Abstract
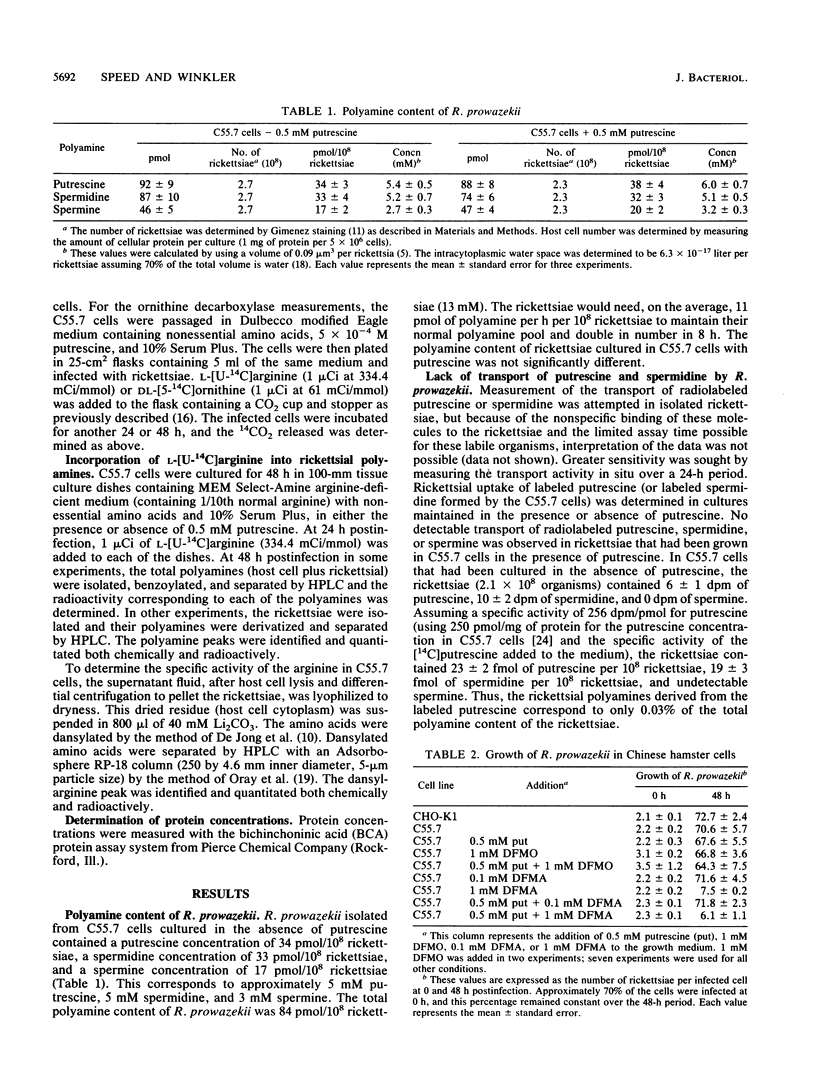
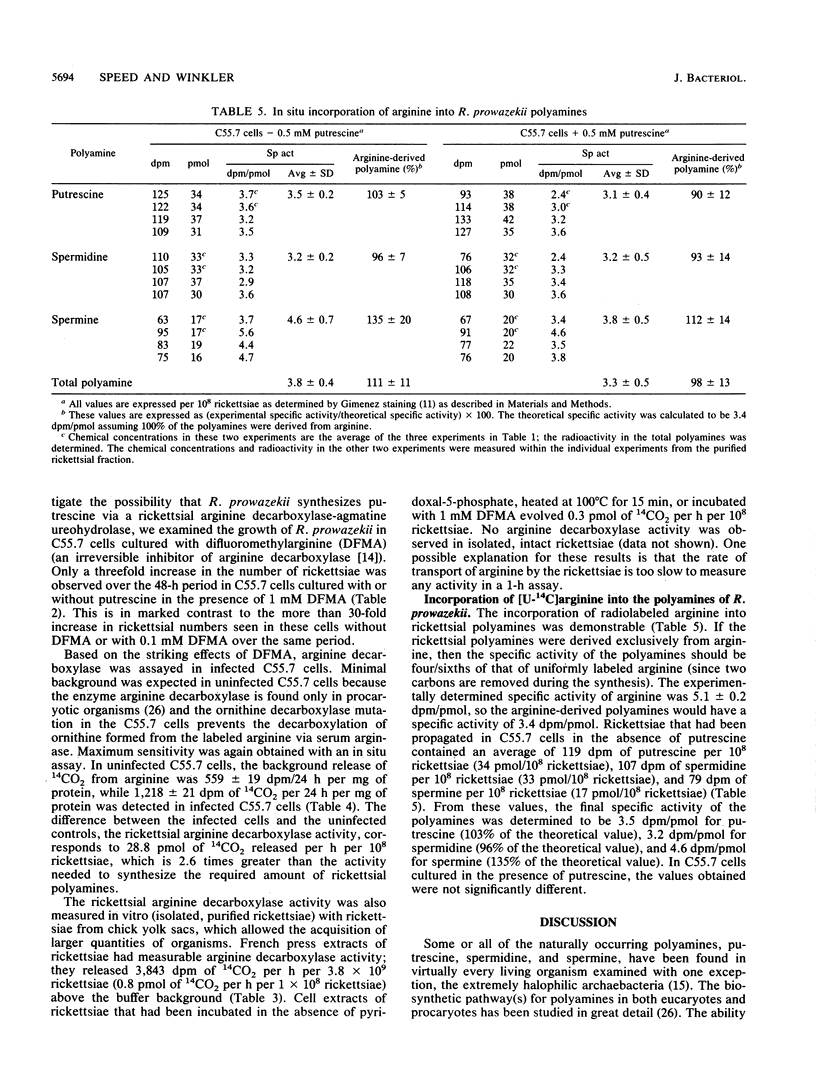
Both the polyamine content and the route of acquisition of polyamines by Rickettsia prowazekii, an obligate intracellular parasitic bacterium, were determined. The rickettsiae grew normally in an ornithine decarboxylase mutant of the Chinese hamster ovary (C55.7) cell line whether or not putrescine, which this host cell required in order to grow, was present. The rickettsiae contained approximately 6 mM putrescine, 5 mM spermidine, and 3 mM spermine when cultured in the presence or absence of putrescine. Neither the transport of putrescine and spermidine by the rickettsiae nor a measurable rickettsial ornithine decarboxylase activity could be demonstrated. However, we demonstrated the de novo synthesis of polyamines from arginine by the rickettsiae. Arginine decarboxylase activity (29 pmol of 14CO2 released per h per 10(8) rickettsiae) was measured in the rickettsiae growing within their host cell. A markedly lower level of this enzymatic activity was observed in cell extracts of R. prowazekii and could be completely inhibited with 1 mM difluoromethylarginine, an irreversible inhibitor of the enzyme. R. prowazekii failed to grow in C55.7 cells that had been cultured in the presence of 1 mM difluoromethylarginine. After rickettsiae were grown in C55.7 in the presence of labeled arginine, the specific activities of arginine in the host cell cytoplasm and polyamines in the rickettsiae were measured; these measurements indicated that 100% of the total polyamine content of R. prowazekii was derived from arginine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum D. M., Dunlap J. C., Morris D. R. Comparison of the biosynthetic and biodegradative ornithine decarboxylases of Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1580–1584. doi: 10.1021/bi00627a008. [DOI] [PubMed] [Google Scholar]
- Applebaum D., Sabo D. L., Fischer E. H., Morris D. R. Biodegradative ornithine decarboxylase of Escherichia coli. Purification, properties, and pyridoxal 5'-phosphate binding site. Biochemistry. 1975 Aug 12;14(16):3675–3681. doi: 10.1021/bi00687a025. [DOI] [PubMed] [Google Scholar]
