Abstract
Purpose
To investigate the effect of the Rho-kinase inhibitor, Y27632, on pig corneal endothelial cell (pCEC) culture, and on inflammation and immune regulation of the responses of human cells to pCECs.
Methods
pCECs were cultured with/without Y27632 to assess cell proliferation and in vitro wound healing assay. The level of MCP-1 and VEGF in pCECs stimulated with human TNF-α were measured. Proliferation of human PBMCs stimulated with pCECs, and cytokine production in human T cells, and monocyte migration after stimulation were investigated.
Results
Y27632 promoted pCEC proliferation, prevented pCEC death, and enhanced in vitro wound healing. After stimulation, there were significantly lower levels of MCP-1 and VEGF measured in pCECs cultured with Y27632, and significantly reduced human PBMC proliferation, cytokine production, and monocyte migration.
Conclusions
The application of the Rho-kinase inhibitor will be beneficial when culturing pCECs, and may provide a novel therapy to reduce inflammation after corneal xenotransplantation.
Keywords: Corneal endothelial cell, Immune response, Inflammation, Pig, Rho kinase, Xenotransplantation
INTRODUCTION
Corneal endothelial cells (CECs) are essential for the maintenance of corneal transparency. Human (h) CECs do not proliferate sufficiently in vivo 1. Conventional penetrating keratoplasty for corneal endothelial dysfunction is rapidly being replaced by newer forms of selective endothelial keratoplasty 2, which provide improved visual outcome, higher graft survival rate, and fewer post-operative complications 3, 4. Since hCECs have been shown to possess proliferative capacity in vitro 5, the replacement of corneal endothelium with cultured hCECs might provide a major advance in the treatment of corneal blindness 6–9. However, (i) primary culture of hCECs is difficult, and a change in the morphology to a fibroblastic-like state cannot always be prevented, (ii) the proliferative capacity of hCECs from older donors is questionable 10, 11, (iii) there is the potential for immunological rejection and/or non-immunological CEC damage, particularly in high-risk patients (highly-sensitized to donor HLA, or with an inflamed/vascularized corneal bed), and (iv) the worldwide need for donor corneas far exceeds supply 12.
The pig (p) might provide an alternative source 12–15. The immune-privileged environment of the cornea may provide a corneal xenograft with some degree of protection 13, and encouraging results of wild-type pig-to-nonhuman primate corneal xenotransplantation suggest that pig corneas may provide an acceptable alternative to deceased human corneas for clinical transplantation 16–18. Furthermore, human humoral and cellular immune responses to corneas from genetically-engineered pigs are significantly reduced compared to those to wild-type pig corneas 19. However, pCECs also do not regenerate in vivo 20, but do proliferate in vitro 11. Cultured pCECs may be an alternative for replacing damaged corneal endothelium 11.
A Rho-kinase inhibitor has been widely used for several types of cell culture, including rabbit 21, monkey 22, and human 23 CECs, and human embryonic and induced pluripotent stem cells 24, but there have been no reports on its effect on pCECs. The inhibition of the Rho/Rho kinase signaling pathway by a specific Rho kinase inhibitor, Y27632, can significantly promote cell adhesion and proliferation, and prevent apoptosis during in vitro CEC culture 25, indicating its potential for CEC culture. Y27632 enhanced corneal endothelial wound healing in vivo in rabbit and monkey models in which the corneal endothelium was partially damaged 25. Furthermore, the administration of a Rho kinase inhibitor to the eye has been considered as a treatment for Fuchs’ endothelial dystrophy 26 and glaucoma 27, 28.
Increased Rho/Rho-kinase activity has been demonstrated in inflammatory diseases 29–31, and the expression and function of the Rho/Rho-kinase signal pathway are up-regulated by inflammatory stimuli in vascular endothelial cells, coronary vascular smooth muscle cells, mesangial cells, and monocytes 29, 32–39.
To our knowledge, however, the role of Rho kinase in inflammation has not been investigated in CECs. We speculated that, as well as promoting CEC proliferation in in vitro culture, Rho kinase inhibition could have an anti-inflammatory effect on CECs after stimulation. The aim of the present study was to investigate in vitro the effect of Y27632 on (i) pCEC culture with regard to cell adhesion, proliferation, and cell viability, and (ii) the modulation of inflammation and the human immune response to pCECs.
MATERIALS AND METHODS
Sources of pig corneas
Corneas were excised from 2 to 6 month-old wild-type pigs (Wally Whippo, Enon Vally, PA). All animal care procedures were in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985).
Sources of human blood and peripheral blood mononuclear cells
Human blood was obtained from 4 healthy human volunteers (including all ABO blood types). Participants gave informed consent per the guidelines of the Institutional Review Board of the University of Pittsburgh. Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats obtained from human donors (Institute for Transfusion Medicine, Pittsburgh, PA), as previously described 19.
Corneal cell cultures
pCECs were cultured, as previously described 11. pCECs between passage 2 to 4 were used in all experiments. Subconfluent pCECs were activated by recombinant pIFN-γ (50ng/mL, R&D system, Minneapolis, MN) or hTNF-α (50ng/mL, Serotec, Raleigh, NC) for adequate periods depending on the objectives of the study. Supernatants obtained from pCECs culture with/without Y27632 (Sigma-Aldrich, St. Louis, MO) 6 hours after hTNF-α stimulation were collected for quantification of monocyte chemotactic protein-1 (MCP-1), and monocyte migration assay.
Cell count assay
Cell count assays were carried out, as previously described 11. Briefly, pCECs were seeded at 5,000 cells in 12-well tissue culture plates (BD Biosciences, San Jose, CA) and cultured for 15d +/− 10μM Y27632. On each day the mean of triplicate results was expressed as a proliferation curve.
Apoptosis assay
In order to determine the effect of Y27632 on pCEC viability, apoptotic/necrotic cells were detected by flow cytometry using Annexin V apoptosis detection kit I (BD), as previously described 11.
In vitro wound distance measuring
pCECs were seeded in 6-well tissue culture plates +/− 10μM Y27632 and cultured until 100% confluent. A linear defect was made in the confluent CECs with a plastic pipette tip (Fisher Scientific, Waltham, MA). pCECs in suspension were washed off with PBS (Invitrogen, Carlsbad, CA), and the culture was continued in medium +/− 10μM Y27632. The wound defect, i.e., the distance between the cells at one edge of the linear defect and those at the opposite edge was measured under the microscope (Nikon, Elgin, IL) using SPOT software 5.1 (SPOT Imaging Solution, Sterling Heights, MI) after 0, 8, 16, and 24h of incubation. The mean of triplicate results was used for analysis.
Bromodeoxyuridine (BrdU) staining
pCECs were cultured with or without 10μM Y27632 until confluent in a 6-well tissue culture plate. A linear defect in the confluent CECs was created with a plastic pipette tip. CECs in suspension were washed off with PBS, and maintained for 2h +/− 10μM Y27632. pCECs were incubated for a further 16h with 10μM BrdU and then stained with anti-BrdU antibody using a BrdU IHC kit (Abcam, Cambridge, MA), according to the manufacturer’s instructions. After fixation with 70% ethanol (Pharmco, Brookfield, CT), the samples were incubated with quenching solution, denaturing solution, and blocking buffer (Abcam). The samples were stained with anti-BrdU antibody (sheep-derived polyclonal, IgG isotype, Abcam) for 1h at room temperature, and counter-stained with hematoxylin. BrdU-positive and negative cells were manually counted in several areas, and the percentage of BrdU-positive cells was compared.
Real-time polymerase chain reaction (PCR)
Total RNA was extracted from non-activated and hTNF-α-activated cultured pCECs +/− Y27632 using Trizol (Invitrogen), and mRNA was reverse-transcribed into cDNA using a cDNA reverse transcription kit (Applied Biosystems, Foster, CA), as previously described 19. cDNA samples were amplified by real-time PCR using Applied Biosystems 7500 (Life Technologies, Grand Island, NY), as previously described 40.
The mRNA level of MCP-1 and vascular endothelial growth factor (VEGF) were quantified in triplicate using SYBR Green (Life Technology), real-time PCR under the following conditions: denaturation at 60°C for 1min, followed by 40 PCR cycles, each cycle consisting of 95°C for 15s, 60°C for 1min. The PCR primers sequences used were as follows:-
pMCP-1: Sense 5′- CTC CCA CAC CGA AGC TTG AA -3′; Antisense 5′- TAA TTG CAT CTG GCT GGG CA -3′,
pVEGF: Sense 5′- CTT GCC TTG CTG CTC TAC CT -3′; Antisense 5′- GTC CAC CAG GGT CTC GAT TG -3′,
pGAPDH: Sense 5′- CGA TGG TGA AGG TCG GAG TG -3′; Antisense 5′- TGC CGT GGG TGG AAT CAT AC -3′.
pMCP-1 and pVCAM-1 mRNA expressions were calculated with pGAPDH as an internal control, and the relative results were expressed as 2−ΔCt.
