ABSTRACT
Despite the effects of CD4+ T cell dysfunction on cognitive and behavioral impairment are well established, the effects of Th2 cytokines on the adult hippocampal neurogenesis and cognitive function in restricted CD4+ T cell receptor (TCR) repertoire model have not been fully elucidate. We found that mice with restricted CD4+ repertoire TCR showed decreased adult hippocampal neurogenesis using OT-II mice. Moreover, we demonstrated that OT-II mice showed increased Th2 cytokine levels in peripheral organs and IL-4 levels in brain. Taken together, altered Th2 cytokine levels may impact learning and memory via impaired adult neurogenesis in restricted CD4+ repertoire TCR mice.
KEYWORDS: adult neurogenesis, CD4+ T cells, learning and memory, OT-II mice, Th2 cytokines
CD4+ T cells and their cytokines are well-known as the most important peripheral immune system. Especially, Th2 cytokines released from type-2 (Th2) CD4+ T cell response are more procognitive than the Th1 cytokines released from type-1 (Th1) CD4+ T cell response.1,2 With these cytokines, peripheral immune cells including CD4+ T cells are able to infiltrate into the central nervous system (CNS) across the rigid blood brain barrier (BBB) that isolates the peripheral immune system from CNS.3-6 Furthermore, the normal functions of peripheral immune cells are important for CNS function, notably learning and memory.2,6 Therefore, in experimental animal models with restricted T cell receptor (TCR) repertoire, impaired function of CD4+ T cells led to a significant cognitive dysfunction. Moreover, inhibition of CD4+ T cells depressed adult hippocampal neurogenesis and further, caused cognitive impairment.7,8
Adult hippocampal neurogenesis has been recognized as a prerequisite for normal cognitive homeostasis, and neurogenesis in the dentate gyrus is critical for learning and memory.9 In addition, previous studies have reported that the cognitive function is highly related to the peripheral immune cells, and CD4+ T cell, one of the major peripheral immune cells, may affect the cognitive function.1,6,10 Despite the underlying mechanism was not fully examined, it has been suggested that CD4+ T cell may play a crucial role in both neurogenesis and the declined memory observed in transgenic animal models with immunodeficiency or restricted CD4+ TCR repertoire.8 Moreover, accumulating evidences supported that Th2 cytokines released by CD4+ T cells are responsible for maintaining cognitive functions and adult hippocampal neurogenesis.6,7 Thus, in this study, we verified the effect of T cell dependent cytokines, especially Th2 cytokines, on cognition and its underlying mechanisms that need to be addressed clearly for the first time. In addition, we used an animal model with restricted CD4+ TCR repertoire to examine the physiological and behavioral changes under the effect.11
Mostly, when the antigen presenting cells presents an antigen to T cells via major histocompatibility complex II on their surfaces, the immune system is triggered to start, and T cells get activated.12 However, OT-II mice have restricted CD4+ TCR repertoire that needs ovalbumin for activation. That is, this nonself-antigen specific CD4+ TCR can only recognize ovalbumin as antigen and cannot be activated without it.13 Interestingly, the naïve CD4+ T cells in OT-II mice lacking prior exposure to ovalbumin showed considerably increased Th2 cytokine levels in the periphery and interleukin-4 (IL-4) level in the CNS. However, this can be accounted for by several supporting studies that OT-II mice showed 4-fold increased ratio in the CD4 to CD8 ratio,14,15 and Th2 cytokine response could be triggered by endogenous IL-4 from naïve CD4+ T cells.16-19 Furthermore, patients with higher ratio of CD4 to CD8 have increased IL-4 level in peripheral blood; the ratio of CD4 to CD8 is more significant factor for production of IL-4 cytokine than the absolute number of CD4+ T cells.20
Based on our in vivo results and previous study suggesting the expression of IL-4 receptors (IL-4Rα) in neurons,2 it can be speculated that overexpression of IL-4 may reduce the proliferation of progenitor cells in hippocampal neurogenesis and impair cognitive functions. Moreover, we conducted in vitro CCK-8 analysis and confirmed the direct inhibiting effect of up-regulated IL-4 on proliferation of neural stem cells. Using proliferating cell nuclear antigen (PCNA) immunoblotting, in part, we demonstrated that upregulated IL-4 inhibits the adult hippocampal neurogenesis by alteration of S-phase in DNA replication. Though further in vivo studies on the effect of IL-4 on neurogenesis in dentate gyrus need to be confirmed, these examinations sufficiently support that IL-4 overexpression in OT-II mice reduces adult hippocampal neurogenesis and cognitive functions.
