Abstract
Bone development and homeostasis require the interplay between several cell types, including mesenchymal osteoblasts and osteocytes, as well as hematopoietic osteoclasts. Recent evidence suggests that cell proliferation, differentiation and apoptosis of both mesenchymal and hematopoietic stem cells, which are fundamental for tissue regeneration and treatment of degenerative diseases, is controlled by P2 receptors (i.e., P2X and P2Y receptors). Both types of P2 receptors are versatile transducers of diverse signals activated by extracellular nucleotides like ATP that are released in response to tissue injury, infection or shear stress. The P2X family of receptors has been shown to mediate multiple signaling events including the influx of calcium, activation of mitogen activated protein kinases (MAPKs) and induction of AP-1 family members known to regulate bone development. Support for the significance of P2X7 in regulating bone development and homeostasis has been provided by several studies focusing on animal models and single nucleotide polymorphisms. P2 receptors are functionally expressed in both bone forming osteoblasts and bone resorbing osteoclasts, while recent findings also suggest that these receptors translate mechanical stimuli in osteocytes. Their ability to respond to external nucleotide analogs renders these cell surface proteins excellent targets for skeletal regenerative therapies. This overview summarizes mechanisms by which nucleotide receptors control skeletal cells and contribute to bone tissue development remodeling and repair.
Keywords: osteoblast, bone, mesenchymal stem cell, osteogenesis
Introduction
Extracellular nucleotides can induce cellular responses by acting as ligands for cell surface receptors (nucleotide receptors). Current studies suggest that these receptors have the ability to modulate differentiation of stem cells, thus providing new avenues by which stem cells can be manipulated for tissue regenerative strategies. Recent studies on pluripotent embryonic stem cells and adult somatic stem cells have focused on the molecular mechanisms that permit retention of an uncommitted phenotype, as well as potential medical applications arising from their ability to morph into specialized cell types. Adult stem cells have emerged as viable therapeutic tools for strategies to repair bone and cartilage tissues in age-related skeletal degenerative diseases (e.g., osteoporosis and osteoarthritis), but also have clinical utility in non-skeletal tissues.
Multipotent adult stem cells, including mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs), are reproducibly harvested from bone marrow, skin, adipose tissue, or blood. Because MSCs give rise to bone-forming osteoblasts and HSCs give rise to macrophages which fuse to form osteoclasts that absorb bone (Owen, 1978; Marie and Fromigue, 2006), these two cell types together are particularly relevant for bone remodeling and degenerative bone diseases. Because human neural stem cells are difficult to access, MSCs and HSCs provide alternative sources for transplantable autologous neurons or glia for treatment of neural diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis (Mezey et al., 2003; Joannides et al., 2004; Ortiz-Gonzalez et al., 2004; Kokai et al., 2005; Toma et al., 2005). MSCs are also considered for treatment of cardiovascular diseases and can be induced to differentiate into cardiomyocytes (Makino et al., 1999; Amado et al., 2005) and to create biological pacemakers (Tomita et al., 2007). During development, MSCs give rise to multiple tissues such as bone, cartilage, muscle, ligament, tendon, adipose, and stroma. Many studies have examined the mechanisms by which MSCs and HSCs differentiate into other cell types and tissues (Pittenger et al., 1999; Dudakovic et al., 2014; Eirin et al., 2014; Dudakovic et al., 2015), which is critical for the development of new regenerative therapies. A significant amount of research is being conducted to provide more complete signaling maps for how MSCs and HSCs differentiate into different bone cell types. This review provides a summary of the role of nucleotide receptors in controlling growth and differentiation of MSCs and HSCs.
Nucleotide Receptor Overview
A potential mechanism of manipulating stem cell differentiation is to activate or inhibit P2 receptors by modulating the levels of extracellular nucleotides or nucleotide analogs. There are ample opportunities for intervening in P2 receptor mediated signaling events because extracellular nucleotides are released in response to tissue injury, infection, shear stress and cell death (Lenertz et al., 2011). There are fifteen P2 receptors, including seven in the P2X family of cation channels (P2X1–7) and eight in the P2Y family of G protein-coupled receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11–14). The P2 receptor class responds to extracellular ATP, ADP, UTP, UDP and UDP-glucose (North, 2002; von Kugelgen, 2006). These receptors have gained considerable interest since their discovery, and many groups are investigating their potential use as therapeutic targets and/or biomarkers for an array of diseases. P2Y receptors have been heavily investigated in the context of thrombosis and heart disease, and P2X receptors have been studied in the context of inflammatory and psychological disorders and in bone homeostasis. This is an exciting time in the nucleotide receptor field as there is growing interest in the potential translational possibilities of exploiting the P2X and P2Y receptors (Lenertz et al., 2011; Barn and Steinhubl, 2012; Kennedy et al., 2013).
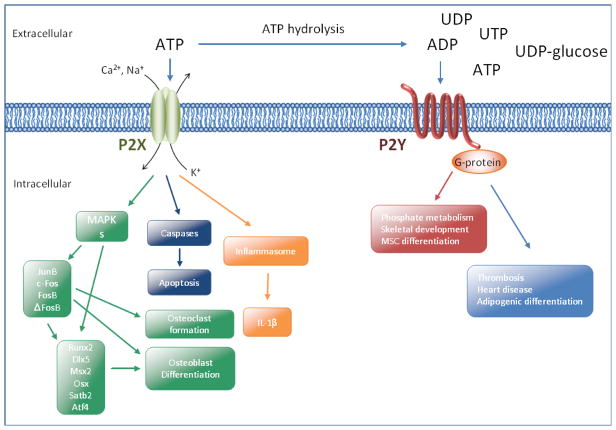
The P2X family of receptors consists of double membrane-spanning proteins with a large extracellular region containing the ATP binding domain. These receptors can homo- or hetero-oligomerize to form cation channels that mediate multiple downstream signaling events. P2X7, which is expressed in numerous cell types including immune, neuronal and bone cells, is activated by millimolar concentrations of extracellular ATP. Upon ligand binding, P2X7 facilitates the influx of Ca2+ and Na+ and the efflux of K+. This can lead to diverse responses including activation of MAPKs and gene transcription, activation of the inflammasome, as well as the subsequent processing and release of the important inflammatory cytokine IL-1β, or sometimes activation of caspases and apoptosis (Lenertz et al., 2011). Specifically, ATP stimulation of P2X7 can lead to the induction and/or activation of the activating protein-1 (AP-1) transcription factors, JunB, c-Fos, FosB and ΔFosB (Gavala et al., 2010). The AP-1 transcription factors, in addition to runt-related transcription factor 2 (Runx2/Cbfa1), distal-less homeobox 5 (Dlx5), mouse segment homeobox 2 (Msx2) and Osterix (Osx), play critical roles in osteoclast and osteoblast formation (Wagner, 2010) (Fig. 1). The roles of P2X7 in bone development and homeostasis are also evident from animal models and single nucleotide polymorphism (SNP) association studies (Lenertz et al., 2011; Rumney et al., 2012), as we will discuss in further detail below.
