Abstract
Receptors for immunoglobulins on animal cells invariably show specificity for Fc regions of the protein and are hence called Fc receptors. The present study shows that immunoglobulin D receptors present an exception to this rule. Binding of IgD-coated erythrocytes to murine IgD-receptor-bearing T-helper cells is competitively inhibited by IgD, by its Fab delta fragments, and by deletion mutants of IgD lacking (i) the first constant domain of the delta heavy chain (KWD1), (ii) that region plus the delta heavy-chain-hinge region (KWD6), or (iii) the third constant domain of the delta heavy chain (Gen. 24). KWD1, Gen. 24, or KWD6 mutants bind to T-helper cells bearing receptors for IgD independently of each other. Furthermore, Gen. 24 and KWD6 mutants also competitively inhibit binding of each other in cross-blocking experiments. These results show that the IgD receptors binds to the Fd delta and the Fc delta and cannot readily be explained by sequence homology between the two parts of the IgD molecule.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Ishizaka K. Enhancement of antibody responses by IgD-binding factors induced by anti-IgD treatment of spleen cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):554–558. doi: 10.1073/pnas.85.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A. R., Coico R. F., Finkelman F., Siskind G. W., Thorbecke G. J. Release of IgD-binding factor by T cells under the influence of interleukin 2, interleukin 4, or cross-linked IgD. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9179–9183. doi: 10.1073/pnas.85.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A. R., Coico R. F., Thorbecke G. J. Characterization and regulation of T-cell IgD receptors and IgD-binding factor. Res Immunol. 1990 Jan;141(1):94–108. doi: 10.1016/0923-2494(90)90110-k. [DOI] [PubMed] [Google Scholar]