Quantification of pMCP-1 protein
Supernatants obtained from pCECs culture with/without Y27632 after hTNF-α stimulation were collected to measure the concentration of pMCP-1 protein by an enzyme-linked immunosorbent assay (ELISA) (Kingfisher Biotech, St. Paul, MN) according to the manufacturer’s protocol. All samples were tested in triplicate and standard curves were generated for calculating the concentration in each assay.
Monocyte migration assay
The pMCP-1 secreted by activated pCECs attracts human monocytes. The number of migrated monocytes was assessed using a 96-well monocyte cell migration assay (EMD Millipore, San Diego, CA) based on the Boyen-chamber principle. Human monocytes were isolated from PBMCs, using monocyte isolation kit II (Miltenyl Biotec, Auburn, CA) as previously described 41. Supernatants obtained from pCECs culture with/without Y27632 after hTNF-α stimulation were collected, and used in the monocytes migration assay. Medium containing exogenous pMCP-1 (100ng/ml) (Abcam) was used as a positive control. Cultured supernatants or control medium were added to the lower chamber, and isolated human monocytes (100,000/well) were placed in the upper chamber, which contained a polycarbonate membrane with a 5μM pore size. After 3 hours of incubation, the cells that had migrated to the lower chamber were collected, and labelled with Calcein-AM. The Calcein-AM-labelled migrated monocytes were measured using a fluorescence plate reader (1420 Multilabel Counter, PerkinElmer, Waltham, MA) at an excitation wavelength of 485nm and an emission wavelength of 535nm.
Intracellular cytokine staining
Two hundred microliters of human whole blood was incubated with Golgistop (BD), 10ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO), and 1μg/ml Ionomycin (Sigma-Aldrich) for 3h at 37°C +/− several concentration of Y27632. After washing with the lysing buffer (BD) followed by PBS, live/dead staining was carried out using a live/dead fixable staining kit (Invitrogen), according to the manufacturer’s instructions. Surface staining was carried out using anti-human CD3 (BD, clone SP34-2), CD4 (BD, clone L200), and CD8 (BD, clone RPA-T8) antibodies, as previously described 19. Intracellular cytokine staining was carried out using a Cytofix/Cytoperm plus Fixation/Permeabilization Kit (BD), according to the manufacturer’s instructions. Briefly, samples were fixed and permeabilized with Cytofix/Cytoperm buffer for 20min at 4°C. Cells were washed with Perm/wash buffer. Intracellular cytokines were stained with anti-human IFN-γ (BD, clone 4S.B3), and TNF-α (iCyt, clone MAb11) antibodies. Surface and intracellular cytokine expression was detected by BD™ LSR II flow cytometer (BD). Data was analyzed using FlowJo software (Treestar Inc. Ashland, OR).
Rho/Rho kinase activity
Rho activity was measured using an active Rho pull-down assay kit (Thermo Scientific, Waltham, MA), according to the manufacturer’s instructions. Briefly, human PBMCs were incubated with 10μg/ml PHA +/− Y27632. The cells were homogenized in ice-cold lysis buffer (Thermo Scientific), centrifuged, then the supernatant was collected and it was incubated for 1h at 4°C with rhotekin agarose (Thermo Scientific) to precipitate guanosine-5′-triphosphate (GTP)-bound Rho. Precipitated samples were washed and eluted with sample buffer for Western blot. They were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot using monoclonal antibody against RhoA at a dilution of 1:670 for the primary (rabbit IgG anti-Rho antibody, Thermo Scientific) and 1:500 for the secondary antibody (stabilized peroxidase conjugated goat anti-rabbit antibody, Thermo Scientific). Myosin phosphatase target subunit 1-phosphorylation (p-MYPT1) was used as a surrogate marker for Rho-kinase activity. It was used at a dilution of 1:500 for the primary (goat polyclonal IgG anti p-MYPT1 [Thr 853], Santa Cruz, Dallas, Tx) and 1:4000 for the secondary (Donkey anti-goat IgG-HRP, Santa Cruz) antibody.
As a control, β-actin was used at a dilution of 1:500 for the primary (Mouse monoclonal IgG, Santa Cruz) and 1:4000 for the secondary (Santa Cruz) antibody.
Determination of nuclear factor-kappa B (NF-κB) p65 phosphorylation in pCECs
Phosphorylation of NF-κB p65 at serine 536 in pCECs after activation was investigated by flow cytometry as previously described 42. Briefly, pCECs were harvested 5 minutes after stimulation with hTNF-α, fixed, permeabilized, and incubated successively with anti-phospho NF-κB p65 antibody (Cell Signaling, Danvers, MA), followed by staining with phycoerythrin-conjugated anti-rabbit IgG antibody (Cell Signaling). Rabbit IgG antibody (isotype) (Cell Signaling) was used as a negative control.
Human PBMC stimulation assay
hPBMCs were isolated for the Rho/Rho kinase activity assessment and stimulation assay. Isolated PBMCs were used as responders, and co-cultured with 10μg/ml phytohaemagglutinin (PHA) (Roche, Basel, Switzerland) or irradiated (2,800cGy) pCECs as stimulators (at responder-stimulator ratios of 10:1) +/− several concentrations of Y27632. pCECs as stimulators were activated with 50μg/ml pIFN-γ for 48h prior to use. hPBMCs were cultured with PHA for 3 days or activated pCECs for 6 days at 37°C in 5% CO2. The mean of triplicate results of cell proliferation was expressed as 3H-thymidine incorporation, as previously described 19.
5-(and 6)-carboxyfluorescein diacetate succinimidyl ester-mixed lymphocyte reaction (CFSE-MLR)
CFSE-MLR was performed as previously described 43. CFSE-labeled hPBMCs were cultured with PHA or activated pCECs for 6 days +/− several concentrations of Y27632. Cells were stained with anti-human CD3, CD4 and CD8 mAbs. Flow cytometry analysis was performed 43.
Statistical methods
The statistical significance of differences was determined by Student’s t or nonparametric tests with Dunnett post-test, as appropriate, using GraphPad Prism version 4 (GraphPad Software, San Diego, CA). Values are presented as mean ± SD. Differences were considered to be significant at p<0.05.
RESULTS
Y27632 enhanced cell proliferation and inhibited apoptosis/necrosis of pCECs in culture
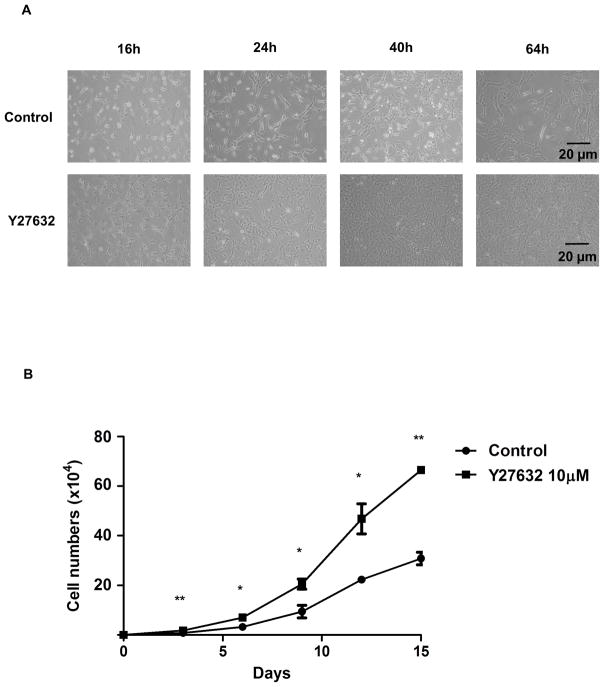
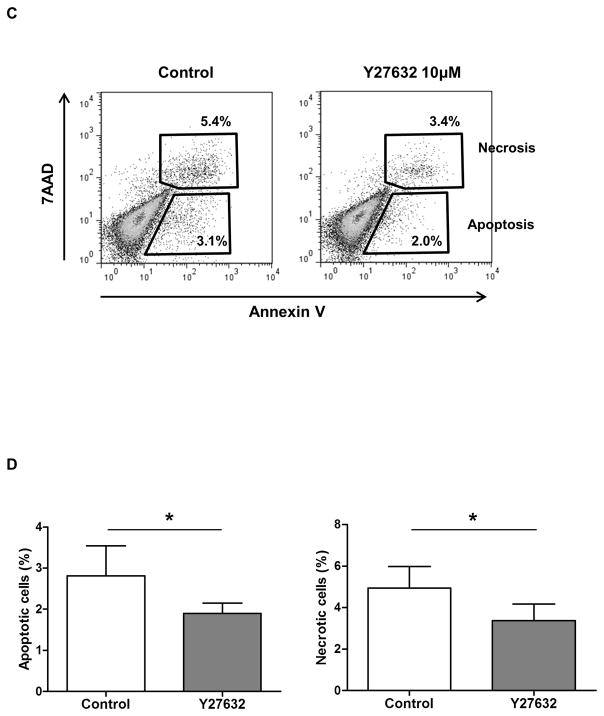
When pCECs were observed visually at several time-points (16, 24, 40, and 64h) under the microscope, Y27632 increased initial attachment of pCECs to the plates, increased pCEC proliferation, and maintained polygonal-shaped morphology (Figure 1A). These results were confirmed by cell count assay, which showed significantly higher (x2-3) cell numbers compared to control (Figure 1B). The cell densities were 1,100/mm2 without Y27632 and 2,373/mm2 with Y27632 at day 15. These results indicated that Y27632 improved culture and promoted in vitro proliferation of pCECs, as it does for CECs from rabbits and monkeys 21, 22. When the Annexin V/7AAD staining was used to detect apoptosis/cell death by flow cytometry (Figure 1C), a significantly lower percentage of apoptosis was found in pCECs in the presence of Y27632 compared to control (p<0.05) (Figure 1D).