Among many biological roles of IL-4 including immune system, the cytokine has both beneficial and harmful effects on our cognitive functions at the same time. The previous studies have demonstrated beneficial effects of IL-4.1,2,21 However, disadvantageous aspects of IL-4 have also been reported. Increased IL-4 levels induced degeneration of hippocampal CA1 region22 and inhibited the proliferation of retinal progenitor cells in the CNS.23 Moreover, IL-4 overexpression was shown in many experimental models of autoimmune disorders such as encephalomyelitis, anemia, glomerulonephritis and arthritis, and vice versa.24-27 Correspondingly, allergic immune responses such as asthma are also associated with IL-4 level and further, affect cognitive function as risk factors.28-30 These studies demonstrate that the regulation of IL-4 expression is critical for managing the adaptive immune functions and CNS homeostasis. Furthermore, the level of IL-4 expression not only reduces adult hippocampal neurogenesis but down-regulates synaptic formation.
Taken together, the neurostructural mechanisms for impaired cognitive function in OT-II mice, an animal model with restricted CD4+ TCR repertoire, seemed to be down-regulated adult hippocampal neurogenesis and synaptic integration under investigation of markers for each; Ki-67, doublecortin, synaptophysin, respectively. These detrimental effects of increased Th2 cytokine levels were confirmed in vitro using CCK-8 analysis and immunoblotting analysis of PCNA. Our results are consistent with numerous studies and thus, regulating the levels of Th2 cytokine are important for maintaining neurogenesis and normal cognition (Fig. 1). However, still further studies are left on other possibilities for indirect effect of elevated Th2 cytokine level on adult hippocampal neurogenesis and cognitive behaviors. Moreover, as previous studies have reported that Th1 cytokine such as Interferon-gamma enhances the adult hippocampal neurogenesis,31,32 it could be valuable to clarify the roles of Th1 cytokines on cognitive functions and to examine the change of Th1/Th2 bias in animal model with restricted CD4+ TCR repertoire. These issues should be addressed soon to verify the main roles of CD4+ T cell related cytokines and the underlying mechanisms.
Figure 1.
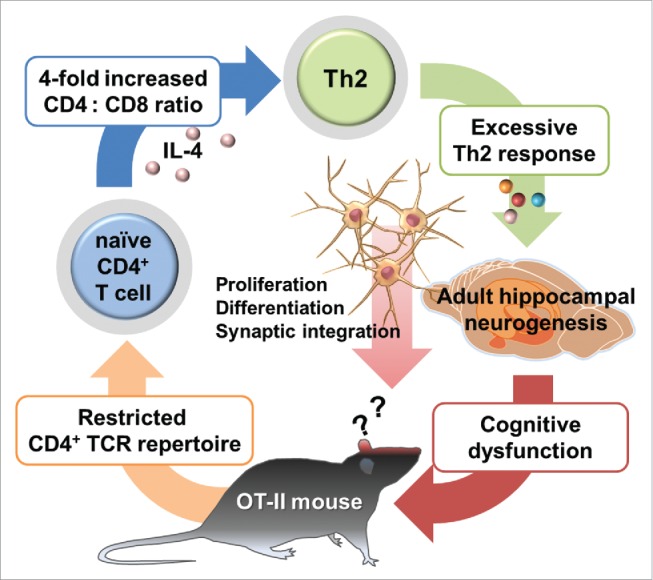
Proposed model of impaired cognitive function induced by alteration of Th2 cytokine levels. Mice with restricted CD4+ T cell receptor repertoire showed significant increased Th2 cytokine levels in peripheral and IL-4 in brain. Altered Th2 cytokine levels decrease the adult hippocampal neurogenesis as well as the cognitive function.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science ICT & Future Planning (NRF-2015R1C1A1A01052732); a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI16C0816).