Fig. 1.
P2X and P2Y receptor family members stimulate multiple downstream events and contribute to cell differentiation and apoptosis. It has been proposed that these receptors are therapeutic targets for an array of diseases. Please see recent review articles for a comprehensive examination of these signaling networks (Idzko et al., 2011; Burnstock et al., 2013)
Function of Nucleotide Receptors in Mesenchymal and Hematopoietic Stem Cells
A number of recent studies indicate that extracellular ATP and P2 receptors regulate self-renewal and cell fate of HSCs and MSCs (Coppi et al., 2007; Yoon et al., 2007; Casati et al., 2011; Zippel et al., 2012). P2X receptors have been found in HSCs and MSCs, and P2X expression is modulated as cells phenotypically commit to a specific lineage (Zippel et al., 2012). In one HSC study, P2X2, P2X3 and P2X5 mRNA levels were found to be low or undetectable, while P2X1, P2X4 and P2X7 mRNAs were detectable (Casati et al., 2011). Yet, other studies presented data suggesting that all P2X receptors, as well as P2Y1 and P2Y2 mRNAs are expressed in HSCs (Lemoli et al., 2004). In a study by Zippel and co-workers, mRNAs for P2X3, P2X4, P2X5, P2X6 and P2X7 and all of the P2Y receptors were found in human MSCs, while the levels of P2Y4 and P2Y12 mRNAs differed between adipose tissue-derived MSCs and ecto-mesenchymal dental follicle cells (Zippel et al., 2012). Adult hematopoietic cells have high levels of P2X1 and P2X7 mRNAs but significantly less P2X4 and P2X5. Interestingly, expression of P2X1, P2X5 and P2X7 in these cells appears to be regulated by ATP. In the presence of extracellular ATP, P2X1 and P2X7 appear to be down-regulated, while P2X5 is up-regulated (Yoon et al., 2007). These findings are indicative of feedback regulation within P2X related pathways.
The relative levels of P2X and P2Y mRNAs and proteins detected before and after differentiation depend on the stem cell source and whether the cells were stimulated towards an adipogenic or osteogenic lineage. For example, upon adipogenic differentiation from adipose tissue-derived MSCs, P2X6 and P2Y11 protein are up-regulated while P2Y4 and P2Y14 are down-regulated. In contrast, P2X6 mRNA and protein levels decrease in adipose tissue-derived MSCs that are differentiated into the osteogenic lineage. Thus, variable expression of P2X6 may be critical for early commitment and differentiation of MSCs (Zippel et al., 2012). Because many of the studies examining P2 receptors in MSCs and HSCs have focused primarily on mRNA levels, more work will be required to fully characterize how cell surface expression of these proteins changes during differentiation into committed cell types.
The role for ATP and P2 receptors in stem cell proliferation and apoptosis is exemplified by the observation that suramin (a nonselective P2X and P2Y receptor antagonist) has cytostatic effects by affecting cell proliferation and apoptosis of HSCs, although these effects are concentration dependent (Yoon et al., 2007). Stimulation of P2 receptors can also increase proliferation of HSCs after treatment with physiologically relevant concentrations of ATP and UTP (Lemoli et al., 2004), or promote HSC differentiation into the myeloid lineage (Barbosa et al., 2011a). Results from future studies may yield a more comprehensive understanding of the biological roles of specific P2X and P2Y family members in stem cell differentiation and further definition of specific downstream targets of these receptors.
Signaling Pathways and Transcriptional Targets of Nucleotide Receptors in Mesenchymal Stem Cells
Interactions of extracellular ATP with nucleotide receptors influence cell proliferation and differentiation of MSCs by controlling the activities of important kinase-dependent signaling pathways and transcription factors. Osteoblast differentiation requires several transcription factors in addition to Runx2 such as Dlx5, Msx2, and Osx. Each of these gene regulatory proteins responds to different extracellular cues required for bone formation including several components of the Wnt signaling pathway (Ducy et al., 1997; Bendall and Abate-Shen, 2000; Nakashima et al., 2002; Robledo et al., 2002; Glass et al., 2005). Differentiation of both osteoblasts and osteoclasts is controlled by paracrine factors, and osteoclastogenesis is at least in part controlled by osteoblasts. Therefore, perturbation of cytokine, hormonal, and mechanical signals that operate on either osteoclasts or osteoblasts may lead to skeletal issues (Martin, 2004). Osteoclastogenesis is stimulated by receptor activator for nuclear factor-κB ligand (RANK-L) (Franzoso et al., 1997) (Takahashi et al., 1988) and attenuated by osteoprotegerin (OPG), which is a decoy receptor (Yasuda et al., 1998a; American Society for and Mineral Research President’s Committee on, 2000), and both proteins are produced by osteoblasts. Similarly, hematopoietic lineage cells may control osteoblast activity, as is indicated by studies showing that monocytes stimulate MSC differentiation into osteoblasts (Nicolaidou et al., 2012).
One central regulatory node in proliferation, differentiation and migration of MSCs is the MAPK signaling pathway. MAPK signaling is directly or indirectly linked to a number of cytokines including tumor necrosis factor-α (TNF-α), hepatocyte growth factor and insulin-like growth factor-1 (IGF-1) that control MSCs (Sitcheran et al., 2003; Forte et al., 2006; Doorn et al., 2013), as well as a large number of downstream effectors of these and other signaling ligands [e.g., high mobility group box 1 (HMGB1) (Meng et al., 2008), and Protein Kinase A (PKA)] (Hwang et al., 2008). Importantly, the MAPK signaling pathway has been linked to osteoblast differentiation (Xiao et al., 2000; Franceschi et al., 2003), because this pathway controls critical gene regulatory factors like Runx2 and peroxisome proliferator activated receptor gamma (PPARγ), which support formation of osteoblasts and adipocytes, respectively (Ducy et al., 1997; Rosen et al., 1999).