- Atkins J. F., Lewis J. B., Anderson C. W., Gesteland R. F. Enhanced differential synthesis of proteins in a mammalian cell-free system by addition of polyamines. J Biol Chem. 1975 Jul 25;250(14):5688–5695. [PubMed] [Google Scholar]
- Austin F. E., Turco J., Winkler H. H. Rickettsia prowazekii requires host cell serine and glycine for growth. Infect Immun. 1987 Jan;55(1):240–244. doi: 10.1128/iai.55.1.240-244.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin F. E., Winkler H. H. Proline incorporation into protein by Rickettsia prowazekii during growth in Chinese hamster ovary (CHO-K1) cells. Infect Immun. 1988 Dec;56(12):3167–3172. doi: 10.1128/iai.56.12.3167-3172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R. Phosphorylation accompanying the oxidation of glutamate by the Madrid E strain of typhus rickettsiae. J Biol Chem. 1956 May;220(1):353–361. [PubMed] [Google Scholar]
- Boeker E. A., Fischer E. H., Snell E. E. Arginine decarboxylase from Escherichia coli. 3. Subunit structure. J Biol Chem. 1969 Oct 10;244(19):5239–5245. [PubMed] [Google Scholar]
- Coolbaugh J. C., Progar J. J., Weiss E. Enzymatic activities of cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Jul;14(1):298–305. doi: 10.1128/iai.14.1.298-305.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- HAYES J. E., HAHN F. E., COHN Z. A., JACKSON E. B., SMADEL J. E. Metabolic studies of rickettsiae. IV. Terminal respiratory enzymes in Rickettsia mooseri. Biochim Biophys Acta. 1957 Dec;26(3):570–576. doi: 10.1016/0006-3002(57)90104-x. [DOI] [PubMed] [Google Scholar]
- Kabus P., Koch G. Quantitative determination of amino acids in tissue culture cells by high performance liquid chromatography. Biochem Biophys Res Commun. 1982 Sep 30;108(2):783–790. doi: 10.1016/0006-291x(82)90897-x. [DOI] [PubMed] [Google Scholar]
- Kallio A., McCann P. P., Bey P. DL-alpha-(Difluoromethyl)arginine: a potent enzyme-activated irreversible inhibitor of bacterial decarboxylases. Biochemistry. 1981 May 26;20(11):3163–3168. doi: 10.1021/bi00514a027. [DOI] [PubMed] [Google Scholar]