References
- [1].Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of t cells. Nat Rev Immunol 2012; 12(9):663-9; PMID:22903149; http://dx.doi.org/ 10.1038/nri3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gadani SP, Cronk JC, Norris GT, Kipnis J. Il-4 in the brain: A cytokine to remember. J Immunol (Baltimore, Md : 1950) 2012; 189(9):4213-9; PMID:23087426; http://dx.doi.org/ 10.4049/jimmunol.1202246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, et al.. Effector t cell interactions with meningeal vascular structures in nascent autoimmune cns lesions. Nature 2009; 462(7269):94-8; PMID:19829296; http://dx.doi.org/ 10.1038/nature08478 [DOI] [PubMed] [Google Scholar]
- [4].Radjavi A, Smirnov I, Derecki N, Kipnis J. Dynamics of the meningeal cd4(+) t-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol Psychiatry 2014; 19(5):531-3; PMID:23752249; http://dx.doi.org/ 10.1038/mp.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci 2013; 33(45):17587-96; PMID:24198349; http://dx.doi.org/ 10.1523/JNEUROSCI.3241-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behavior Immunity 2011; 25(2):181-213; PMID:20970492; http://dx.doi.org/ 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
- [7].Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. Cd4-positive t lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol (Baltimore, Md : 1950) 2009; 182(7):3979-84; http://dx.doi.org/ 10.4049/jimmunol.0801218 [DOI] [PubMed] [Google Scholar]
- [8].Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 2006; 9(2):268-75; http://dx.doi.org/ 10.1038/nn1629 [DOI] [PubMed] [Google Scholar]
- [9].Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 2010; 11(5):339-50; PMID:20354534; http://dx.doi.org/ 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marin I, Kipnis J. Learning and memory. And the immune system. Learning Memory (Cold Spring Harbor, NY) 2013; 20(10):601-6; http://dx.doi.org/ 10.1101/lm.028357.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jeon SG, Kim KA, Chung H, Choi J, Song EJ, Han SY, Oh MS, Park JH, Kim JI, Moon M. Impaired memory in ot-ii transgenic mice is associated with decreased adult hippocampal neurogenesis possibly induced by alteration in th2 cytokine levels. Mol Cells 2016; 39(8):603-10; PMID:27432189; http://dx.doi.org/ 10.14348/molcells.2016.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392(6673):245-52; PMID:9521319; http://dx.doi.org/ 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- [13].Barnden MJ, Allison J, Heath WR, Carbone FR. Defective tcr expression in transgenic mice constructed using cdna-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 1998; 76(1):34-40; PMID:9553774; http://dx.doi.org/ 10.1046/j.1440-1711.1998.00709.x [DOI] [PubMed] [Google Scholar]
- [14].Leung S, Smith D, Myc A, Morry J, Baker JR Jr. Ot-ii tcr transgenic mice fail to produce anti-ovalbumin antibodies upon vaccination. Cell Immunol 2013; 282(2):79-84; PMID:23770715; http://dx.doi.org/ 10.1016/j.cellimm.2012.12.006 [DOI] [PubMed] [Google Scholar]
- [15].Palumbo ML, Canzobre MC, Pascuan CG, Rios H, Wald M, Genaro AM. Stress induced cognitive deficit is differentially modulated in balb/c and c57bl/6 mice: Correlation with th1/th2 balance after stress exposure. J Neuroimmunol 2010; 218(1-2):12-20; PMID:19942299; http://dx.doi.org/ 10.1016/j.jneuroim.2009.11.005 [DOI] [PubMed] [Google Scholar]
- [16].Bullens DM, Rafiq K, Kasran A, Van Gool SW, Ceuppens JL. Naive human t cells can be a source of il-4 during primary immune responses. Clin Exp Immunol 1999; 118(3):384-91; PMID:10594556; http://dx.doi.org/ 10.1046/j.1365-2249.1999.01072.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive t helper 2 cell-mediated allergic lung inflammation. Immunity 2014; 40(3):425-35; PMID:24613091; http://dx.doi.org/ 10.1016/j.immuni.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Noben-Trauth N, Hu-Li J, Paul WE. Il-4 secreted from individual naive cd4+ t cells acts in an autocrine manner to induce th2 differentiation. Eur J Immunol 2002; 32(5):1428-33; http://dx.doi.org/ 10.1002/1521-4141(200205)32:5%3c1428::AID-IMMU1428%3e3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- [19].Noben-Trauth N, Hu-Li J, Paul WE. Conventional, naive cd4+ t cells provide an initial source of il-4 during th2 differentiation. J Immunol (Baltimore, Md : 1950) 2000; 165(7):3620-5; PMID:11034364; http://dx.doi.org/ 10.4049/jimmunol.165.7.3620 [DOI] [PubMed] [Google Scholar]