Several studies support a role for the P2X7 receptor and MAPK signaling in osteogenic differentiation of human MSCs. Treatment with an inhibitor of P2X7 (KN-62, which also blocks Ca2+/calmodulin-dependent kinase type II), or a non-selective P2 antagonist (PPADS) suppresses p38/MAPK dependent osteogenic differentiation of MSCs in response to shockwaves and ATP treatment (Sun et al., 2013a). Furthermore, extracellular ATP prevents serum deprivation-induced apoptosis of human MSCs through MAPK-related signaling pathways (Berlier et al., 2015). The P2X7 antagonist A438079 blocks expression of Runx2 in MC3T3-E1 osteoblastic cells subjected to tension force (Kariya et al., 2015). Studies by Ciciarello and colleagues showed that Runx2 mRNA levels are lower in ATP-differentiated human bone marrow-derived MSCs (BM-hMSCs) treated with inhibitors against the ecto-NTPDase CD39 and the ecto-5′-nucleotidase CD73. This finding indicates that Runx2 expression may be regulated by the ATP breakdown product adenosine instead of ATP. The authors of this report also suggested that the P2Y family members P2Y1 and P2Y4 are involved in adipogenic differentiation of BM-hMSCs (Ciciarello et al., 2013a). Other studies have revealed a role for the P2X4 receptor in chondrocytic lineage commitment. For example, the P2X4 inhibitor 5-BDBD attenuates differentiation of MSCs into the chondrocyte lineage, while inhibitors for other P2 family members do not have chondrogenic effects (Kwon, 2012). Furthermore, extracellular ATP and P2X4 are involved in chondrogenic differentiation of chicken high density mesenchymal cell cultures (Fodor et al., 2009). While there is increasing evidence for the importance of P2X and P2Y receptors in MSC differentiation, additional studies will be required to fully elucidate the contributions of each receptor family member to cell fate determination in the mesenchymal lineage.
Downstream Molecular Targets of Nucleotide Receptors in Hematopoietic Stem Cells
Extracellular nucleotides such as ATP also activate P2 nucleotide receptors that control differentiation of multipotent HSCs into various blood cell types, including monocytic, B and T cells (Spangrude et al., 1988). P2 receptors ultimately impinge on the activities of different classes of lineage-specific hematopoietic transcription factors (Wontakal et al., 2012) that are also controlled by cytokines. In the macrophage lineage, RANK-L and OPG play an integral role in osteoclastogenesis (Yasuda et al., 1998a; Yasuda et al., 1998b). HSC self-renewal and differentiation towards the lymphoid lineage (Wang et al., 2012) is controlled by the ATF-like basic leucine zipper transcription factor, BATF and Wnt signaling (e.g., Wnt-3a)(Fleming et al., 2008; Luis et al., 2009). Furthermore, P2 receptors regulate hematopoietic progenitor cell function together with a number of cytokines, including stem cell factor (SCF), granulocyte macrophage-colony stimulating factor (GM-CSF), and interleukins 1, 3, 6 and 11 (Burgess and Metcalf, 1980; Migliaccio et al., 1991; Albella et al., 1999). Several studies have provided evidence of the role of nucleotide ligands in proliferation, differentiation, and death of hematopoietic progenitor cells (Lemoli et al., 2004; Yoon et al., 2007; Barbosa et al., 2011a). Treatment of hematopoietic cells from mouse bone marrow with high concentrations of ATP (>5 mM) reduces proliferation (Yoon et al., 2007), while lower concentrations (<1 μM ATP) increase proliferation (Lemoli et al., 2004). These findings are consistent with the concept that the concentration of ATP dictates onset of apoptosis or cell survival (Lenertz et al., 2011), and these findings have potential ramifications for the therapeutic use of HSCs in bone marrow transplants (Weissman, 2000).
Calcium Oscillations, Mesenchymal and Hematopoietic Stem Cells
P2X receptors also are involved in regulating the ability of cells to recycle intracellular concentrations of calcium, a process that is important for stem cell differentiation (Tonelli et al., 2012). Intracellular calcium oscillations are known to occur during a number of processes including embryogenesis and the associated signals are implicated in cell differentiation (Tonelli et al., 2012). These autocrine and paracrine functions involve the calcium-dependent transcription factor nuclear factor of activated T cells (NFAT), which is known to be induced by extracellular nucleotides, at least in microglia, human MSCs and osteoblasts (Shiratori et al., 2010; Glaser et al., 2013; Grol et al., 2013). NFAT activation is critical for gene regulation because it is activated during differentiation of thymocytes and may have an integral role in hematopoietic cell growth and differentiation (Adachi et al., 2000).
Elevated concentrations of ATP in the extracellular space represent a potent signal for cells. ATP not only plays a role in stem cell proliferation, differentiation and apoptosis but it is also involved in ion fluxes through the release of ATP and subsequent autocrine or paracrine activity (Casati et al., 2011; Arslan et al., 2013; Cao et al., 2013; Ciciarello et al., 2013a; Sun et al., 2013b). ATP is stored in vesicles associated with the membrane that undergo exocytosis triggered by an increase in the concentration of cytosolic Ca2+ (Trueta and De-Miguel, 2012). This extracellular ATP can then bind to P2X receptors, causing an influx of Ca2+ and important cell signaling responses that activate gene transcription (Lenertz et al., 2011). Thus, stimulation of P2X receptors increases the intracellular calcium concentration, and self-regulation through P2 receptors allows cells to modify intracellular concentrations of calcium and downstream events (Abbracchio and Verderio, 2006). For example, human MSCs exposed to 10 μM ATP display decreased proliferation, while application of the unselective P2 antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonate (PPADS) or the P2Y1 antagonist 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate (MRS 2179) increase cell number.
Two types of responses to extracellular ATP appear to be present in human MSCs. The first is an outward current followed by an inward current of lesser magnitude; this response is most pronounced at the end of stimulation (5 min). This ion current is mediated by calcium-dependent potassium channels which become activated following activation of P2Y1 receptors. The second is an inward current that reaches a maximal magnitude within 3 min upon which cell becomes desensitized to this signal. This event is mediated by a yet to be specified P2X receptor with a defined time course of action that permits the influx of non-specific cations that depolarize the cell. Both currents can be present in cells with the outward current overshadowing the inward current (Coppi et al., 2007).
