period of enhanced learning about environmental factors that are made more salient during adolescence and are further accompanied by reduced extinction. As a result, associations that occur during adolescence or beforehand will become an important part of the individual’s foundation for the rest of his/her life.
4. Sex differences and puberty onset
Schulz and Sisk (this edition, Schulz and Sisk, 2016) remind us that gonadal hormones strongly influence adolescent brain development. Dogma taught us that gonadal hormones influence the brain at two discrete time periods with two unique functions: perinatal hormones organize sex differences and pubertal hormones activate sex differences (Phoenix et al., 1959). However, the current thinking is that the inter-relationship of brain development and sex steroids is more continuous and represents a protracted sensitive period of development. From this perspective, paying attention to sex differences as they continue to emerge across childhood into adolescence is key for proper development. Puberty is a time of reproductive maturation (Schulz and Sisk, 2016), and mammals prepare by increasing risk-taking and social behaviors. The gonadal hormones play a role in shaping the adolescent brain. Imaging studies demonstrate sex differences in cortical gray matter (Giedd et al., 1997), and more detailed cell counts in the PFC of rats indicate estrogen increases neuronal loss during puberty that is prevented by ovariectomy (Juraska and Markham, 2004; Markhamet al., 2007). Alterations in gonadal hormone expression leading up to adolescence as a result of early life stress (ELS; e.g., such as delayed puberty; Wilson et al., 2013) may have profound effects on brain structure. The review by Romeo (2016) discusses important sex differences to external stressors as a function of age and how they influence later adult function.
5. Early life stress and delayed clinical outcomes
Early life stress in the form of childhood abuse affects approximately one-third of the population, making it not only one of the most prevalent issues facing society, but also one of the most preventable. Four possible outcomes to ELS have been described: resilient, recovering, delayed, or chronically internalizing (e.g., anxiety or depression) or externalizing behaviors (e.g., impulsivity, aggression, self harm) (Bonanno and Mancini, 2008; D’Andrea et al., 2012). Together, these four potential outcomes imply different trajectories. The periadolescent period (11–12 years of age) is when abused and controls start to diverge in their developmental trajectories (Andersen and Teicher, 2008; Widom et al., 2007). A number of authors in this edition discuss how ELS produces altered trajectories that manifest during adolescence.
Studies on the effects of ELS on behavior and brain development suggest that a delay occurs in their manifestation, which is typically adolescence. For example, few depressive symptoms are observed in childhood following ELS, but are delayed an average of 9 years after the initiation of abuse (Widom et al., 2007; Teicher et al., 2009). The average of addiction, an externalizing disorder, can be as young as 12–13 years of age in children with low protective factors (including exposure to ELS and increases prevalence in non-vulnerable populations (Andersen and Teicher, 2009; Harrington et al., 2011)). A number of antecedent factors influence maldevelopment (Fig. 1) that lead up to an adolescent manifestation. Such a delay provides a window of opportunity for a targeted, preventative intervention for children exposed to ELS. However, we need more research on which factors can predict the trajectory of ELS. Anxiety maybe one such factor to predict the internalizing disorder of depression. Approximately two-thirds of children with generalized anxiety disorder will develop depression in early adolescence (Beesdo et al., 2007). This same has been observed in victims of abuse (Andersen and Teicher, 2008).
Typical adolescents demonstrate progressively increasing social awareness that is observable in both behavior and with brain imaging studies (reviewed by Kilford et al., 2016 [this edition]). For example, typical adolescents demonstrate a hyper-responsivity to emotional faces (Monk et al., 2003; Killgore et al., 2001). Individuals with a history of ELS, however, show elevated amygdala activity to faces at <11 years old (Malter Cohen et al., 2013; Marusak et al., 2015), suggesting early maturation of this trajectory. Tottenham and Galvan (this edition; Tottenham and Galvan, 2016) comprehensively describe the inter-relationships between the amygdala and the prefrontal cortex in normal and abused subjects.
Altered trajectories have also been described in brain structure. Exposure to stress shows a loss in hippocampal volume that does not manifest until adolescence (De Bellis et al., 2001). With a more fine-tuned analysis of the timeline, synaptic density fails to peak at postnatal day 60 (P60) in a rat model of ELS (Andersen and Teicher, 2004). Changes in brain structure following ELS have been associated with different sensitive periods in the brain, where ages of active growth for a region are most vulnerable if the abuse occurs during that time (Andersen et al., 2008; Pechtel et al., 2014). Differences in myelination in general and more specifically, microglia (Schwarz and Brenhouse, 2016; Calcia et al., 2016), have also been described following ELS (Teicher et al., 2004). The relationship between altered myelination or functional connectivity are found in major depressive disorder or addiction; a meta-analysis of this inter-relationship is described in this edition by (Lichenstein et al., 2016).