- [20].Lee SY, Kim SJ, Kwon SS, Kim YK, Kim KH, Moon HS, Song JS, Park SH. Distribution and cytokine production of cd4 and cd8 t-lymphocyte subsets in patients with acute asthma attacks. Ann Allergy Asthma Immunol 2001; 86(6):659-64; PMID:11428739; http://dx.doi.org/ 10.1016/S1081-1206(10)62295-8 [DOI] [PubMed] [Google Scholar]
- [21].Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: A key role for il-4. J Exp Med 2010; 207(5):1067-80; PMID:20439540; http://dx.doi.org/ 10.1084/jem.20091419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Park KW, Baik HH, Jin BK. Interleukin-4-induced oxidative stress via microglial nadph oxidase contributes to the death of hippocampal neurons in vivo. Curr Aging Sci 2008; 1(3):192-201; PMID:20021392; http://dx.doi.org/ 10.2174/1874609810801030192 [DOI] [PubMed] [Google Scholar]
- [23].Serre K, Mohr E, Gaspal F, Lane PJ, Bird R, Cunningham AF, Maclennan IC. Il-4 directs both cd4 and cd8 t cells to produce th2 cytokines in vitro, but only cd4 t cells produce these cytokines in response to alum-precipitated protein in vivo. Mol Immunol 2010; 47(10):1914-22; PMID:20392496; http://dx.doi.org/ 10.1016/j.molimm.2010.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lafaille JJ, Keere FV, Hsu AL, Baron JL, Haas W, Raine CS, Tonegawa S. Myelin basic protein-specific t helper 2 (th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J Exp Med 1997; 186(2):307-12; PMID:9221760; http://dx.doi.org/ 10.1084/jem.186.2.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor β, interleukin 4, and prostaglandin e expression in the brain. J Exp Med 1992; 176(5):1355-64; PMID:1383385; http://dx.doi.org/ 10.1084/jem.176.5.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rivas D, Mozo L, Zamorano J, Gayo A, Torre-Alonso JC, Rodriguez A, Gutierrez C. Upregulated expression of il-4 receptors and increased levels of il-4 in rheumatoid arthritis patients. J Autoimmun 1995; 8(4):587-600; PMID:7492352; http://dx.doi.org/ 10.1016/0896-8411(95)90010-1 [DOI] [PubMed] [Google Scholar]
- [27].Erb KJ, Ruger B, von Brevern M, Ryffel B, Schimpl A, Rivett K. Constitutive expression of interleukin (il)-4 in vivo causes autoimmune-type disorders in mice. J Exp Med 1997; 185(2):329-39; PMID:9016881; http://dx.doi.org/ 10.1084/jem.185.2.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Caldera-Alvarado G, Khan DA, Defina LF, Pieper A, Brown ES. Relationship between asthma and cognition: The cooper center longitudinal study. Allergy 2013; 68(4):545-8; PMID:23409872; http://dx.doi.org/ 10.1111/all.12125 [DOI] [PubMed] [Google Scholar]
- [29].Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H. Attenuation of allergic airway inflammation in il-4 deficient mice. Clin Exp Allergy 1994; 24(1):73-80; PMID:8156448; http://dx.doi.org/ 10.1111/j.1365-2222.1994.tb00920.x [DOI] [PubMed] [Google Scholar]
- [30].Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med 2004; 10(10):493-9; PMID:15464449; http://dx.doi.org/ 10.1016/j.molmed.2004.08.004 [DOI] [PubMed] [Google Scholar]
- [31].Baron R, Nemirovsky A, Harpaz I, Cohen H, Owens T, Monsonego A. Ifn-gamma enhances neurogenesis in wild-type mice and in a mouse model of alzheimer's disease. Faseb J 2008; 22(8):2843-52; PMID:18390924; http://dx.doi.org/ 10.1096/fj.08-105866 [DOI] [PubMed] [Google Scholar]
- [32].Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by il-4 or ifn-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 2006; 31(1):149-60; PMID:16297637; http://dx.doi.org/ 10.1016/j.mcn.2005.10.006 [DOI] [PubMed] [Google Scholar]