During hematopoiesis, purinergic signals regulate numerous cell functions like pro-inflammatory activity, chemotaxis or platelet aggregation. Recent findings suggest that purinergic signaling may affect hematopoietic stem cells by directly binding P2Rs on HPSCs or by targeting cells that are part of the HSC niche (such as MSCs). Upon insults inducing cellular stress, cells can release nucleotides into the extracellular environment and take part in the different steps of inflammatory responses (e.g., acting as a danger signal and alerting the immune system). Subsequently, nucleotides can bind to P2 receptors exposed on the cell membrane of target cells and induce granulocyte and macrophage chemotactic attraction toward the inflammatory focus, as well as activation of antigen-presenting cells inducing the activation of the innate and the adaptive immune system (Rossi et al., 2012). Interestingly, purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to the chemokine CXCL12, a key factor that hematopoietic stem cells in the bone marrow niche, as well as increases the homing capacity and production of pro-inflammatory cytokines. MSCs are resistant to the cytotoxic effects of ATP. ATP-stimulated MSCs undergo cell proliferation and ATP induces cell migration by potentiating the chemotactic response of MSCs to the chemokine CXCL12, and increases their spontaneous migration (Ferrari et al., 2011). Riddle and colleagues found that treating BMSCs with ATP, but not other nucleotides, increases cellular proliferation and that extracellular ATP is a prerequisite for fluid flow-induced increases in intracellular calcium concentration, activation of calcineurin, the nuclear translocation of NFATc1, and cellular proliferation (Riddle et al., 2007). Furthermore, purinergic signals were also shown to induce cell death and cell differentiation in hematopoietic stem cells. The findings collected over the past decade suggest that purinergic signaling may promote the expansion of hematopoietic progenitors at the expense of more immature HSC subsets (Barbosa et al., 2011b) (Rossi et al., 2012).
P2 Receptors and Bone
Nucleotide receptors play important roles in the functions of both osteoclasts and osteoblasts. In vitro cultures of osteoblasts and osteoclasts support a role of ATP and nucleotide receptors in their development from, respectively, MSCs and HSCs. P2 knockout animals display skeletal deformities, and genetic studies have found correlations between P2 polymorphisms and increased risk of osteoporosis. P2 receptors and bone have been the focus of a few recent comprehensive reviews (Orriss et al., 2011; Wesselius et al., 2011; Rumney et al., 2012), thus only a small subset of the work about nucleotide receptors and bone development will be highlighted here.
The significance of P2 receptors in skeletal development has been illustrated through the use of knockout animals. Multiple studies have shown that P2X7, P2Y1, P2Y2, P2Y6 and P2Y13 knockout mice exhibit skeletal abnormalities (Ke et al., 2003; Li et al., 2005; Orriss et al., 2011; Syberg et al., 2012). For example, Ke and colleagues observed that both male and female P2X7 knockout mice have narrower femurs that are otherwise similar in length compared to littermate controls (Ke et al., 2003). In a later study by a different group, it was also found that wild-type and P2X7 knockout mice have similar femur lengths. In addition, the ulnas in both male and female knockout mice are less responsive to mechanical loading than their wild-type counterparts (Li et al., 2005).
In both of these P2X7 knockout studies, mice generated by Pfizer were used. The Pfizer knockout was created by inserting the neomycin resistance gene after amino acid 505 located in the C-terminal tail of P2X7 (Solle et al., 2001). The strain of mice used to create these knockouts has a naturally-occurring SNP at amino acid 451. Cells derived from mice with the P451L polymorphism have reduced P2X7 function (Adriouch et al., 2002). Syberg et al. generated a P2X7 knockout mouse using animals with Proline at amino acid 451 and found that the skeletal abnormalities found in the knockout mice in comparison to their littermate controls are more striking than those observed in mice with the P451L polymorphism (Syberg et al., 2012). Therefore, it appears that P2X7 plays an even larger role in bone formation than was previously thought. Furthermore, it was very recently shown that P2X7 functions as a receptor for the novel paracrine coupling factor Serum Amyloid A3 (Saa3), which is highly expressed by osteocytes and controls bone homeostasis. Saa3 binds to P2X7 inducing a pathway which leads to the up-regulation of Mmp13, an important extracellular matrix remodeling enzyme in bone (Thaler et al., 2013; Thaler et al., 2014).
Associations between P2X polymorphisms and osteoporosis risk have also been investigated. A study by Wesselius and colleagues examined three non-synonymous P2X4 polymorphisms in a Dutch cohort of over 900 patients who had a bone fracture. The Y315C polymorphism was associated with an increased risk of osteoporosis and lower lumbar spine bone mineral density (BMD) values (Wesselius et al., 2013c). This same research group also investigated any associations between P2Y2 and P2X7 polymorphisms and osteoporosis risk. There appears to be an association between the P2Y2 L46P polymorphism and increased BMD in the hip, lumbar spine and femoral neck (Wesselius et al., 2013b). This study also found an association between two P2X7 loss-of-function polymorphisms, E496A and G150R, with decreased hip BMD values but increased BMD values in the lumbar spine in patients with the P2X7 A348T gain-of-function variant (Wesselius et al., 2013a). In a different study involving post-menopausal women who were examined ten years after obtaining a baseline BMD value, the P2X7 R307Q and I568N loss-of-function polymorphisms were associated with increased bone loss while the Q460R polymorphism was considered protective from bone loss (Jorgensen et al., 2012b). The P2X7 E496A polymorphism strongly inhibits osteoclast apoptosis in vitro (Ohlendorff et al., 2007) which could explain the decreased hip BMD values in patients carrying this polymorphism (Wesselius et al., 2013a). In a further study, it was demonstrated that the P2X7 receptor plays an essential role in osteoclast formation. Macrophage-colony stimulating factor (M-CSF) and RANK-L -stimulated fusion of human monocytes is fully prevented after functional repression of P2X7 (Pellegatti et al., 2011).
In addition to the P2X7 investigations, the association between the use of the P2Y12 inhibitor clopidogrel (Plavix), a platelet aggregation inhibitor that is used to prevent coronary heart disease, and fracture risk was investigated in a study involving over 75,000 people who took this drug. In this study, individuals who took clopidogrel for more than one year had an increased risk of fracture while patients who took the drug for less than one year had a lower risk of developing a fracture compared to those who had never taken the drug (Jorgensen et al., 2012a). The reason for the time-dependent differences in results is not yet known, but this large study demonstrates that P2Y12 is likely an important mediator of normal bone maintenance. Collectively, these studies support the idea that P2 single nucleotide polymorphisms may be useful biomarkers for assessing a patient’s risk of developing osteoporosis and/or a fracture.