Figure 1. Enhanced cell proliferation and Inhibition of apoptosis/necrosis of pCECs in the presence of the Rho kinase inhibitor Y27632.
(A) Representative figures of cultured pCECs after being seeded on the plate with/without Rho kinase inhibitor. pCECs were cultured +/− 10μM Y27632, and observed at several time-points (16, 24, 40, and 64h) under the microscope. Compared to control cells, pCECs cultured with Y27632 enhanced initial attachment to the plates, proliferation, and maintained a polygonal-shaped phenotype for 64h. (B) The proliferative capacity of pCECs +/− 10μM Y27632 was compared by direct cell counting for 15 days. Cell numbers were significantly higher at all time-points (2-fold on days 3, 6, 9, 15, and 3-fold on day 12, *p<0.05, **p<0.01). Data are expressed as mean ± SD (n=3). (C) Representative figures of Annexin V/7AAD staining of pCECs (after 70–80% confluence) with/without 10μM Y27632. Annexin V-negative/7AAD-negative cells were identified as live cells. Annexin V-positive/7AAD-negative cells were identified as apoptotic, whereas Annexin V-positive/7AAD-positive cells were identified as necrotic or dead. (D) During culture, there were significantly lower percentages of apoptosis (*p<0.05) and dead cells (*p<0.05) in pCECs in the presence of 10μM Y27632 compared to control. Data expressed as the mean ± SD (n=3).
Y27632 accelerated wound healing of pCECs during culture
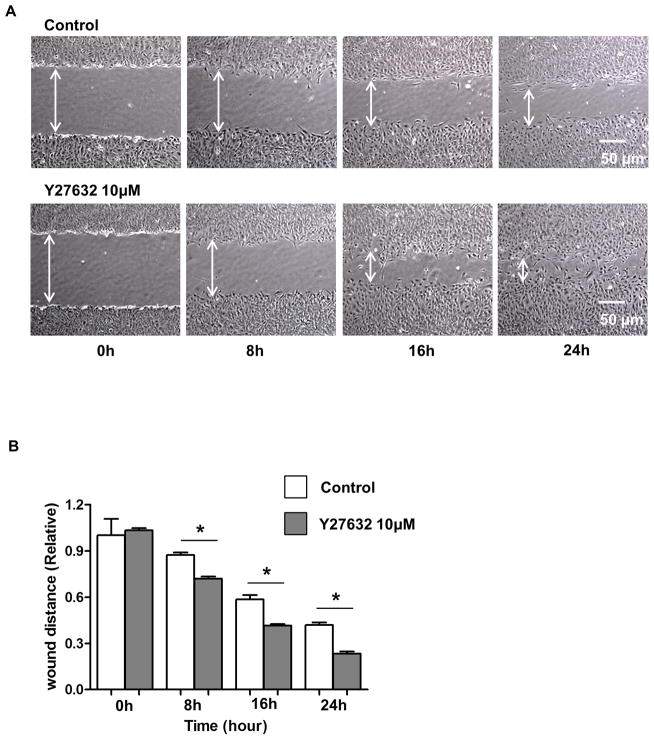
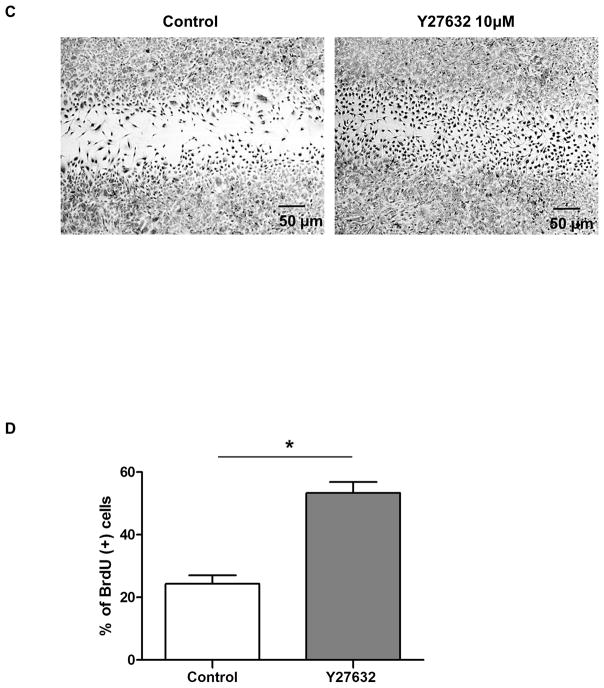
To determine the role of Y27632 in pCEC wound healing, a linear defect was created in confluent pCECs with a sterile pipette tip. pCECs were then cultured +/− 10μM Y27632. The defect was measured at several time-points (0, 8, 16, and 24h) (Figure 2A). Compared to controls, the defect was significantly reduced in the Y27632-treated group at all time-points (87% vs. 72%, 59% vs. 41%, 42% vs. 23%, respectively; as a ratio of the initial width of the defect, p<0.01) (Figure 2B). To confirm that the enhanced pCEC wound healing was not associated with pCECs spreading in the region of the defect rather than from pCEC proliferation, BrdU staining was carried out (Figure 2C, D). When a defect was created in confluent cells, the cells became positive for BrdU, especially in the region of the defect (Figure 2C). In the presence of Y27632, a significantly higher % of BrdU-positive cells were found in the region of the defect compared to the control group (p<0.01) (Figure 2D). Once the pCECs became confluent, proliferation stopped and nearly all of the cells became negative for BrdU. There was no significant difference in the number of BrdU-positive cells between the control and Y27632-treated groups (data not shown). These results indicated that Y27632 promoted pCEC wound healing by promoting cell proliferation.
Figure 2. Enhanced proliferation resulted in accelerated healing of cultured pCECs with Y27632 in vitro.
(A) Representative figures of in vitro healing in pCECs +/− 10μM Y27632. A linear defect was created with a pipette tip in confluent pCECs. The healing process was observed at several time-points (0, 8, 16, and 24h) under the microscope. Compared to control, pCECs cultured with Y27632 demonstrated accelerated healing. (B) The defect was significantly reduced in the Y27632 group at all time-points (87% vs. 72%, 59% vs. 41%, 42% vs. 23%, respectively, as a ratio of the width of the initial defect, *p<0.01). Data expressed as mean ± SD (n= 3). (C) Representative figures of BrdU-staining of in vitro wound healing in pCECs +/− 10μM Y27632. BrdU stained nuclei of proliferating cells (Black nuclei) and majority of the BrdU-positive cells were located near the defect. There were more BrdU-positive cells in the area of the defect and a reduced wound width distance was observed in the Y27632-treated group. (D) The BrdU-positive cells were counted manually in randomly-chosen same-sized squares. Y27632 significantly increased the % of BrdU-positive pCECs compared to control (*p<0.01). Data expressed as mean ± SD (n=3).