- Bourgois A., Abney E. R., Parkhouse R. M. Mouse immunoglobulin receptors on lymphocytes: identification of IgM and IgD molecules by tryptic cleavage and a postulated role for cell surface IgD. Eur J Immunol. 1977 Apr;7(4):210–213. doi: 10.1002/eji.1830070404. [DOI] [PubMed] [Google Scholar]
- Coico R. F., Berzofsky J. A., York-Jolley J., Ozaki S., Siskind G. W., Thorbecke G. J. Physiology of IgD. VII. Induction of receptors for IgD on cloned T cells by IgD and interleukin 2. J Immunol. 1987 Jan 1;138(1):4–6. [PubMed] [Google Scholar]
- Coico R. F., Finkelman F., Swenson C. D., Siskind G. W., Thorbecke G. J. Exposure to crosslinked IgD induces receptors for IgD on T cells in vivo and in vitro. Proc Natl Acad Sci U S A. 1988 Jan;85(2):559–563. doi: 10.1073/pnas.85.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coico R. F., Siskind G. W., Thorbecke G. J. Role of IgD and T delta cells in the regulation of the humoral immune response. Immunol Rev. 1988 Oct;105:45–67. doi: 10.1111/j.1600-065x.1988.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Coico R. F., Xue B., Wallace D., Pernis B., Siskind G. W., Thorbecke G. J. T cells with receptors for IgD. Nature. 1985 Aug 22;316(6030):744–746. doi: 10.1038/316744a0. [DOI] [PubMed] [Google Scholar]
- Coico R. F., Xue B., Wallace D., Siskind G. W., Thorbecke G. J. Physiology of IgD. VI. Transfer of the immunoaugmenting effect of IgD with T delta-containing helper cell populations. J Exp Med. 1985 Dec 1;162(6):1852–1861. doi: 10.1084/jem.162.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Kessler S. W., Mushinski J. F., Potter M. IgD-secreting murine plasmacytomas: identification and partial characterization of two IgD myeloma proteins. J Immunol. 1981 Feb;126(2):680–687. [PubMed] [Google Scholar]