A P2 receptor that gained more attention recently is P2Y13. Interestingly, P2Y13 knockout mice show an age-dependent bone phenotype that is governed by changes in phosphate metabolism and hormone levels. While young P2Y13 knockout mice have more trabecular bone, more osteoblasts, fewer osteoclasts, and thicker growth plates, mature P2Y13 knockout mice show the opposite bone phenotype including less trabecular bone and lower osteoblast and osteoclast numbers. This age-dependent phenotype was correlated with serum fibroblast growth factor-23 (FGF-23) and phosphorus levels that were higher in young knockout mice but remained unchanged in mature mice (Wang et al., 2014). Additionally, P2Y13 was shown to be a physiological determinant of MSCs differentiation as P2Y13 knockout-MSCs show a decreased osteogenic, but an increased adipogenic differentiation potential (Biver et al., 2013). A similar role was also suggested for the P2Y1 and P2Y4 receptors. It was demonstrated that in MSCs, ATP stimulates adipogenesis via its triphosphate form by the engagement of P2Y1 and P2Y4, while osteogenic differentiation is induced by the nucleoside adenosine, resulting from ATP degradation induced by CD39 and CD73 ectonucleotidases expressed on the MSC membrane (Ciciarello et al., 2013b).
Osteocytes, which are thought to be major mechanosensors in bone, respond to mechanical stimuli by exhibiting unique calcium (Ca2+) oscillations to fluid shear. Such Ca2+ oscillations are significantly reduced by P2 nucleotide receptor inhibition. This finding provides direct evidence that osteocytes respond to in situ mechanical loading by Ca2+ oscillations, which are dependent on the P2 receptors (Jing et al., 2014). Known roles of P2X and P2Y receptors in bone development and homeostasis are summarized in Table 1.
Tab. 1.
P2X and P2Y receptors and their roles in bone development and homeostasis
| Receptor | Nucleotide binding | G-protein coupling | skeletal development related functions, phenotypes | References |
|---|---|---|---|---|
| P2X1 | ATP | negative regulation of bone mineralization | [83] | |
| P2X2 | ATP | |||
| P2X3 | ATP | expressed in human MSCs | [56] | |
| P2X4 | ATP | SNP associates with an increased risk for osteoporosis | [72] | |
| P2X5 | ATP | expressed in human MSCs and osteoblasts | [56] | |
| P2X6 | ATP | upregulated in adipocytes, low expressed in osteoblasts | [56] | |
| P2X7 | ATP | Important role in bone development and homeostasis | [63, 70, 71, 74–77] | |
| P2Y1 | ADP | Gq/11 | decreased bone mass in P2Y1−/− mice | [84] |
| P2Y2 | ATP, ADP | Gq/11 | increased bone mass in P2Y2−/− mice | [84] |
| P2Y4 | UTP | Gi and Gq/11 | ||
| P2Y6 | UDP | Gq/11 | decreased bone resorption in P2Y6−/− mice | [85] |
| P2Y11 | ATP | Gs and Gq/11 | ||
| P2Y12 | ADP | Gi | Important mediator of normal bone maintenance | [86, 87] |
| P2Y13 | ADP | Gi | decreased bone formation and bone resorption in P2Y13−/− mice | [84] |
| P2Y14 | UDP-glucose | Gi |
Conclusions
Proper bone development and homeostasis rely on numerous biochemical and cellular interactions in and between undifferentiated stem cells, bone forming and bone resorbing cells. Alterations in these complex processes can lead to the development of several degenerative diseases like osteoporosis or osteoarthritis. Therefore, understanding the nature of MSCs and HSCs is of primary importance as these stem cells, which retain a certain degree of plasticity, can easily be harvested from bone marrow, skin, adipose tissue, or blood and may thus allow defining new approaches and new medical applications in regenerative medicine.
The increased research focus on P2 receptors during the last years has elucidated associations between nucleotide signaling and a growing number of musculoskeletal disorders. P2 receptors were shown to regulate cell proliferation, differentiation and apoptosis in HSCs as well as in MSCs and as such they play a central role in bone development and homeostasis. Indeed, various P2 receptor polymorphisms, as well as diverse P2 receptor knock out mice models, have been associated with alterations in bone development and/or homeostasis by deregulation of the the functional deregulation between osteoblasts, osteoclast and osteocytes. Strategies aiming to target modulation of P2 receptor function may represent a promising possibility for future therapies.
Research Highlights.
Function of Nucleotide Receptors in Mesenchymal and Hematopoietic Stem Cells
Transcriptional Targets of Nucleotide Receptors in Mesenchymal Stem Cells
Downstream Molecular Targets of Nucleotide Receptors in Hematopoietic Stem Cells
Calcium Oscillations, Mesenchymal and Hematopoietic Stem Cells
P2 Receptors and Bone
Acknowledgments
Funding: This work was supported in part by National Institutes of Health grant R01 AR049069 (AJvW). Additional support was provided by intramural grants from the Center for Regenerative Medicine at Mayo Clinic (AJvW & RT). We also deeply appreciate William and Karen Eby for generous philanthropic support.