The effect of Y27632 on reducing inflammatory response to stimulated pCECs
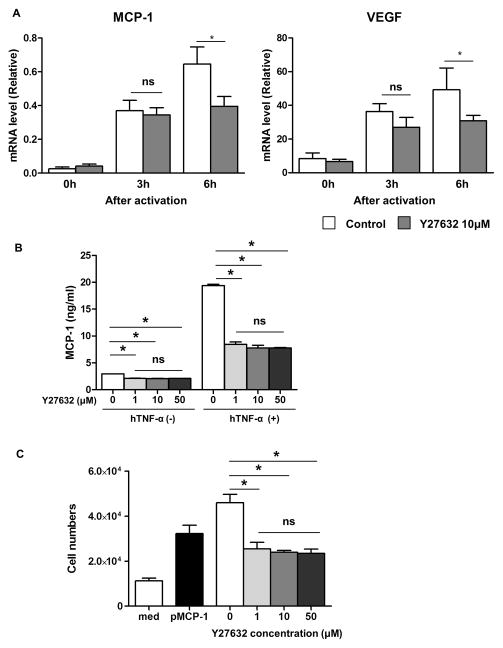
TNF-α is a potent inflammatory mediator, which increases monocyte chemotactic protein-1 (MCP-1) (which induces macrophage infiltration) and vascular endothelial growth factor (VEGF) (which stimulates angiogenesis) leading to neovascularization in the cornea during inflammation 44. Since these molecules have an essential role in corneal graft rejection 13, 45, 46, suppression of them is important in preventing rejection. It is likely that these two molecules are produced by both host (e.g., corneal stroma) and donor (e.g., transplanted graft) cells following endothelial cell transplantation. Therefore, it would be important to investigate MCP-1 and VEGF in pCECs following stimulation. There were significant increases in both MCP-1 and VEGF in pCECs after stimulation with hTNF-α in a dose- and time-dependent manner (data not shown). However, compared to controls, culture with 10μM Y27632 significantly reduced both MCP-1 and VEGF mRNA expression in pCECs 6h after activation (43% and 37% reduction, respectively, p<0.05) (Figure 3A). These results indicated that Y27632 might reduce the inflammatory response induced by hTNF-α in pCECs. In addition to mRNA, MCP-1 protein concentration in the pCECs culture supernatant was also significantly decreased with Y27632 in the absence and presence of hTNF-α stimulation (Figure 3B). The higher concentration of Y27632 did not further suppress the MCP-1 protein concentration. In order to confirm whether secreted MCP-1 from pCECs could attract human monocytes, a monocyte migration assay was performed. Correlating with the lower level of pMCP-1, the migrated monocytes were significantly decreased (by more than >50%) when monocytes were incubated with culture medium obtained from activated pCECs in the presence of Y27632 compared to that in the absence of Y27632 (Figure 3C).
Figure 3. Suppression of inflammatory chemokines from pCECs.
(A) MCP-1 and VEGF mRNA levels in pCECs with/without 10μM Y27632 were measured by real-time PCR after hTNF-α activation. Although there were increases in MCP-1 and VEGF mRNA in pCECs after activation, Y27632 significantly reduced MCP-1 and VEGF mRNA expression after 6h of activation (43% and 37% reduction respectively, *p<0.05, ns = not significant, n = 4). (B) MCP-1 protein in the pCEC culture supernatant with/without several concentration of Y27632 was measured by ELISA, with/without hTNF-α activation. Y27632 significantly suppressed MCP-1 production from pCECs with/without hTNF-α activation, however there was no differences in the Y27632 group with different concentrations (data was triplicated and from at least 3 independent experiments, *p<0.05). (C) MCP-1 secreted from activated pCEC attracts human monocytes through the 5μM sized-pores. In the presence of Y27632, the migrated monocytes were significantly reduced. However there was no difference in the Y27632 group with different concentrations. (*p<0.05, n=4)
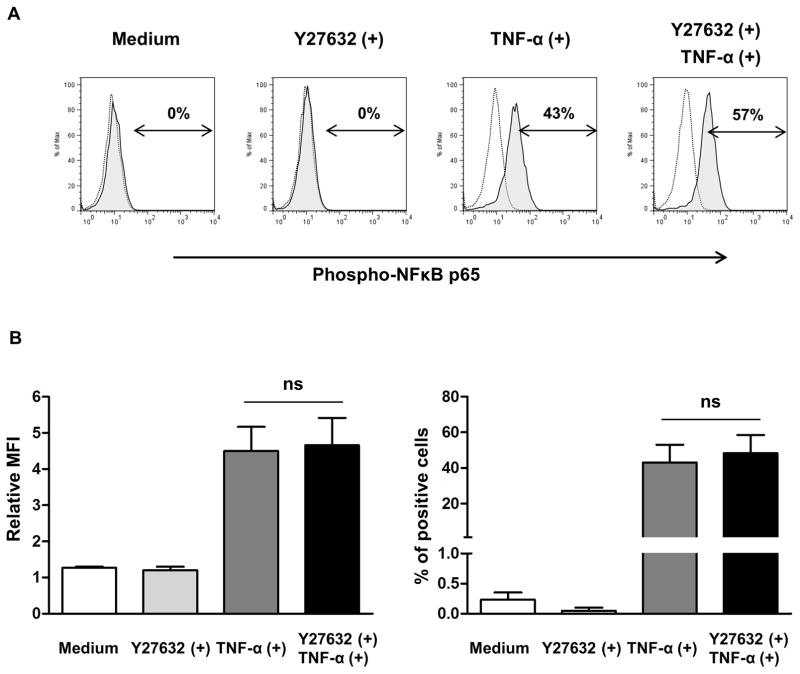
Y27632 did not inhibit the phosphorylation of NF-κB p65 in pCECs
To investigate the suppressive mechanism of MCP expression by Y27632, phosphorylation of NF-κB p65 in pCECs after stimulation with hTNF-α was measured by flow cytometry (Figure 4). Although there was no detectable phosphorylation of NF-κB p65 in nonactivated cells with/without Y27632, phosphorylation of NF-κB p65 were increased after stimulation with hTNF-α (Figure 4A). However, Y27632 did not significantly inhibit the phosphorylation of NF-κB p65 in pCECs after stimulation with hTNF-α compared to control pCECs without Y27632 (Figure 4B).
Figure 4. Phosphorylation of NF-κB p65 in pCECs after stimulation with hTNF-α.
(A) Determination of phosphorylated NF-κB p65 in pCECs by flow cytometry. pCECs were cultured with/without Y27632, and then stimulated with hTNF-α for 5 minutes. Black dotted lines show staining with isotype control. Shaded areas show staining with anti-phosphorylation of NF-κB p65 antibody. Data are representative of results of three independent experiments. (B) There was no significant difference in relative MFI and % phosphorylated NF-κB p65 positive cells between pCECs cultured with/without Y27632. Data expressed as the mean ± SD (n=3). (ns = not significant vs. without Y27632)
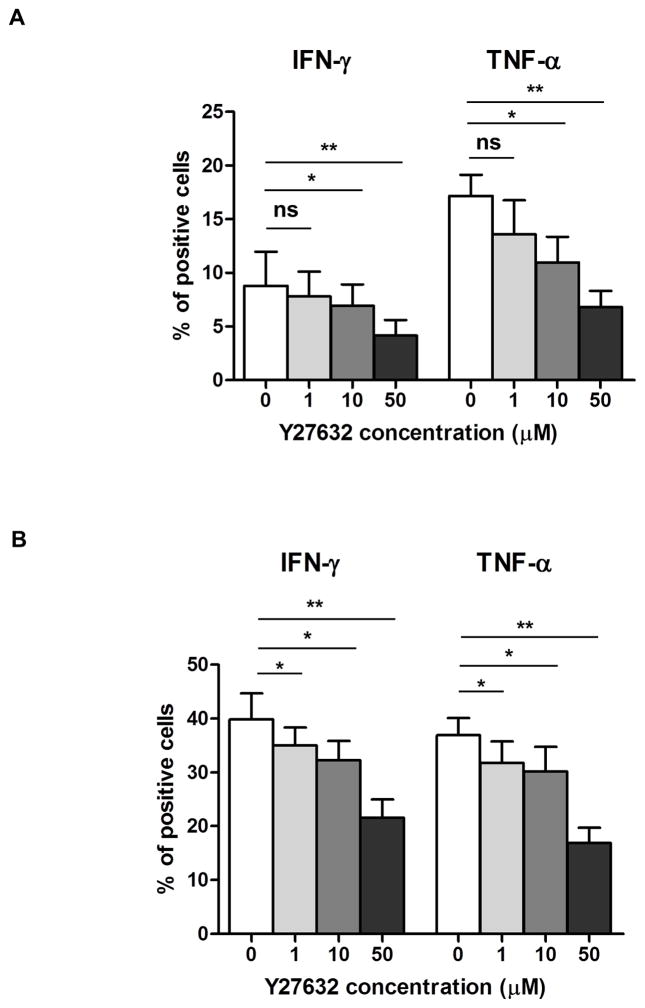
Y27632 inhibited inflammatory cytokine production by human T cells
T cells are the predominant cells in corneal graft rejection, and produce inflammatory cytokines, e.g., IFN-γ and TNF-α 47. To investigate the effect of Y27632 on the inhibition of inflammatory cytokines produced by T cells, human whole blood was stimulated with PMA in the presence of ionomycin +/− Y27632. Production of inflammatory cytokines was assessed by intracellular cytokine staining. In the presence of Y27632, there were significant dose-dependent decreases in IFN-γ and TNF-α secretion in CD4+ (Figure 5A) and CD8+ (Figure 5B) T cells compared to controls (10μM p<0.05; 50μM p<0.01).
Figure 5. Suppression of inflammatory cytokines from human whole blood.