- Goroff D. K., Stall A., Mond J. J., Finkelman F. D. In vitro and in vivo B lymphocyte-activating properties of monoclonal anti-delta antibodies. I. Determinants of B lymphocyte-activating properties. J Immunol. 1986 Apr 1;136(7):2382–2392. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kinet J. P. Antibody-cell interactions: Fc receptors. Cell. 1989 May 5;57(3):351–354. doi: 10.1016/0092-8674(89)90910-0. [DOI] [PubMed] [Google Scholar]
- Mathur A., Lynch R. G., Köhler G. Expression, distribution and specificity of Fc receptors for IgM on murine B cells. J Immunol. 1988 Sep 15;141(6):1855–1862. [PubMed] [Google Scholar]
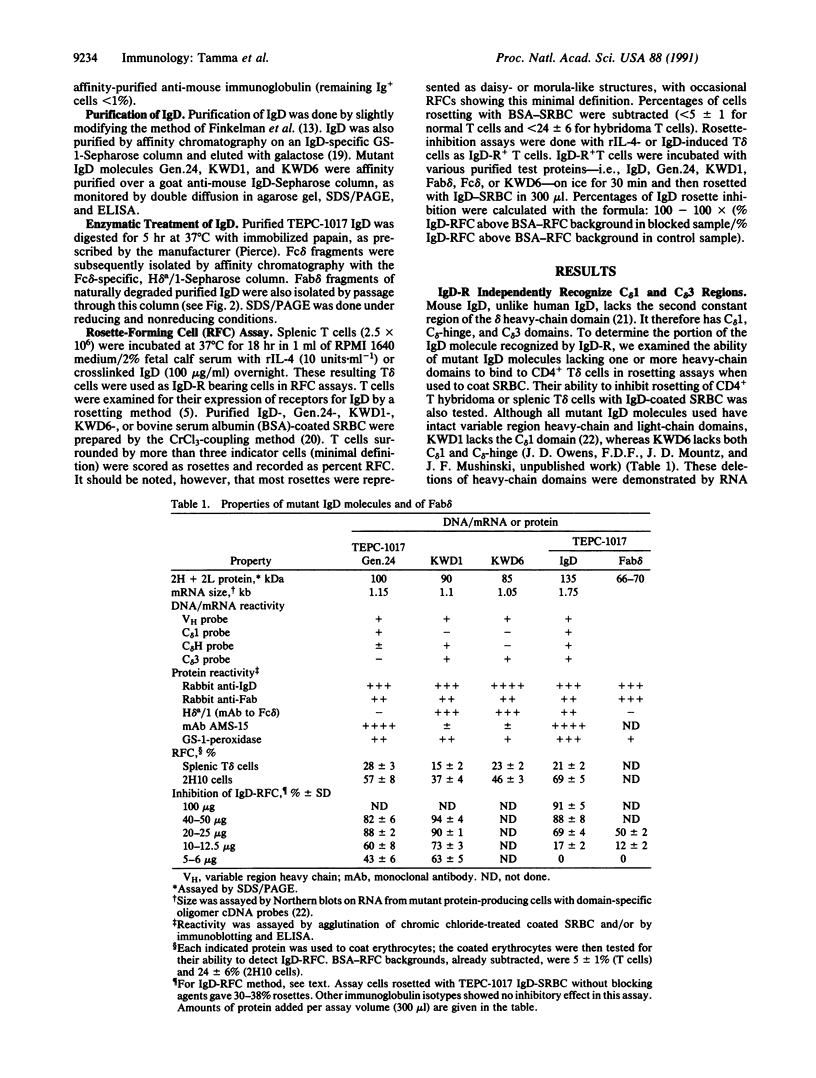
- Mathur A., Lynch R. G., Köhler G. The contribution of constant region domains to the binding of murine IgM to Fc mu receptors on T cells. J Immunol. 1988 Jan 1;140(1):143–147. [PubMed] [Google Scholar]
- Metzger H., Kinet J. P. How antibodies work: focus on Fc receptors. FASEB J. 1988 Jan;2(1):3–11. doi: 10.1096/fasebj.2.1.3275562. [DOI] [PubMed] [Google Scholar]
- Mountz J. D., Mushinski J. F., Owens J. D., Finkelman F. D. The in vivo generation of murine IgD-secreting cells is accompanied by deletion of the C mu gene and occasional deletion of the gene for the C delta 1 domain. J Immunol. 1990 Sep 1;145(5):1583–1591. [PubMed] [Google Scholar]
- Oppenheim J. D., Amin A. R., Thorbecke G. J. A rapid one step purification procedure for murine IgD based on the specific affinity of Bandeiraea (Griffonia) simplicifolia-1 for N-linked carbohydrates on IgD. J Immunol Methods. 1990 Jul 3;130(2):243–250. doi: 10.1016/0022-1759(90)90054-y. [DOI] [PubMed] [Google Scholar]