6. Animal models and stress responsiveness
Preclinically, animal studies can model early adversity, including early shock treatments, maternal separation, and social isolation and defeat paradigms (reviewed by Andersen (2015)). Resultant behaviors following exposure to these paradigms are relevant to clinical findings described above (Andersen, 2015). For example, exposure to maternal separation increases depressive-like behaviors during adolescence that includes a reduction in synaptic density and myelin basic protein (Leussis and Andersen, 2008; Leussis et al., 2012). Animal modeling of a complex issue like child abuse in the human condition provides a simpler system to dissect out contributions of different systems that may contribute to psychopathology. The review by Romeo (this edition; Romeo (2016)) tells us that stress responses (measured by adrenocorticotropin hormone [ACTH] and corticosterone) in pre-pubertal rats (28 days of age) are protracted, but then becomes increasingly regulated by emerging adulthood (77 days of age). Here, the typical trajectory is one of increased stress regulation with maturation between peri-adolescence to adulthood. Sex differences also are important for stress effects. Females tend to show elevated stress responses following prior stress exposure and males show reduced stress responses, which are discussed in detail. Romeo reviews the stress responsiveness data from various animal models of stress exposure in this edition. These data highlight the importance of a derailed trajectory that can be used to guide an intervention by determining when regulatory processes diverge between conditions.
7. Sex differences in immune response to ELS
Immune responses are elevated in individuals with a history of ELS in humans (Carpenter et al., 2010) and animals (Brenhouse and Andersen, 2011b; Hennessy et al., 2015). The review provided by Schwarz and Brenhouse (2016) emphasizes how important sex differences can be for the expression of early life events. Adolescent activity in neuro-immune signaling pathways provides a novel mechanism possibly underlying sex differences associated with ELS. The majority of research focuses on the inflammatory markers of interleukin-6 (IL-6) and IL-1 beta, which are both increased following ELS exposure (Brenhouse and Andersen, 2011b). Following ELS, males express more interleukin-10 (IL-10) that protects against neuroinflammation than females (Schwarz and Brenhouse, 2016). Inflammatory responses have been associated with depression in adult human studies (Lindqvist et al., 2009) and these data suggest a potential protective factor against depression that is present in males. Here, we should not only focus on potential resilience factors, but utilize them strategically. By examining how such factors diverge across sex, sex differences can provide a novel perspective for prevention efforts. Preventative interventions are possible. For example, Wieck et al. (2013) have reported that central administration of IL-10 can prevent GABAergic losses that are found in animals exposed to ELS. Similar anti-inflammatory intervention studies where humans (Licinio and Wong, 1999) and animals (Brenhouse and Andersen, 2011b; Hennessy et al., 2015) are treated with an anti-inflammatory have reduced depression or improved working memory.
8. Preventative interventions
As the study of adolescence moves forward, there needs to be a greater emphasis on prevention. Adolescence is the pinnacle of brain development and may be the last point for any permanent change before a sensitive period closes. For example, teens do not leave their house before the brain has reached such a peak, giving parents a last chance to provide both structure and guidance to their offspring. As information about typical and atypical behavior and brain development is presented in this edition, consider how knowing the number of well-validated, evidenced based factors that are used in prevention research can be applied to developing better interventions to prevent later dysfunction. The factors that improve interventions are: 1) age-related patterns of competence and disorder; 2) developmental-appropriate tasks; 3) multiple contexts to increase generalization; and 4) interactions among biological, psychological, and social factors. For example, cognitive behavior therapy is helpful for insight regarding trauma and how to move forward. These same skills can be taught to children and adolescents at any stage to improve everyday problem solving. The process of mentalising, perspective-taking, and social decision-making all use different parts of the brain that are increasing activated during adolescence (described by Kilford et al., 2016 [this edition]). Resultant endpoints include empathy, trust, perspective, reading social cues, and other social cognitive behaviors that lead to socially appropriate level of behavior. Trauma-informed therapies (Fig. 1) can bridge the divide between negative environmental factors that influence development and the positive ones that optimize it. Individuals with a history of ELS have difficulty in these domains and for good reason. The increasing social awareness (salience) that emerges during adolescence may be part of the trigger for the appearance of psychopathology during this period. However, such increased awareness may also be used therapeutically to alter brain connectivity by guided interventions. Exercise is another potential preventative intervention. Both animal and human studies show reduced rewarding effects of drugs and improved mood (Zlebnik et al., 2012; Norris et al., 1992; Nabkasorn et al., 2006) following exercise. Alternatively, reducing other risk factors, such as inflammation, can reduce the effects of ELS (Schwarz and Brenhouse, 2016; Brenhouse and Andersen, 2011b). Inflammation can be controlled by improved sleep, diet, exercise, or medication (Cotman and Berchtold, 2002; Kiecolt-Glaser et al., 2015; Schuch et al., 2016). The first two factors may also contribute to additional vulnerabilities of even typical adolescents.