Inflammatory cytokines, IFN-γ and TNF-α, were measured by intracellular staining from human whole blood after activation with PMA-Ionomycin in the presence or absence of Y27632 at several concentrations. In the presence of Y27632, cytokine levels from (A) CD4+ and (B) CD8+ T cells were significantly decreased. Data expressed as mean ± SD (*p<0.05, **p<0.01, ns = not significant, n=3).
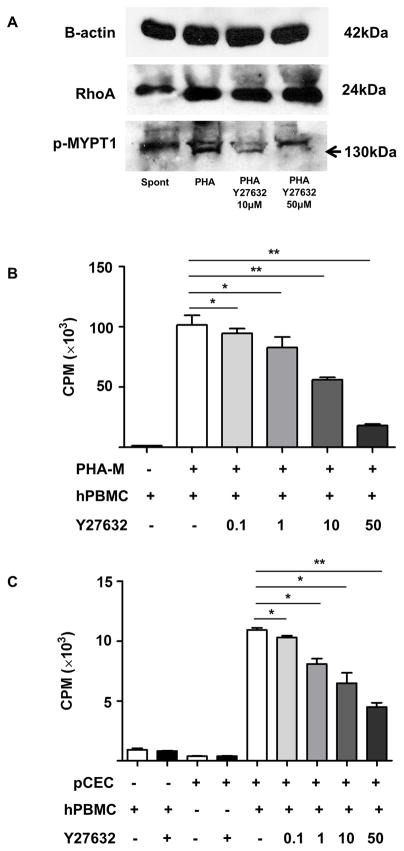
Y27632 suppressed T cell proliferation
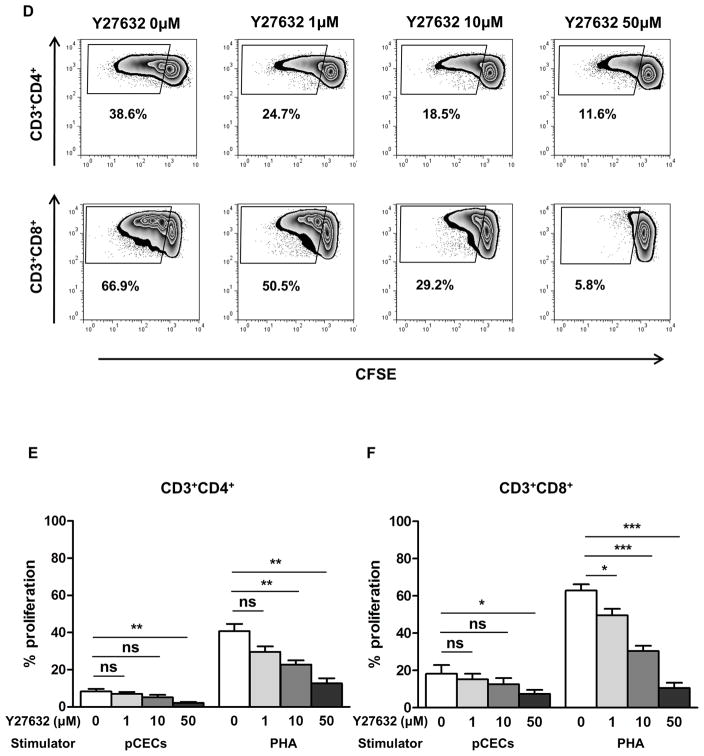
To determine whether the Rho/Rho kinase pathway stimulates T cell proliferation and its inhibition can suppress the human cellular immune response, we first tested an active Rho pull-down assay and western blot with p-MYPT1 antibody. MYPT1 is downstream of the Rho pathway and it is phosphorylated by the Rho kinase. Stimulation of hPBMCs by PHA increased activation of the Rho/Rho kinase cascade (confirmed by an increase in the intensity of both active RhoA and p-MYPT1 [Figure 6A]). In the presence of Y27632, the intensity of p-MYPT1 was decreased (Figure 6A), cell proliferation after stimulation with PHA was significantly reduced in a dose-dependent manner (10 and 50μM Y27632, p<0.01) (Figure 6B). Y27632 significantly attenuated cell proliferation when hPBMCs were co-cultured with pCECs in a dose-dependent manner (10μM p<0.05; 50μM p<0.01) (Figure 6C). Y27632 had no effect on baseline proliferation or viability of cells, confirmed by Annexin V/7AAD staining (data not shown). To further investigate the proliferative T cell subsets, CFSE-MLR was performed (Figure 6D). After co-culture with either pCECs or PHA, proliferation of both CD3+CD4+ T cell (Figure 6D, E) and CD3+CD8+ T cell (Figure 6D, F) was significantly reduced by Y27632 in a dose-dependent manner. These results indicated that the Rho kinase pathway plays a role in the T cell immune response and that its inhibition suppresses this response.
Figure 6. Y27632 significantly suppresses proliferation of hPBMCs.
(A) RhoA (upstream of Rho kinase) was up-regulated in hPBMCs activated by PHA. Phosphorylated myosin phosphatase target subunit 1 (p-MYPT1, 130kDa), which is downstream of Rho kinase was also up-regulated by PHA stimulation, and dose-dependently down-regulated by 10 and 50μM of Y27632. (B) Y27632 significantly reduced proliferation of hPBMCs stimulated with PHA after 72h in a dose-dependent manner (*p<0.05, **p<0.01 vs. PHA alone; n=3). (C) Y27632 significantly reduced proliferation of hPBMC stimulated by activated pCECs after 6 days in a dose-dependent manner (*p<0.05, **p<0.01 vs. without Y27632; n=3). Results are expressed as 3H-thymidine incorporation (counts per minute). (D) Representative figures of CFSE staining of CD3+CD4+ (upper row) and CD3+CD8+ (lower row) T cells after co-culture with activated pCECs with/without several concentration of Y27632. Proliferating cells decrease in a dose-dependent manner. (E) CD3+CD4+ T cell proliferation was significantly suppressed in a dose dependent manner in a presence of Y27632, after co-culture with activated pCECs or PHA. (F) CD3+CD8+ T cell proliferation was significantly suppressed in a dose dependent manner in a presence of Y27632, after co-culture with activated pCECs or PHA. (*p<0.05, **p<0.01, ***p<0.001, ns=not significant vs. without Y27632; n=4).
DISCUSSION
hCECs are considered to be nonproliferative in vivo because they are arrested in the G1-phase of the cell cycle 5. Several factors are involved in anti-proliferative mechanisms 5, 48, including (i) the presence of transforming growth factor- β2 in the aqueous humor that suppresses S phase entry, possibly involving its effect on p27Kip1 and prostaglandin E2, (ii) inhibition of cell-cell contact, possibly mediated by the cyclin-dependent kinase inhibitor p27Kip1, (iii) deficiency or absence of positive growth factor stimulation, and (iv) age-related increased expression of G1-phase inhibitors (cyclin-dependent kinase inhibitor p21Cip1, and p16INK4a) that leads the cells to stress-induced premature senescence. In contrast, hCECs can be cultured and grown successfully in vitro, indicating that they retain proliferative capacity.
The replacement of damaged corneal endothelium with cultured hCECs has been considered as an alternative method for treatment of corneal blindness 3, 9, 25. Several studies in animal models have demonstrated the possibility of transplanting them as a cellular sheet with a carrier 8 or by injecting CECs directly into the anterior chamber 25. However, difficulties in primary hCEC culture have been reported with a change in the morphology to a fibroblastic-like phenotype 23 and less proliferative capacity, especially in hCECs from older donors 10, 11.
The Rho kinase pathway is involved in regulating the actin cytoskeleton, cell migration, apoptosis, and proliferation 49–51. In addition, Rho GTPases play a critical role in suppressing cell-cycle progression 49, 51. Several studies have demonstrated that inhibition of Rho/Rho kinase signaling by the specific Rho kinase inhibitor, Y27632, promotes CEC proliferation and wound healing in vitro 22 and in vivo 25, 52, 53. Furthermore, dissociation-induced apoptosis of CECs could be inhibited 22. Okumura et al. demonstrated the molecular mechanism of Y27632 on cell proliferation using monkey CECs. Y27632 facilitates degradation of p27Kip1 (negative G1 regulator) and promotes the expression of cyclin D (positive G1 regulator) via phosphatidylinositol 3-kinase signaling 54. Both activities are required for G1/S progression, resulting in promoting CEC proliferation.