- Poston R. N. A buffered chromic chloride method of attaching antigens to red cells: use in haemagglutination. J Immunol Methods. 1974 May;5(1):91–96. doi: 10.1016/0022-1759(74)90050-7. [DOI] [PubMed] [Google Scholar]
- Rowe D. S., Hug K., Forni L., Pernis B. Immunoglobulin D as a lymphocyte receptor. J Exp Med. 1973 Oct 1;138(4):965–972. doi: 10.1084/jem.138.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
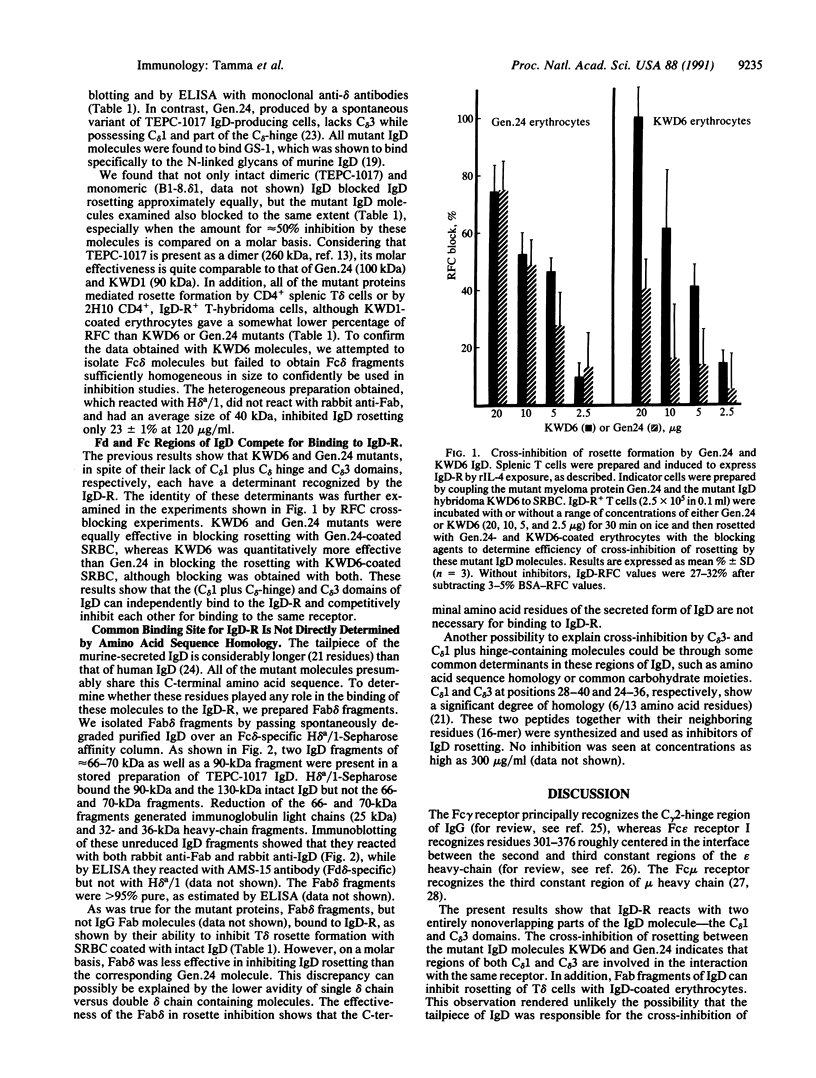
- Swenson C. D., Van Vollenhoven R. F., Xue B., Siskind G. W., Thorbecke G. J., Coico R. F. Physiology of IgD. IX. Effect of IgD on immunoglobulin production in young and old mice. Eur J Immunol. 1988 Jan;18(1):13–20. doi: 10.1002/eji.1830180104. [DOI] [PubMed] [Google Scholar]
- Thiele C. J., Owens J. D., Finkelman F. D., Mushinski J. F. Mouse IgD half molecules with shortened IgD heavy chain result from alterations within C delta locus. J Immunol. 1985 Feb;134(2):1251–1256. [PubMed] [Google Scholar]
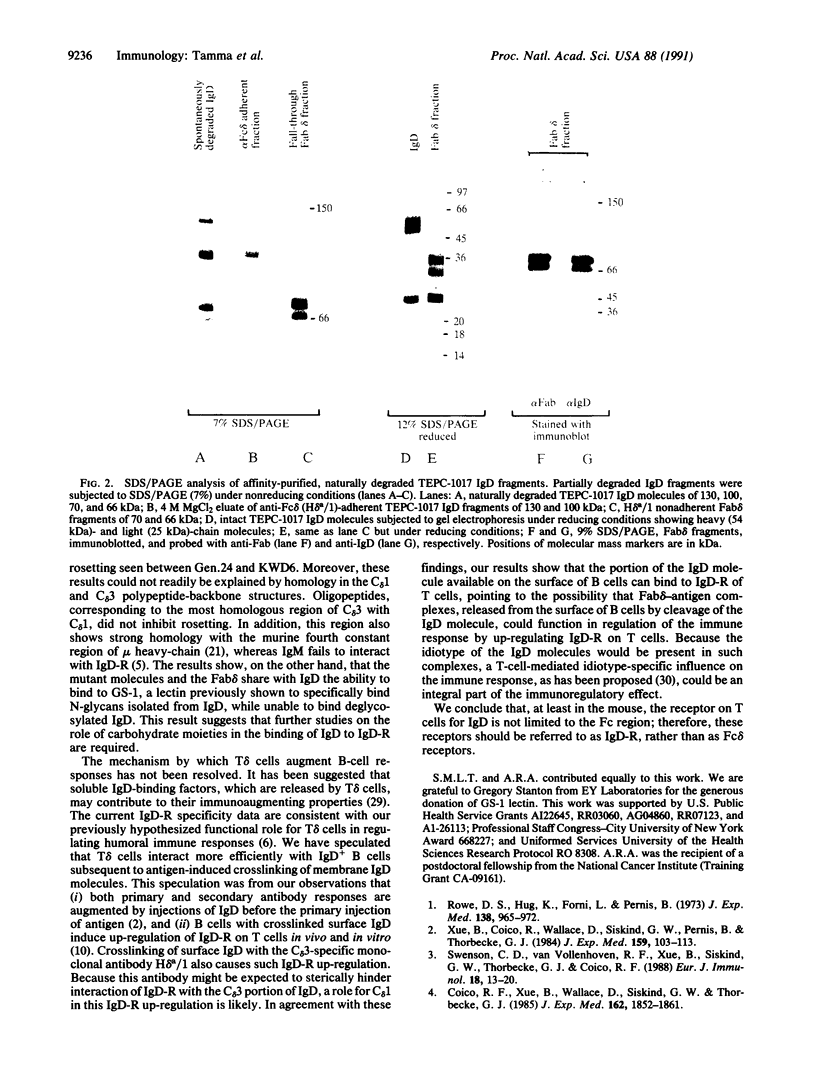
- Tucker P. W., Liu C. P., Mushinski J. F., Blattner F. R. Mouse immunoglobulin D: messenger RNA and genomic DNA sequences. Science. 1980 Sep 19;209(4463):1353–1360. doi: 10.1126/science.6968091. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Coico R., Wallace D., Siskind G. W., Pernis B., Thorbecke G. J. Physiology of IgD. IV. Enhancement of antibody production in mice bearing IgD-secreting plasmacytomas. J Exp Med. 1984 Jan 1;159(1):103–113. doi: 10.1084/jem.159.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitron I. M., Clevinger B. L. Regulation of murine B cells through surface immunoglobulin. I. Monoclonal anti-delta antibody that induces allotype-specific proliferation. J Exp Med. 1980 Nov 1;152(5):1135–1146. doi: 10.1084/jem.152.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]