9. Future considerations
If the goal of a researcher is to understand a phenomenon, and the goal of the clinician is to use any and all information to improve the well-being of their patient, then our fields need to move closer together to streamline this process. First, more cross talk is needed between these different groups to better understand the problem. Second, we need to find methodological points that facilitate cross talk among different species. Neuroimaging holds such promise for this goal, although task-based assessments in small mammals have not been achieved to the same level as humans. Saliva sampling represents another point for cross talk. Saliva sampling has been used at different ages (infants to the elderly) to measure cortisol/corticosterone or estrogen/testosterone have been assayed. Genetic and epigenetic measures are equally possible from saliva samples. Blood/plasma sampling represents another point of communication. Third, behavior provides another bridge and the Research Domain Criteria (RDocS) of the National Institute of Mental Health can guide cross talk. Brain/behavior changes that characterize adolescence are universal, and it is possible to alter the trajectory.
References
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci. Biobehav. Rev. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J. Neuropsychiatry Clin. Neurosci. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Exposure to early adversity: points of cross-species translation that can lead to improved understanding of depression. Dev. Psychopathol. 2015;27(2):477–491. doi: 10.1017/S0954579415000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Bisby M, Richardson R. Imparied fear extinction in adolescent rodents: behavioral and neural analyses. Neurosci. Biobehav. Rev. 2016;70:59–73. doi: 10.1016/j.neubiorev.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Hofler M, Lieb R. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch. Gen. Psychiatry. 2007;64(8):903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Mancini AD. The human capacity to thrive in the face of potential trauma. Pediatrics. 2008;121(2):369–375. doi: 10.1542/peds.2007-1648. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav. Neurosci. 2008;122(2):460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev. 2011a;35(8):1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol. Psychiatry. 2011b;70(5):434–440. doi: 10.1016/j.biopsych.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J. Neurosci. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Thompson BS, Sonntag KC, Andersen SL. Extinction and reinstatement to cocaine-associated cues in male and female juvenile rats and the role of D1 dopamine receptor. Neuropharmacology. 2015;95:22–28. doi: 10.1016/j.neuropharm.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Tseng K. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci. Biobehav. Rev. 2016;70:4–12. doi: 10.1016/j.neubiorev.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl.) 2016;233(9):1637–1650. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- D’Andrea W, Ford J, Stolbach B, Spinazzola J, van der Kolk BA. Understanding interpersonal trauma in children: why we need a developmentally appropriate trauma diagnosis. Am. J. Orthopsychiatry. 2012;82(2):187–200. doi: 10.1111/j.1939-0025.2012.01154.x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol. Psychiatry. 2001;50(4):305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27(1–2):155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404(6774):183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303(5664):1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Flege JE, Munro MJ, MacKay IR. Factors affecting strength of perceived foreign accent in a second language. J. Acoust. Soc. Am. 1995;97(5 Pt 1):3125–3134. doi: 10.1121/1.413041. [DOI] [PubMed] [Google Scholar]
- Freund N, Thompson BS, Sonntag K, Meda S, Andersen SL. When the party is over: depressive-like states in rats following termination of cortical D1 receptor overexpression. Psychopharmacology (Berl.) 2016 doi: 10.1007/s00213-015-4200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 1997;21(8):1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325(5945):1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Gomes F, Rincon-Cortes M, Grace AA. Adolescence as a period of vulnerability and intervention in schizophrenia: insights from the MAM model. Neurosci. Biobehav. Rev. 2016;70:260–270. doi: 10.1016/j.neubiorev.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J. Neurosci. 1999;19(22):RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington M, Robinson J, Bolton SL, Sareen J, Bolton J. A longitudinal study of risk factors for incident drug use in adults: findings from a representative sample of the US population. Cana. J. Psychiatry Rev. Can. Psychiatrie. 2011;56(11):686–695. doi: 10.1177/070674371105601107. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Stafford NP, Yusko-Osborne B, Schiml PA, Xanthos ED, Deak T. Naproxen attenuates sensitization of depressive-like behavior and fever during maternal separation. Physiol. Behav. 2015;139:34–40. doi: 10.1016/j.physbeh.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P. Synaptic density in human frontal cortex developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Markham JA. The cellular basis for volume changes in the rat cortex during puberty: white and gray matter. Ann. N. Y. Acad. Sci. 2004;1021:431–435. doi: 10.1196/annals.1308.058. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am. J. Psychiatry. 2015;172(11):1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilford E, Garrett E, Blakemore S-J. The development of social cognition in adolescence: an integrated perspective. Neurosci. Biobehav. Rev. 2016;70:106–120. doi: 10.1016/j.neubiorev.