Further studies showed that modulation of cell adhesion by the Rho kinase inhibitor enhances CEC engraftment in a primate model of endothelial dysfunction 21. In contrast, Pipparelli et al. recently reported that Y27632 had no effect on the proliferative capacity of hCECs although it increased cell adhesion and enhanced wound healing ex vivo and in vitro 23. This suggests that the action of the inhibitor is related to activation of the cell cycle in terms of induction of proliferation; this might be different in humans from animal models. However, in Pipparelli’s study, hCECs from older donors (mean age 73y) were used, whereas experimental animals are generally younger and correspond to younger donors (e.g., most models used 3–5y-old primates, equivalent to a human age of 5–20y) 25. In contrast, Okumura et al., recently showed that Rho-kinase inhibitor enhanced hCECs proliferation, although no information was provided regarding the human CEC donor’s age 54. Cultivated hCECs from older donors have lower proliferative ability, a senescent cell phenotype, and transformed cell morphology, suggesting less functional ability than those derived from younger donors 10, 11, 55. This is associated with a significant increase with age of two cyclin kinase inhibitors, p21Cip1 and p16INK4, which are important negative regulators for the G1-phase of the cell cycle 56. Although hCECs from young subjects are ideal for in vitro expansion and to provide CECs for transplantation, it will be difficult to obtain sufficient young human corneas to provide the CECs required 12.
In the present study, we confirmed that Y27632 is beneficial for pCEC culture as it enhances cell proliferation and maintains a polygonal morphology phenotype, which is crucial for function. Moreover, Y27632 can significantly accelerate healing of a linear defect in confluent cells. Like CECs from monkeys and humans, pCECs do not proliferate in vivo, but regain their proliferative capacity during in vitro culture. However, once they are confluent, they stop proliferating and become BrdU-negative even in the presence of Y27632. This suggests that Y27632 is unlikely to result in uncontrolled CEC proliferation.
When a defective area of CECs was present, the CECs in the vicinity of the defect (but not those distant from the defect) began to divide and close the defect. This suggests that, if cultured CECs are transplanted, they might also proliferate in vivo (particularly if in the presence of a Rho kinase inhibitor) and replace the cell loss 26 associated with mechanical and/or immunological injury following the transplantation procedure. Therefore, CECs treated with a Rho kinase inhibitor and/or the topical application of a Rho kinase inhibitor might reduce the annual rate of loss of hCEC density. Following penetrating keratoplasty, the loss of CECs over 5 years is reported to be 4.2%, whereas the loss that occurs with age in a healthy cornea is only around 0.6% 2, 57. The significant post-transplantation reduction in CEC density (<500 cells/mm2) leads to loss of function, resulting in corneal swelling and opacification.
We previously reported that (i) young pCECs (<2 months-old), which are approximately equivalent to a 3–5 years-old human, had a similar proliferative capacity to young hCECs (<39 years), (ii) pCECs showed less cell death during culture compared to hCECs 11, and (iii) genetic-modification did not affect the proliferative capacity and cell viability of pCECs suggesting a beneficial effect of using pCECs, especially genetically-engineered pCECs, for corneal transplantation. The advantages include (i) the greater availability of corneas from young pigs 11, and (ii) the reduced human immune response to genetically-engineered pCECs 19. Further studies are required to investigate the expression pattern of function-related proteins, e.g., ZO-1 and Na+/K+-ATPase, in pCECs cultured with a Rho kinase inhibitor.
The Rho/Rho kinase signal pathway plays an important role in inflammation, immune regulation, and VEGF-induced angiogenesis 37, 58–62. Increased Rho kinase activity has been implicated in endothelial dysfunction and vascular inflammation in cardiovascular and pulmonary diseases and cancer 59, 61. In the present study, we found that Y27632 had beneficial anti-inflammatory and immunomodulatory functions. MCP-1 recruits monocytes, memory T cells, and dendritic cells to an inflamed area that can mediate graft rejection. VEGF stimulates angiogenesis in the cornea, which is usually avascular. Macrophage/monocyte infiltration and neovascularization are both important signs of corneal graft rejection 13. It has been shown that several inflammatory cytokines (e.g., TNF-α, interleukin-1β), growth factors (e.g., GM-CSF) and chemokines (e.g., MCP-1) are significantly elevated in the aqueous humor of patients with corneal rejection 63 or even in corneas with chronic inflammation 64. Kvanta et al., demonstrated that MCP-1 and VEGF have a role in the development of inflammation-associated corneal angiogenesis 65, suggesting that preventing these molecules would be necessary for long term corneal graft survival. Furthermore, using a pig-to-monkey corneal xenotransplantation model, an increase in TNF-α was detected in the aqueous humor of rejected xenografts in monkeys 16. After stimulation with inflammatory cytokines, Y27632 significantly decreased the level of the MCP-1 and VEGF in pCECs, as well as monocytes migration, suggesting that it may help to reduce inflammation and neovascularization, thus promoting graft survival. Moreover, it suppressed the T cell response to pCECs and reduced inflammatory cytokine production, suggesting an effect in reducing the T cell immune response. Although the mechanisms of these effects have yet to be elucidated, several reports have discussed potential mechanisms 37, 38.
TNF-α is a potent stimulant of several inflammatory signals, including NF-κB 31, mitogen-activated protein kinase (MAPK) and the Rho/Rho kinase pathway 37. Y27632 inhibits TNF-α-induced MCP-1 expression, secretion, and function through inhibition of Rho-kinase and p38MAPK activity in mesangial cells 37. Rho kinase inhibitors also ameliorate TNF-α-mediated monocyte migration to mesangial cells 37. MAPK signaling pathways have been recognized as crucial mediators of the Rho/Rho kinase signaling pathway, and also play an important role in regulating MCP-1 gene expression in various cell types 66. Y27632 attenuates activation of NF-κB, a central regulator of cellular gene regulation, in response to proinflammatory stimuli 38. The MCP-1 promoter contains NF-κB binding sites. Therefore, NF-κB inhibition by Rho kinase inhibition may result in decreased NF-κB binding to the MCP-1 promoter, leading to reduced expression of the MCP-1 gene 38. However, we demonstrated that Y27632 did not inhibit the phosphorylation of NF-κB p65 in pCECs after stimulation with hTNF-α suggesting another mechanism, such as through MAPK signaling pathways, might be involved in reducing pCECs activation.
Small GTPase is an important regulator involved in a number of intracellular signaling pathways. It is one of the key components in antigen, costimulatory, cytokine, and chemokine receptors, actin stress fiber formation, cell proliferation, and transcriptional regulation to regulate the immune response 58, 67–69. Increasing evidence indicates that an important role of Rho/Rho kinase signaling is to regulate immune and inflammatory responses 70–72. A Rho kinase inhibitor prolonged cardiac allograft survival in mice 58, 73. Inhibition by fasudil suppressed the increase in Rho kinase activity in allografts, the accumulation of inflammatory cells into the allograft, inflammatory cytokines, and the development of cardiac allograft vasculopathy 70.
The present in vitro study and previous accumulated reports suggest that the Rho/Rho kinase pathway may be a new target for prevention of the inflammatory and immune responses following corneal transplantation. Interestingly it has been shown that Y27632 has varying effects depending on cell types and possibly on the species. It has been demonstrated that Y27632 suppresses epithelial cell proliferation 74, while in contrast increases corneal endothelial cell proliferation in humans 54. The clinical use of fasudil has already been approved in Japan and China for prevention and treatment of cerebral vasospasm following surgery for subarachnoid hemorrhage, and has been used in over 124,000 patients in Japan 59. In addition, Y39983, another Rho kinase inhibitor, eye drops have been developed for the treatment of glaucoma (reducing intraocular pressure) 75, and are currently undergoing clinical trials 28, 59. Corticosteroids have been widely used for the prevention and treatment of corneal graft rejection. However, corticosteroid-related complications, e.g., an increase in intraocular pressure (glaucoma) may be problematic. The effect of Rho kinase inhibition in reducing intraocular pressure suggests a further advantage of its use after corneal transplantation, possibly in combination with corticosteroids.
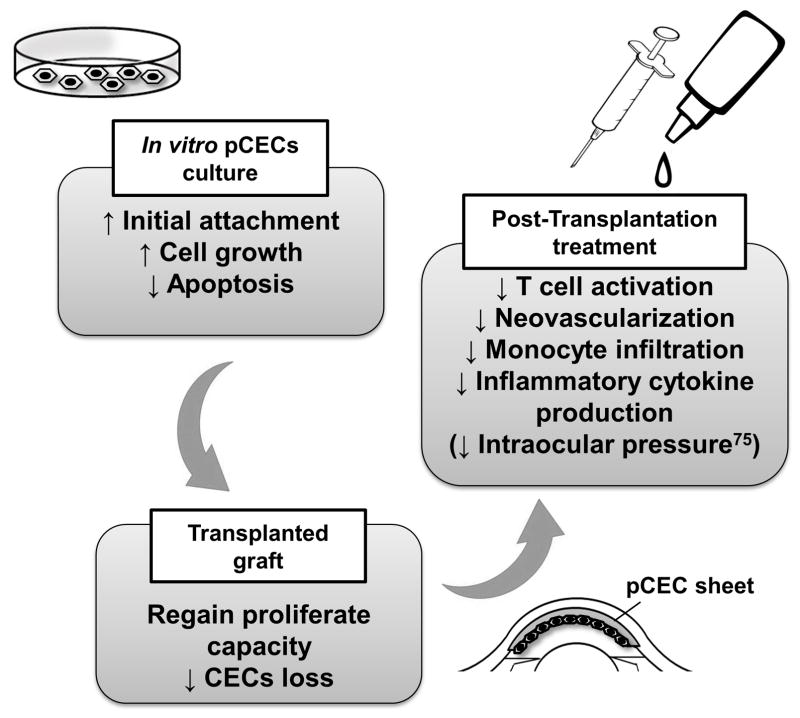
In conclusion, Y27632 yields significant improvements in many aspects, thereby enhancing cultured pCEC-based therapy (e.g., pCEC transplantation with/without a carrier). Local administration of the specific inhibitor Y27632 or fasudil may provide a new pre- and post-operative therapeutic modality for corneal xenotransplantation (Figure 7).
