Abstract
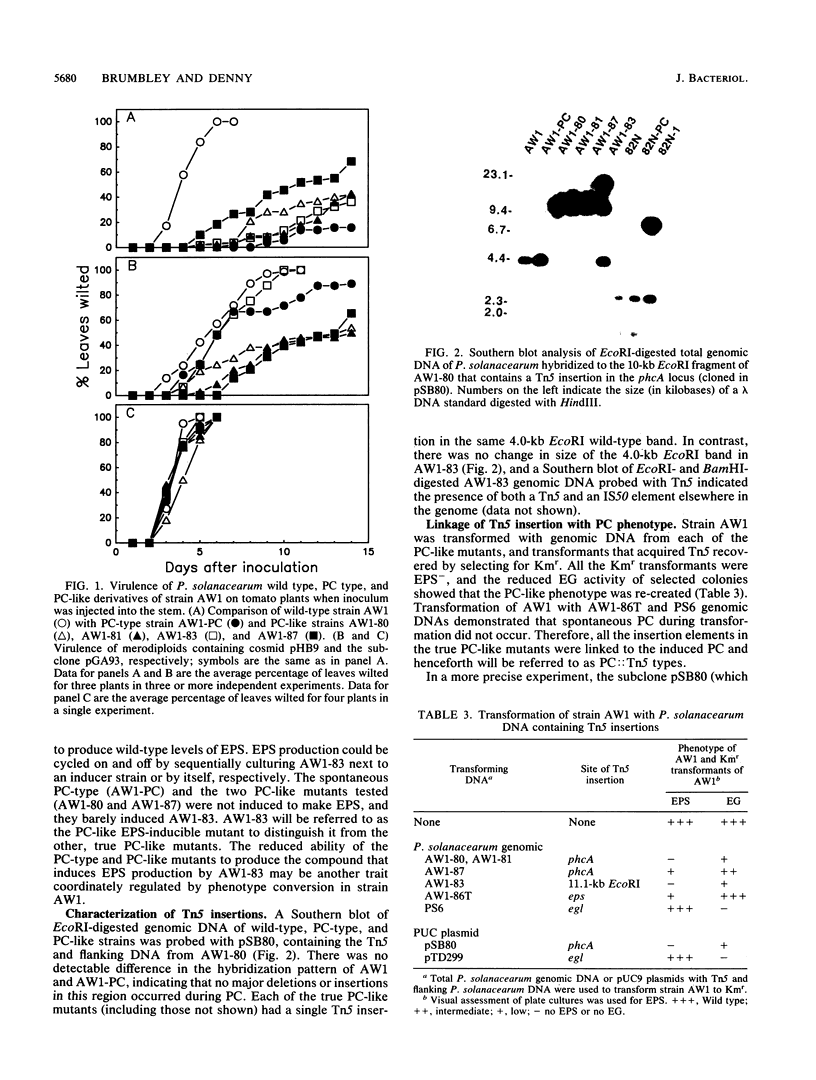
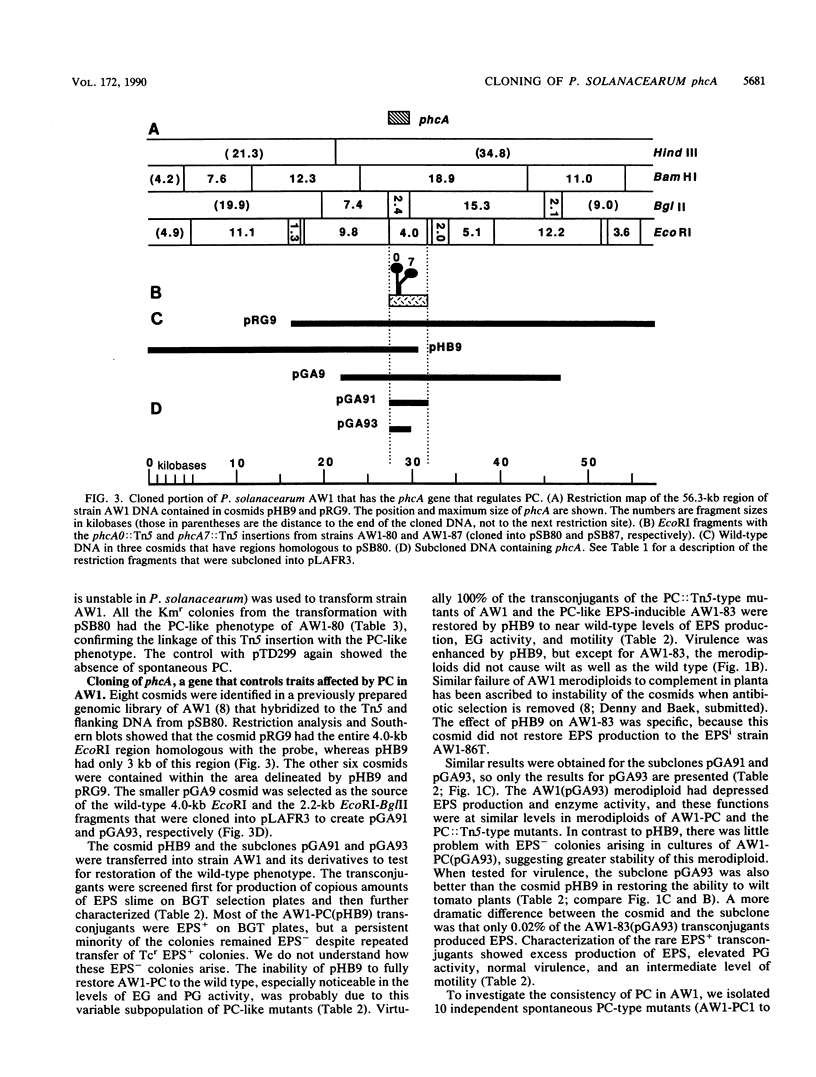
Pseudomonas solanacearum undergoes a spontaneous mutation that pleiotropically reduces extracellular polysaccharide (EPS) production, endoglucanase activity, and virulence and increases motility. We refer to the process that coordinately affects these traits as phenotype conversion (PC) and the resulting mutants as PC types. Previous research with the wild-type strain AW1 suggested that inactivation of a single locus could mimic phenotype conversion (T. P. Denny, F. W. Makini, and S. M. Brumbley, Mol. Plant-Microbe Interact. 1:215-223, 1988). Additional Tn5 mutagenesis of AW1 generated three more mutants (AW1-81, AW1-82, and AW1-84) that were indistinguishable from the PC type and one slightly leaky mutant (AW1-87); all four had single insertions in the same 4.0-kilobase (kb) EcoRI fragment that were responsible for the PC-like phenotype. Another insertion mutant, AW1-83, which lacks an insertion in this 4.0-kb fragment, resembled the PC type except that it was reversibly induced to produce wild-type levels of EPS when cultured adjacent to AW1. The wild-type region containing the gene that controls traits affected by phenotype conversion in AW1, designated phcA, was cloned on a 2.2-kb DNA fragment that restored all the phcA::Tn5 mutants and 11 independent spontaneous PC-type derivatives of AW1 to wild-type status. Homology with the phcA region was found in diverse wild-type strains of P. solanacearum, although restriction fragment length polymorphisms were seen. No major DNA alterations were observed in the phcA homologous region of PC types from strain AW1 or 82N. PC types from 7 of 11 conjugal strains of P. solanacearum were restored to EPS+ by phcA from AW1; however, only some PC types of strain K60 were restored, whereas others were not. We believe that a functional phcA gene is required to maintain the wild-type phenotype in P. solanacearum, and for most strains phenotype conversion results from a loss of phcA gene expression or the function of its gene product.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Boucher C. A., Van Gijsegem F., Barberis P. A., Arlat M., Zischek C. Pseudomonas solanacearum genes controlling both pathogenicity on tomato and hypersensitivity on tobacco are clustered. J Bacteriol. 1987 Dec;169(12):5626–5632. doi: 10.1128/jb.169.12.5626-5632.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney B. F., Denny T. P. A cloned avirulence gene from Pseudomonas solanacearum determines incompatibility on Nicotiana tabacum at the host species level. J Bacteriol. 1990 Sep;172(9):4836–4843. doi: 10.1128/jb.172.9.4836-4843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drigues P., Demery-Lafforgue D., Trigalet A., Dupin P., Samain D., Asselineau J. Comparative studies of lipopolysaccharide and exopolysaccharide from a virulent strain of Pseudomonas solanacearum and from three avirulent mutants. J Bacteriol. 