Abbreviation list
- AP-1
activating protein-1
- BATF
transcription factor basic leucine zipper transcription factor, ATF-like
- BMD
bone mineral density
- Dlx5
distal-less homeobox 5
- FGF-23
fibroblast growth factor-23
- GATA-1
GATA-binding factor 1/erythroid transcription factor
- GM-CSF
granulocyte macrophage-colony stimulating factor
- HSCs
hematopoietic stem cells
- IGF-1
insulin-like growth factor-1
- KLF1
Kruppel-like factor 1
- M-CSF
Macrophage-colony stimulating factor
- MRS 2179
2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate
- MSCs
mesenchymal stem cells
- Msx2
mouse segment homeobox 2
- NFAT
calcium-dependent transcription factor nuclear factor of activated T cells
- OPG
osteoprotegerin
- Osx
Osterix
- P2X, P2Y
Purinergic receptors
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulfonate
- PPARγ
peroxisome proliferator activated receptor gamma
- RANK-L
receptor activator of nuclear factor kappa-B ligand
- Runx2/Cbfa1
runt-related transcription factor 2
- Scl
Stem cell leukemia
- SNP
single nucleotide polymorphism
- TNF-α
tumor necrosis factor-α
Footnotes
Conflict of Interest: Not applicable.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 2006;276:91–103. discussion 103–12, 275–81. [PubMed] [Google Scholar]
- Adachi S, Amasaki Y, Miyatake S, Arai N, Iwata M. Successive expression and activation of NFAT family members during thymocyte differentiation. J Biol Chem. 2000;275:14708–16. doi: 10.1074/jbc.275.19.14708. [DOI] [PubMed] [Google Scholar]
- Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–12. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- Albella B, Segovia JC, Guenechea G, Pragnell IB, Bueren JA. Preserved long-term repopulation and differentiation properties of hematopoietic grafts subjected to ex vivo expansion with stem cell factor and interleukin 11. Transplantation. 1999;67:1348–57. doi: 10.1097/00007890-199905270-00010. [DOI] [PubMed] [Google Scholar]
- Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–9. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society for B; Mineral Research President’s Committee on NProposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption. The American Society for Bone and Mineral Research President’s Committee on Nomenclature. J Bone Miner Res. 2000;15:2293–6. doi: 10.1359/jbmr.2000.15.12.2293. [DOI] [PubMed] [Google Scholar]
- Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–12. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Barbosa CM, Leon CM, Nogueira-Pedro A, Wasinsk F, Araujo RC, Miranda A, Ferreira AT, Paredes-Gamero EJ. Differentiation of hematopoietic stem cell and myeloid populations by ATP is modulated by cytokines. Cell Death Dis. 2011a;2:e165. doi: 10.1038/cddis.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa CM, Leon CM, Nogueira-Pedro A, Wasinsk F, Araujo RC, Miranda A, Ferreira AT, Paredes-Gamero EJ. Differentiation of hematopoietic stem cell and myeloid populations by ATP is modulated by cytokines. Cell death & disease. 2011b;2:e165. doi: 10.1038/cddis.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barn K, Steinhubl SR. A brief review of the past and future of platelet P2Y12 antagonist. Coron Artery Dis. 2012;23:368–74. doi: 10.1097/MCA.0b013e3283564930. [DOI] [PubMed] [Google Scholar]
- Bendall AJ, Abate-Shen C. Roles for Msx and Dlx homeoproteins in vertebrate development. Gene. 2000;247:17–31. doi: 10.1016/s0378-1119(00)00081-0. [DOI] [PubMed] [Google Scholar]
- Berlier JL, Rigutto S, Dalla Valle A, Lechanteur J, Soyfoo MS, Gangji V, Rasschaert J. Adenosine Triphosphate Prevents Serum Deprivation-Induced Apoptosis in Human Mesenchymal Stem Cells via Activation of the MAPK Signaling Pathways. Stem Cells. 2015;33:211–8. doi: 10.1002/stem.1831. [DOI] [PubMed] [Google Scholar]
- Biver G, Wang N, Gartland A, Orriss I, Arnett TR, Boeynaems JM, Robaye B. Role of the P2Y13 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Stem cells. 2013;31:2747–58. doi: 10.1002/stem.1411. [DOI] [PubMed] [Google Scholar]
- Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56:947–58. [PubMed] [Google Scholar]
- Cao X, Li LP, Qin XH, Li SJ, Zhang M, Wang Q, Hu HH, Fang YY, Gao YB, Li XW, Sun LR, Xiong WC, Gao TM, Zhu XH. Astrocytic ATP release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells. 2013 doi: 10.1002/stem.1408. [DOI] [PubMed] [Google Scholar]
- Casati A, Frascoli M, Traggiai E, Proietti M, Schenk U, Grassi F. Cell-autonomous regulation of hematopoietic stem cell cycling activity by ATP. Cell Death Differ. 2011;18:396–404. doi: 10.1038/cdd.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciciarello M, Zini R, Rossi L, Salvestrini V, Ferrari D, Manfredini R, Lemoli RM. Extracellular purines promote the differentiation of human bone marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem Cells Dev. 2013a;22:1097–111. doi: 10.1089/scd.2012.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciciarello M, Zini R, Rossi L, Salvestrini V, Ferrari D, Manfredini R, Lemoli RM. Extracellular purines promote the differentiation of human bone marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem cells and development. 2013b;22:1097–111. doi: 10.1089/scd.2012.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppi E, Pugliese AM, Urbani S, Melani A, Cerbai E, Mazzanti B, Bosi A, Saccardi R, Pedata F. ATP modulates cell proliferation and elicits two different electrophysiological responses in human mesenchymal stem cells. Stem Cells. 2007;25:1840–9. doi: 10.1634/stemcells.2006-0669. [DOI] [PubMed] [Google Scholar]
- Doorn J, Roberts SJ, Hilderink J, Groen N, van Apeldoorn A, van Blitterswijk C, Schrooten J, de Boer J. Insulin-like growth factor-I enhances proliferation and differentiation of human mesenchymal stromal cells in vitro. Tissue Eng Part A. 2013;19:1817–28. doi: 10.1089/ten.TEA.2012.0522. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Dudakovic A, Camilleri E, Riester SM, Lewallen EA, Kvasha S, Chen X, Radel DJ, Anderson JM, Nair AA, Evans JM, Krych AJ, Smith J, Deyle DR, Stein JL, Stein GS, Im HJ, Cool SM, Westendorf JJ, Kakar S, Dietz AB, van Wijnen AJ. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. Journal of cellular biochemistry. 2014;115:1816–28. doi: 10.1002/jcb.24852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakovic A, Camilleri ET, Lewallen EA, McGee-Lawrence ME, Riester SM, Kakar S, Montecino M, Stein GS, Ryoo HM, Dietz AB, Westendorf JJ, van Wijnen AJ. Histone deacetylase inhibition destabilizes the multi-potent state of uncommitted adipose-derived mesenchymal stromal cells. Journal of cellular physiology. 2015;230:52–62. doi: 10.1002/jcp.24680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, van Wijnen AJ, Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551:55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Gulinelli S, Salvestrini V, Lucchetti G, Zini R, Manfredini R, Caione L, Piacibello W, Ciciarello M, Rossi L, Idzko M, Ferrari S, Di Virgilio F, Lemoli RM. Purinergic stimulation of human mesenchymal stem cells potentiates their chemotactic response to CXCL12 and increases the homing capacity and production of proinflammatory cytokines. Experimental hematology. 