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12(2):427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Kuhn C. Emergence of sex differences in the development of substance use and abuse during adolescence. Pharmacol. Ther. 2015;153:55–78. doi: 10.1016/j.pharmthera.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hohn A, Shatz CJ. Dynamic regulation of BDNF and NT-3 expression during visual system development. J. Comp. Neurol. 2000;420(1):1–18. doi: 10.1002/(sici)1096-9861(20000424)420:1<1::aid-cne1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62(1):22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Freund N, Brenhouse HC, Thompson BS, Andersen SL. Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Dev. Neurosci. 2012;34(2–3):210–217. doi: 10.1159/000339162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berl.) 2009;204(4):725–733. doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Lichenstein S, Verstynen T, Forbes E. Adolescent brain devlopment and psychopathology: a case for connectivity of the anterior cingulate cortex in affective and substance use disorders. Neurosci. Biobehav. Rev. 2016;70:271–287. doi: 10.1016/j.neubiorev.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol. Psychiatry. 1999;4(4):317–327. doi: 10.1038/sj.mp.4000586. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry. 2009;66(3):287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40(5):1250–1258. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Nabkasorn C, Miyai N, Sootmongkol A, Junprasert S, Yamamoto H, Arita M. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur. J. Public Health. 2006;16(2):179–184. doi: 10.1093/eurpub/cki159. [DOI] [PubMed] [Google Scholar]
- Norris R, Carroll D, Cochrane R. The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. J. Psychosom. Res. 1992;36(1):55–65. doi: 10.1016/0022-3999(92)90114-h. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. NeuroImage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Romeo R. Adoelscence and the ontogeny of the hormonal stress response. Neurosci. Biobehav. Rev. 2016 doi: 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch FB, Deslandes AC, Stubbs B, Gosmann NP, Silva CT, Fleck MP. Neurobiological effects of exercise on major depressive disorder: a systematic review. Neurosci. Biobehav. Rev. 2016;61:1–11. doi: 10.1016/j.neubiorev.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Schulz K, Sisk C. Organizing actions of adolescent gonadal steroid hormones on brain and behaivoral development. Neurosci. Biobehav. Rev. 2016;70:148–158. doi: 10.1016/j.neubiorev.2016.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J, Brenhouse HC. Immunoadolescence: neuroimmune development and adolescent behavior. Neurosci. Biobehav. Rev. 2016;70:288–299. doi: 10.1016/j.neubiorev.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. U. S. A. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag KC, Brenhouse HC, Freund N, Thompson BS, Puhl M, Andersen SL. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents. Psychopharmacology (Berl.) 2014;231(8):1615–1626. doi: 10.1007/s00213-013-3399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanis JJ, Andersen SL. Reducing substance use during adolescence: a translational framework for prevention. Psychopharmacology (Berl.) 2014;231(8):1437–1453. doi: 10.1007/s00213-013-3393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg LA. Social neuroscience perspective on adolescent risk-taking. Dev. Rev.: DR. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH. Scars that won’t heal: the neurobiology of child abuse. Sci. Am. 2002;286(3):68–75. doi: 10.1038/scientificamerican0302-68. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol. Psychiatry. 2004;56(2):80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J. Clin. Psychiatry. 2009;70(5):684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnessen E, Svendsen IS, Olsen IC, Guttormsen A, Haugen T. Performance development in adolescent track and field athletes according to age, sex and sport discipline. PLoS One. 2015;10(6):e0129014. doi: 10.1371/journal.pone.0129014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Galvan A. Stress and the adolescent brain: amygdala-prefrontal cortex and ventral striatum as developmental targets. Neurosci. Biobehav. Rev. 2016;70:217–227. doi: 10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor LJ. Are there critical periods for musical development? Dev. Psychobiol. 2005;46(3):262–278. doi: 10.1002/dev.20059. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde A, Peters S, Braams B, Crone E. What motivates adolescents? Neuroal responses to rewards and the influence on adolescent drug taking, learning, and cognitive control. Neurosci. Biobehav. Rev. 2016;70:135–147. doi: 10.1016/j.neubiorev.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol. Psychiatry. 2002;7(9):985–994. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grownup. Arch. Gen. Psychiatry. 2007;64(1):49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Wieck A, Andersen SL, Brenhouse HC. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain Behav. Immun. 2013;28:218–226. doi: 10.1016/j.bbi.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Bounar S, Godfrey J, Michopoulos V, Higgins M, Sanchez M. Social and emotional predictors of the tempo of puberty in female rhesus monkeys. Psychoneuroendocrinology. 2013;38(1):67–83. doi: 10.1016/j.psyneuen.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology (Berl.) 2012;224(3):387–400. doi: 10.1007/s00213-012-2760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]