Figure 7. Potential application of a Rho kinase inhibitor to corneal xenotransplantation.
Y27632 enables cultured pCEC-based therapy for (i) in vitro pCEC culture (e.g., enhanced pCEC proliferation, reduced apoptosis), (ii) in vivo graft function (e.g., reproliferation of the pCECs after transplantation), and (iii) in vivo immune regulation (e.g., prevention of graft rejection).
Acknowledgments
Research on xenotransplantation at the University of Pittsburgh is funded in part by NIH Grants #1RO3A1096296-01 (HH), #IU19A1090959-01 (DKCC), #U01A1066331 (DKCC), and #5P01 HL107152-02 (DKCC), by an Ocular Tissue Engineering and Regenerative Ophthalmology (OTERO) Postdoctoral Fellowship (WL), and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Blacksburg, VA.
ABBREVIATIONS
- CEC
corneal endothelial cell
- CFSE
5-(and 6)-carboxyfluorescein diacetate succinimidyl ester
- ELISA
Enzyme-linked immunosorbent assay
- h
human
- MCP-1
monocyte chemotactic protein-1
- PBMC
peripheral blood mononuclear cell
- p
pig
- VEGF
vascular endothelial growth factor
Footnotes
DECLARATION OF INTEREST
No authors have a conflict of interest.
AUTHORS’ SPECIFIC CONTRIBUTIONS
WL1 participated in the performance of the research, interpretation of the results, and in writing of the manuscript. YM1, CL1and MZ1 participated in the performance of the research, and in review of the manuscript. DKCC1 participated in research design, in interpretation of the results, and in writing of the manuscript. HH1 participated in research design, in the performance of the research, interpretation of the results, and in writing of the manuscript.
References
- 1.Joyce NC, Meklir B, Joyce SJ, et al. Cell cycle protein expression and proliferative status in human corneal cells. Invest Ophthalmol Vis Sci. 1996;37(4):645–655. [PubMed] [Google Scholar]
- 2.Lee SE, Mehra R, Fujita M, et al. Characterization of porcine corneal endothelium for xenotransplantation. Semin Ophthalmol. 2014;29(3):127–135. doi: 10.3109/08820538.2013.787104. [DOI] [PubMed] [Google Scholar]
- 3.Tan DT, Dart JK, Holland EJ, et al. Corneal transplantation. Lancet. 2012;379(9827):1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 4.Shimazaki J. The evolution of lamellar keratoplasty. Curr Opin Ophthalmol. 2000;11(4):217–223. doi: 10.1097/00055735-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22(3):359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 6.Engelmann K, Bednarz J, Valtink M. Prospects for endothelial transplantation. Exp Eye Res. 2004;78(3):573–578. doi: 10.1016/s0014-4835(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 7.Sumide T, Nishida K, Yamato M, et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. Faseb J. 2006;20(2):392–394. doi: 10.1096/fj.04-3035fje. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi N, Sakamoto Y, Okumura N, et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Invest Ophthalmol Vis Sci. 2007;48(10):4519–4526. doi: 10.1167/iovs.07-0567. [DOI] [PubMed] [Google Scholar]
- 9.Peh GS, Beuerman RW, Colman A, et al. Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation. 2011;91(8):811–819. doi: 10.1097/TP.0b013e3182111f01. [DOI] [PubMed] [Google Scholar]
- 10.Zhu C, Joyce NC. Proliferative response of corneal endothelial cells from young and older donors. Invest Ophthalmol Vis Sci. 2004;45(6):1743–1751. doi: 10.1167/iovs.03-0814. [DOI] [PubMed] [Google Scholar]
- 11.Fujita M, Mehra R, Lee SE, et al. Comparison of proliferative capacity of genetically-engineered pig and human corneal endothelial cells. Ophthalmic Res. 2013;49(3):127–138. doi: 10.1159/000342978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara H, Cooper DK. Xenotransplantation-the future of corneal transplantation? Cornea. 2011;30(4):371–378. doi: 10.1097/ICO.0b013e3181f237ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara H, Cooper DK. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17(5):338–349. doi: 10.1111/j.1399-3089.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim MK, Wee WR, Park CG, et al. Xenocorneal transplantation. Curr Opin Organ Transplant. 2011;16(2):231–236. doi: 10.1097/MOT.0b013e328344870c. [DOI] [PubMed] [Google Scholar]
- 15.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379(9816):672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 16.Pan Z, Sun C, Jie Y, et al. WZS-pig is a potential donor alternative in corneal xenotransplantation. Xenotransplantation. 2007;14(6):603–611. doi: 10.1111/j.1399-3089.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi HJ, Kim MK, Lee HJ, et al. Efficacy of pig-to-rhesus lamellar corneal xenotransplantation. Invest Ophthalmol Vis Sci. 2011;52(9):6643–6650. doi: 10.1167/iovs.11-7273. [DOI] [PubMed] [Google Scholar]
- 18.Li A, Pan Z, Jie Y, et al. Comparison of immunogenicity and porcine-to-rhesus lamellar corneal xenografts survival between fresh preserved and dehydrated porcine corneas. Xenotransplantation. 2011;18(1):46–55. doi: 10.1111/j.1399-3089.2011.00626.x. [DOI] [PubMed] [Google Scholar]
- 19.Hara H, Koike N, Long C, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest Ophthalmol Vis Sci. 2011;52(8):5278–5286. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholls SM, Mitchard LK, Laycock GM, et al. A model of corneal graft rejection in semi-inbred NIH miniature swine: significant T-cell infiltration of clinically accepted allografts. Invest Ophthalmol Vis Sci. 2012;53(6):3183–3192. doi: 10.1167/iovs.11-9106. [DOI] [PubMed] [Google Scholar]
- 21.Okumura N, Koizumi N, Ueno M, et al. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012;181(1):268–277. doi: 10.1016/j.ajpath.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009;50(8):3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- 23.Pipparelli A, Arsenijevic Y, Thuret G, et al. ROCK inhibitor enhances adhesion and wound healing of human corneal endothelial cells. PLoS One. 2013;8(4):e62095. doi: 10.1371/journal.pone.0062095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76(8):722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koizumi N, Okumura N, Kinoshita S. Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp Eye Res. 2012;95(1):60–67. doi: 10.1016/j.exer.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Okumura N, Koizumi N, Kay EP, et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013;54(4):2493–2502. doi: 10.1167/iovs.12-11320. [DOI] [PubMed] [Google Scholar]
- 27.Inoue T, Tanihara H. Rho-associated kinase inhibitors: A novel glaucoma therapy. Prog Retin Eye Res. 2013:371–12. doi: 10.1016/j.preteyeres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Challa P, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Investig Drugs. 2014;23(1):81–95. doi: 10.1517/13543784.2013.840288. [DOI] [PubMed] [Google Scholar]
- 29.Hippenstiel S, Soeth S, Kellas B, et al. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood. 2000;95(10):3044–3051. [PubMed] [Google Scholar]
- 30.Segain JP, Raingeard de la Bletiere D, Sauzeau V, et al. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology. 2003;124(5):1180–1187. doi: 10.1016/s0016-5085(03)00283-x. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Xu H, Liang L, et al. Antiinflammatory effect of Rho kinase blockade via inhibition of NF-kappaB activation in rheumatoid arthritis. Arthritis Rheum. 2008;58(11):3366–3376. doi: 10.1002/art.23986. [DOI] [PubMed] [Google Scholar]
- 32.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145(6):1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takemoto M, Sun J, Hiroki J, et al. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106(1):57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 34.Chen LY, Zuraw BL, Liu FT, et al. IL-1 receptor-associated kinase and low molecular weight GTPase RhoA signal molecules are required for bacterial lipopolysaccharide-induced cytokine gene transcription. J Immunol. 2002;169(7):3934–3939. doi: 10.4049/jimmunol.169.7.3934. [DOI] [PubMed] [Google Scholar]
- 35.Hiroki J, Shimokawa H, Higashi M, et al. Inflammatory stimuli upregulate Rho-kinase in human coronary vascular smooth muscle cells. J Mol Cell Cardiol. 2004;37(2):537–546. doi: 10.1016/j.yjmcc.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Anwar KN, Fazal F, Malik AB, et al. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol. 2004;173(11):6965–6972. doi: 10.4049/jimmunol.173.11.6965. [DOI] [PubMed] [Google Scholar]