2011;39:360–74. 374 e1–5. doi: 10.1016/j.exphem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–83. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor J, Matta C, Juhasz T, Olah T, Gonczi M, Szijgyarto Z, Gergely P, Csernoch L, Zakany R. Ionotropic purinergic receptor P2X4 is involved in the regulation of chondrogenesis in chicken micromass cell cultures. Cell Calcium. 2009;45:421–30. doi: 10.1016/j.ceca.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, Prat M, Di Nardo P. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res. 2003;44(Suppl 1):109–16. [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–96. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavala ML, Hill LM, Lenertz LY, Karta MR, Bertics PJ. Activation of the transcription factor FosB/activating protein-1 (AP-1) is a prominent downstream signal of the extracellular nucleotide receptor P2RX7 in monocytic and osteoblastic cells. J Biol Chem. 2010;285:34288–98. doi: 10.1074/jbc.M110.142091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Resende RR, Ulrich H. Implications of purinergic receptor-mediated intracellular calcium transients in neural differentiation. Cell Commun Signal. 2013;11:12. doi: 10.1186/1478-811X-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Grol MW, Pereverzev A, Sims SM, Dixon SJ. P2 receptor networks regulate signaling duration over a wide dynamic range of ATP concentrations. J Cell Sci. 2013;126:3615–26. doi: 10.1242/jcs.122705. [DOI] [PubMed] [Google Scholar]
- Hwang KC, Kim JY, Chang W, Kim DS, Lim S, Kang SM, Song BW, Ha HY, Huh YJ, Choi IG, Hwang DY, Song H, Jang Y, Chung N, Kim SH, Kim DW. Chemicals that modulate stem cell differentiation. Proc Natl Acad Sci U S A. 2008;105:7467–71. doi: 10.1073/pnas.0802825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing D, Baik AD, Lu XL, Zhou B, Lai X, Wang L, Luo E, Guo XE. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:1582–92. doi: 10.1096/fj.13-237578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364:172–8. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- Jorgensen NR, Grove EL, Schwarz P, Vestergaard P. Clopidogrel and the risk of osteoporotic fractures: a nationwide cohort study. J Intern Med. 2012a;272:385–93. doi: 10.1111/j.1365-2796.2012.02535.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen NR, Husted LB, Skarratt KK, Stokes L, Tofteng CL, Kvist T, Jensen JE, Eiken P, Brixen K, Fuller S, Clifton-Bligh R, Gartland A, Schwarz P, Langdahl BL, Wiley JS. Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. Eur J Hum Genet. 2012b;20:675–81. doi: 10.1038/ejhg.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya T, Tanabe N, Shionome C, Manaka S, Kawato T, Zhao N, Maeno M, Suzuki N, Shimizu N. Tension force-induced ATP promotes osteogenesis through P2X7 receptor in osteoblasts. J Cell Biochem. 2015;116:12–21. doi: 10.1002/jcb.24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–67. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Chootip K, Mitchell C, Syed NI, Tengah A. P2X and P2Y nucleotide receptors as targets in cardiovascular disease. Future Med Chem. 2013;5:431–49. doi: 10.4155/fmc.13.6. [DOI] [PubMed] [Google Scholar]
- Kokai LE, Rubin JP, Marra KG. The potential of adipose-derived adult stem cells as a source of neuronal progenitor cells. Plast Reconstr Surg. 2005;116:1453–60. doi: 10.1097/01.prs.0000182570.62814.e3. [DOI] [PubMed] [Google Scholar]
- Kwon HJ. Extracellular ATP signaling via P2X(4) receptor and cAMP/PKA signaling mediate ATP oscillations essential for prechondrogenic condensation. J Endocrinol. 2012;214:337–48. doi: 10.1530/JOE-12-0131. [DOI] [PubMed] [Google Scholar]
- Lemoli RM, Ferrari D, Fogli M, Rossi L, Pizzirani C, Forchap S, Chiozzi P, Vaselli D, Bertolini F, Foutz T, Aluigi M, Baccarani M, Di Virgilio F. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood. 2004;104:1662–70. doi: 10.1182/blood-2004-03-0834. [DOI] [PubMed] [Google Scholar]
- Lenertz LY, Gavala ML, Zhu Y, Bertics PJ. Transcriptional control mechanisms associated with the nucleotide receptor P2X7, a critical regulator of immunologic, osteogenic, and neurologic functions. Immunol Res. 2011;50:22–38. doi: 10.1007/s12026-011-8203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–9. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- Luis TC, Weerkamp F, Naber BA, Baert MR, de Haas EF, Nikolic T, Heuvelmans S, De Krijger RR, van Dongen JJ, Staal FJ. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–54. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie PJ, Fromigue O. Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen Med. 2006;1:539–48. doi: 10.2217/17460751.1.4.539. [DOI] [PubMed] [Google Scholar]
- Martin TJ. Paracrine regulation of osteoclast formation and activity: milestones in discovery. J Musculoskelet Neuronal Interact. 2004;4:243–53. [PubMed] [Google Scholar]
- Meng E, Guo Z, Wang H, Jin J, Wang J, Wang H, Wu C, Wang L. High mobility group box 1 protein inhibits the proliferation of human mesenchymal stem cells and promotes their migration and differentiation along osteoblastic pathway. Stem Cells Dev. 2008;17:805–13. doi: 10.1089/scd.2007.0276. [DOI] [PubMed] [Google Scholar]
- Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci U S A. 2003;100:1364–9. doi: 10.1073/pnas.0336479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G, Migliaccio AR, Valinsky J, Langley K, Zsebo K, Visser JW, Adamson JW. Stem cell factor induces proliferation and differentiation of highly enriched murine hematopoietic cells. Proc Natl Acad Sci U S A. 1991;88:7420–4. doi: 10.1073/pnas.88.16.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM, Cope AP, Horwood NJ. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One. 2012;7:e39871. doi: 10.1371/journal.pone.0039871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ohlendorff SD, Tofteng CL, Jensen JE, Petersen S, Civitelli R, Fenger M, Abrahamsen B, Hermann AP, Eiken P, Jorgensen NR. Single nucleotide polymorphisms in the P2X7 gene are associated to fracture risk and to effect of estrogen treatment. Pharmacogenetics and genomics. 2007;17:555–67. doi: 10.1097/FPC.0b013e3280951625. [DOI] [PubMed] [Google Scholar]
- Orriss I, Syberg S, Wang N, Robaye B, Gartland A, Jorgensen N, Arnett T, Boeynaems JM. Bone phenotypes of P2 receptor knockout mice. Front Biosci (Schol Ed) 2011;3:1038–46. doi: 10.2741/208. [DOI] [PubMed] [Google Scholar]
- Ortiz-Gonzalez XR, Keene CD, Verfaillie CM, Low WC. Neural induction of adult bone marrow and umbilical cord stem cells. Curr Neurovasc Res. 2004;1:207–13. doi: 10.2174/1567202043362342. [DOI] [PubMed] [Google Scholar]