- Keysary A., McCaul T. F., Winkler H. H. Roles of the Fc receptor and respiratory burst in killing of Rickettsia prowazekii by macrophagelike cell lines. Infect Immun. 1989 Aug;57(8):2390–2396. doi: 10.1128/iai.57.8.2390-2396.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- Slocum R. D., Flores H. E., Galston A. W., Weinstein L. H. Improved method for HPLC analysis of polyamines, agmatine and aromatic monoamines in plant tissue. Plant Physiol. 1989;89:512–517. doi: 10.1104/pp.89.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglich C., Grens A., Scheffler I. E. Chinese hamster cells deficient in ornithine decarboxylase activity: reversion by gene amplification and by azacytidine treatment. Somat Cell Mol Genet. 1985 Jan;11(1):11–23. doi: 10.1007/BF01534730. [DOI] [PubMed] [Google Scholar]
- Steglich C., Scheffler I. E. An ornithine decarboxylase-deficient mutant of Chinese hamster ovary cells. J Biol Chem. 1982 Apr 25;257(8):4603–4609. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Rickettsial hemolysis: rapid method for enumeration of metabolically active typhus rickettsiae. J Clin Microbiol. 1979 May;9(5):645–647. doi: 10.1128/jcm.9.5.645-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Dobson M. E., Dasch G. A. Biochemistry of rickettsiae: recent advances. Acta Virol. 1987 May;31(3):271–286. [PubMed] [Google Scholar]
- Winkler H. H., Daugherty R. M. Proline transport and metabolism in Rickettsia prowazekii. J Bacteriol. 1984 May;158(2):460–463. doi: 10.1128/jb.158.2.460-463.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Inhibitory and restorative effects of adenine nucleotides on rickettsial adsorption and hemolysis. Infect Immun. 1974 Jan;9(1):119–126. doi: 10.1128/iai.9.1.119-126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J Biol Chem. 1973 Mar 10;248(5):1687–1695. [PubMed] [Google Scholar]
- Young D. V., Srinivasan P. R. Regulation of macromolecular synthesis by putrescine in a conditional Escherichia coli putrescine auxotroph. J Bacteriol. 1972 Oct;112(1):30–39. doi: 10.1128/jb.112.1.30-39.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