1985 May;162(2):504–509. doi: 10.1128/jb.162.2.504-509.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Ohman D. E. Use of a gene replacement cosmid vector for cloning alginate conversion genes from mucoid and nonmucoid Pseudomonas aeruginosa strains: algS controls expression of algT. J Bacteriol. 1988 Jul;170(7):3228–3236. doi: 10.1128/jb.170.7.3228-3236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick C. A., Sequeira L. Lipopolysaccharide-Defective Mutants of the Wilt Pathogen Pseudomonas solanacearum. Appl Environ Microbiol. 1984 Jul;48(1):94–101. doi: 10.1128/aem.48.1.94-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. H., Schell M. A. DNA sequence analysis of pglA and mechanism of export of its polygalacturonase product from Pseudomonas solanacearum. J Bacteriol. 1990 Jul;172(7):3879–3887. doi: 10.1128/jb.172.7.3879-3887.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. Z., Sukordhaman M., Schell M. A. Excretion of the egl gene product of Pseudomonas solanacearum. J Bacteriol. 1989 Jul;171(7):3767–3774. doi: 10.1128/jb.171.7.3767-3774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman A., Hruschka J. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth cultures of Pseudomonas solanacearum. J Gen Microbiol. 1973 May;76(1):177–188. doi: 10.1099/00221287-76-1-177. [DOI] [PubMed] [Google Scholar]
- RHODES M. E. The cytology of Pseudomonas spp. as revealed by a silver-plating staining method. J Gen Microbiol. 1958 Jun;18(3):639–648. doi: 10.1099/00221287-18-3-639. [DOI] [PubMed] [Google Scholar]
- Roberts D. P., Denny T. P., Schell M. A. Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. J Bacteriol. 1988 Apr;170(4):1445–1451. doi: 10.1128/jb.170.4.1445-1451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. A. Purification and Characterization of an Endoglucanase from Pseudomonas solanacearum. Appl Environ Microbiol. 1987 Sep;53(9):2237–2241. doi: 10.1128/aem.53.9.2237-2241.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira L. Surface components involved in bacterial pathogen-plant host recognition. J Cell Sci Suppl. 1985;2:301–316. doi: 10.1242/jcs.1985.supplement_2.16. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Whatley M. H., Hunter N., Cantrell M. A., Hendrick C., Keegstra K., Sequeira L. Lipopolysaccharide Composition of the Wilt Pathogen, Pseudomonas solanacearum: CORRELATION WITH THE HYPERSENSITIVE RESPONSE IN TOBACCO. Plant Physiol. 1980 Mar;65(3):557–559. doi: 10.1104/pp.65.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P. L., Leong S., Sequeira L. Molecular cloning of genes that specify virulence in Pseudomonas solanacearum. J Bacteriol. 1988 Feb;170(2):617–622. doi: 10.1128/jb.170.2.617-622.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]