- Owen M. Histogenesis of bone cells. Calcif Tissue Res. 1978;25:205–7. doi: 10.1007/BF02010770. [DOI] [PubMed] [Google Scholar]
- Pellegatti P, Falzoni S, Donvito G, Lemaire I, Di Virgilio F. P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:1264–74. doi: 10.1096/fj.10-169854. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Taylor AF, Rogers JR, Donahue HJ. ATP release mediates fluid flow-induced proliferation of human bone marrow stromal cells. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2007;22:589–600. doi: 10.1359/jbmr.070113. [DOI] [PubMed] [Google Scholar]
- Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–7. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Rossi L, Salvestrini V, Ferrari D, Di Virgilio F, Lemoli RM. The sixth sense: hematopoietic stem cells detect danger through purinergic signaling. Blood. 2012;120:2365–75. doi: 10.1182/blood-2012-04-422378. [DOI] [PubMed] [Google Scholar]
- Rumney RM, Wang N, Agrawal A, Gartland A. Purinergic signalling in bone. Front Endocrinol (Lausanne) 2012;3:116. doi: 10.3389/fendo.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K. P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem. 2010;114:810–9. doi: 10.1111/j.1471-4159.2010.06809.x. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Cogswell PC, Baldwin AS., Jr NF-kappaB mediates inhibition of mesenchymal cell differentiation through a posttranscriptional gene silencing mechanism. Genes Dev. 2003;17:2368–73. doi: 10.1101/gad.1114503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–32. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Sun D, Junger WG, Yuan C, Zhang W, Bao Y, Qin D, Wang C, Tan L, Qi B, Zhu D, Zhang X, Yu T. Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem cells. 2013a;31:1170–80. doi: 10.1002/stem.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Junger WG, Yuan C, Zhang W, Bao Y, Qin D, Wang C, Tan L, Qi B, Zhu D, Zhang X, Yu T. Shockwaves Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells through ATP Release and Activation of P2X7 Receptors. Stem Cells. 2013b doi: 10.1002/stem.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syberg S, Petersen S, Beck Jensen JE, Gartland A, Teilmann J, Chessell I, Steinberg TH, Schwarz P, Jorgensen NR. Genetic Background Strongly Influences the Bone Phenotype of P2X7 Receptor Knockout Mice. J Osteoporos. 2012;2012:391097. doi: 10.1155/2012/391097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Thaler R, Sturmlechner I, Spitzer S, Riester SM, Rumpler M, Zwerina J, Klaushofer K, van Wijnen AJ, Varga F. Acute-phase protein serum amyloid A3 is a novel paracrine coupling factor that controls bone homeostasis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.14-265512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler R, Zwerina J, Rumpler M, Spitzer S, Gamsjaeger S, Paschalis EP, Klaushofer K, Varga F. Homocysteine induces serum amyloid A3 in osteoblasts via unlocking RGD-motifs in collagen. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2013;27:446–63. doi: 10.1096/fj.12-208058. [DOI] [PubMed] [Google Scholar]
- Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Makino S, Hakuno D, Hattan N, Kimura K, Miyoshi S, Murata M, Ieda M, Fukuda K. Application of mesenchymal stem cell-derived cardiomyocytes as bio-pacemakers: current status and problems to be solved. Med Biol Eng Comput. 2007;45:209–20. doi: 10.1007/s11517-007-0163-4. [DOI] [PubMed] [Google Scholar]
- Tonelli FM, Santos AK, Gomes DA, da Silva SL, Gomes KN, Ladeira LO, Resende RR. Stem cells and calcium signaling. Adv Exp Med Biol. 2012;740:891–916. doi: 10.1007/978-94-007-2888-2_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueta C, De-Miguel FF. Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front Physiol. 2012;3:319. doi: 10.3389/fphys.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–32. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Wagner EF. Bone development and inflammatory disease is regulated by AP-1 (Fos/Jun) Ann Rheum Dis. 2010;69(Suppl 1):i86–88. doi: 10.1136/ard.2009.119396. [DOI] [PubMed] [Google Scholar]
- Wang J, Sun Q, Morita Y, Jiang H, Gross A, Lechel A, Hildner K, Guachalla LM, Gompf A, Hartmann D, Schambach A, Wuestefeld T, Dauch D, Schrezenmeier H, Hofmann WK, Nakauchi H, Ju Z, Kestler HA, Zender L, Rudolph KL. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148:1001–14. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Wang N, Robaye B, Gossiel F, Boeynaems JM, Gartland A. The P2Y13 receptor regulates phosphate metabolism and FGF-23 secretion with effects on skeletal development. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:2249–59. doi: 10.1096/fj.13-243626. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–6. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- Wesselius A, Bours MJ, Agrawal A, Gartland A, Dagnelie PC, Schwarz P, Jorgensen NR. Role of purinergic receptor polymorphisms in human bone. Front Biosci. 2011;16:2572–85. doi: 10.2741/3873. [DOI] [PubMed] [Google Scholar]
- Wesselius A, Bours MJ, Henriksen Z, Syberg S, Petersen S, Schwarz P, Jorgensen NR, van Helden S, Dagnelie PC. Association of P2X7 receptor polymorphisms with bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Osteoporos Int. 2013a;24:1235–46. doi: 10.1007/s00198-012-2059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselius A, Bours MJ, Henriksen Z, Syberg S, Petersen S, Schwarz P, Jorgensen NR, van Helden S, Dagnelie PC. Association of P2Y(2) receptor SNPs with bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Purinergic Signal. 2013b;9:41–9. doi: 10.1007/s11302-012-9326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselius A, Bours MJ, Jorgensen NR, Wiley J, Gu B, van Helden S, van Rhijn L, Dagnelie PC. Non-synonymous polymorphisms in the P2RX (4) are related to bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Purinergic Signal. 2013c;9:123–30. doi: 10.1007/s11302-012-9337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wontakal SN, Guo X, Smith C, MacCarthy T, Bresnick EH, Bergman A, Snyder MP, Weissman SM, Zheng D, Skoultchi AI. A core erythroid transcriptional network is repressed by a master regulator of myelo-lymphoid differentiation. Proc Natl Acad Sci U S A. 2012;109:3832–7. doi: 10.1073/pnas.1121019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–9. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998a;139:1329–37. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998b;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MJ, Lee HJ, Lee YS, Kim JH, Park JK, Chang WK, Shin HC, Kim DK. Extracellular ATP is involved in the induction of apoptosis in murine hematopoietic cells. Biol Pharm Bull. 2007;30:671–6. doi: 10.1248/bpb.30.671. [DOI] [PubMed] [Google Scholar]
- Zippel N, Limbach CA, Ratajski N, Urban C, Luparello C, Pansky A, Kassack MU, Tobiasch E. Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cells Dev. 2012;21:884–900. doi: 10.1089/scd.2010.0576. [DOI] [PubMed] [Google Scholar]