- 37.Matoba K, Kawanami D, Ishizawa S, et al. Rho-kinase mediates TNF-alpha-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem Biophys Res Commun. 2010;402(4):725–730. doi: 10.1016/j.bbrc.2010.10.093. [DOI] [PubMed] [Google Scholar]
- 38.Kawanami D, Matoba K, Kanazawa Y, et al. Thrombin induces MCP-1 expression through Rho-kinase and subsequent p38MAPK/NF-kappaB signaling pathway activation in vascular endothelial cells. Biochem Biophys Res Commun. 2011;411(4):798–803. doi: 10.1016/j.bbrc.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Peng W, Jian W, et al. ROCK inhibitor fasudil attenuated high glucose-induced MCP-1 and VCAM-1 expression and monocyte-endothelial cell adhesion. Cardiovasc Diabetol. 2012:1165. doi: 10.1186/1475-2840-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara H, Witt W, Crossley T, et al. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140(1):39–46. doi: 10.1111/imm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long C, Hara H, Pawlikowski Z, et al. Genetically engineered pig red blood cells for clinical transfusion: initial in vitro studies. Transfusion. 2009;49(11):2418–2429. doi: 10.1111/j.1537-2995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 42.Firaguay G, Nunes JA. Analysis of signaling events by dynamic phosphoflow cytometry. Sci Signal. 2009;2(86):pl3. doi: 10.1126/scisignal.286pl3. [DOI] [PubMed] [Google Scholar]
- 43.Iwase H, Ekser B, Satyananda V, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol. 2015;32(2):99–108. doi: 10.1016/j.trim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamagami H, Yamagami S, Inoki T, et al. The effects of proinflammatory cytokines on cytokine-chemokine gene expression profiles in the human corneal endothelium. Invest Ophthalmol Vis Sci. 2003;44(2):514–520. doi: 10.1167/iovs.02-0498. [DOI] [PubMed] [Google Scholar]
- 45.Torres PF, Slegers TP, Peek R, et al. Changes in cytokine mRNA levels in experimental corneal allografts after local clodronate-liposome treatment. Invest Ophthalmol Vis Sci. 1999;40(13):3194–3201. [PubMed] [Google Scholar]
- 46.Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126(1):71–77. doi: 10.1001/archopht.126.1.71. [DOI] [PubMed] [Google Scholar]
- 47.Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007;32(12):1005–1016. doi: 10.1080/02713680701767884. [DOI] [PubMed] [Google Scholar]
- 48.Joyce NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res. 2012;95(1):16–23. doi: 10.1016/j.exer.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269(5228):1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 50.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 51.Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol. 2004;5(5):355–366. doi: 10.1038/nrm1365. [DOI] [PubMed] [Google Scholar]
- 52.Okumura N, Koizumi N, Ueno M, et al. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. Br J Ophthalmol. 2011;95(7):1006–1009. doi: 10.1136/bjo.2010.194571. [DOI] [PubMed] [Google Scholar]
- 53.Okumura N, Koizumi N, Ueno M, et al. The new therapeutic concept of using a rho kinase inhibitor for the treatment of corneal endothelial dysfunction. Cornea. 2011;30(Suppl):1S54–59. doi: 10.1097/ICO.0b013e3182281ee1. [DOI] [PubMed] [Google Scholar]
- 54.Okumura N, Nakano S, Kay EP, et al. Involvement of cyclin D and p27 in cell proliferation mediated by ROCK inhibitors Y-27632 and Y-39983 during corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2014;55(1):318–329. doi: 10.1167/iovs.13-12225. [DOI] [PubMed] [Google Scholar]
- 55.Joyce NC, Zhu CC. Human corneal endothelial cell proliferation: potential for use in regenerative medicine. Cornea. 2004;23(8 Suppl):S8–S19. doi: 10.1097/01.ico.0000136666.63870.18. [DOI] [PubMed] [Google Scholar]
- 56.Enomoto K, Mimura T, Harris DL, et al. Age differences in cyclin-dependent kinase inhibitor expression and rb hyperphosphorylation in human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2006;47(10):4330–4340. doi: 10.1167/iovs.05-1581. [DOI] [PubMed] [Google Scholar]
- 57.Ing JJ, Ing HH, Nelson LR, et al. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105(10):1855–1865. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- 58.Tharaux PL, Bukoski RC, Rocha PN, et al. Rho kinase promotes alloimmune responses by regulating the proliferation and structure of T cells. J Immunol. 2003;171(1):96–105. doi: 10.4049/jimmunol.171.1.96. [DOI] [PubMed] [Google Scholar]
- 59.Liao JK, Seto M, Noma K. Rho kinase (ROCK) inhibitors. J Cardiovasc Pharmacol. 2007;50(1):17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin L, Morishige K, Takahashi T, et al. Fasudil inhibits vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Mol Cancer Ther. 2007;6(5):1517–1525. doi: 10.1158/1535-7163.MCT-06-0689. [DOI] [PubMed] [Google Scholar]
- 61.Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20(2):242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okamoto H, Yoshio T, Kaneko H, et al. Inhibition of NF-kappaB signaling by fasudil as a potential therapeutic strategy for rheumatoid arthritis. Arthritis Rheum. 2010;62(1):82–92. doi: 10.1002/art.25063. [DOI] [PubMed] [Google Scholar]
- 63.Funding M, Hansen TK, Gjedsted J, et al. Simultaneous quantification of 17 immune mediators in aqueous humour from patients with corneal rejection. Acta Ophthalmol Scand. 2006;84(6):759–765. doi: 10.1111/j.1600-0420.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 64.Spandau UH, Toksoy A, Verhaart S, et al. High expression of chemokines Gro-alpha (CXCL-1), IL-8 (CXCL-8), and MCP-1 (CCL-2) in inflamed human corneas in vivo. Arch Ophthalmol. 2003;121(6):825–831. doi: 10.1001/archopht.121.6.825. [DOI] [PubMed] [Google Scholar]
- 65.Kvanta A, Sarman S, Fagerholm P, et al. Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization. Exp Eye Res. 2000;70(4):419–428. doi: 10.1006/exer.1999.0790. [DOI] [PubMed] [Google Scholar]
- 66.Kaur J, Woodman RC, Kubes P. P38 MAPK: critical molecule in thrombin-induced NF-kappa B-dependent leukocyte recruitment. Am J Physiol Heart Circ Physiol. 2003;284(4):H1095–1103. doi: 10.1152/ajpheart.00016.2002. [DOI] [PubMed] [Google Scholar]
- 67.Costello PS, Walters AE, Mee PJ, et al. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappaB pathways. Proc Natl Acad Sci U S A. 1999;96(6):3035–3040. doi: 10.1073/pnas.96.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JR, Ha YJ, Kim HJ. Cutting edge: induced expression of a RhoA-specific guanine nucleotide exchange factor, p190RhoGEF, following CD40 stimulation and WEHI 231 B cell activation. J Immunol. 2003;170(1):19–23. doi: 10.4049/jimmunol.170.1.19. [DOI] [PubMed] [Google Scholar]
- 69.Salazar-Fontana LI, Barr V, Samelson LE, et al. CD28 engagement promotes actin polymerization through the activation of the small Rho GTPase Cdc42 in human T cells. J Immunol. 2003;171(5):2225–2232. doi: 10.4049/jimmunol.171.5.2225. [DOI] [PubMed] [Google Scholar]
- 70.Hattori T, Shimokawa H, Higashi M, et al. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94(1):46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- 71.Liu M, Gu M, Wu Y, et al. Therapeutic effect of Y-27632 on chronic allograft nephropathy in rats. J Surg Res. 2009;157(1):e117–127. doi: 10.1016/j.jss.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 72.Poosti F, Yazdani S, Dolman ME, et al. Targeted inhibition of renal Rho kinase reduces macrophage infiltration and lymphangiogenesis in acute renal allograft rejection. Eur J Pharmacol. 2012;694(1–3):111–119. doi: 10.1016/j.ejphar.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Ohki S, Iizuka K, Ishikawa S, et al. A highly selective inhibitor of Rho-associated coiled-coil forming protein kinase, Y-27632, prolongs cardiac allograft survival of the BALB/c-to-C3H/He mouse model. J Heart Lung Transplant. 2001;20(9):956–963. doi: 10.1016/s1053-2498(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 74.Yin J, Yu FS. Rho kinases regulate corneal epithelial wound healing. Am J Physiol Cell Physiol. 2008;295(2):C378–387. doi: 10.1152/ajpcell.90624.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tokushige H, Inatani M, Nemoto S, et al. Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest Ophthalmol Vis Sci. 2007;48(7):3216–3222. doi: 10.1167/iovs.05-1617. [DOI] [PubMed] [Google Scholar]