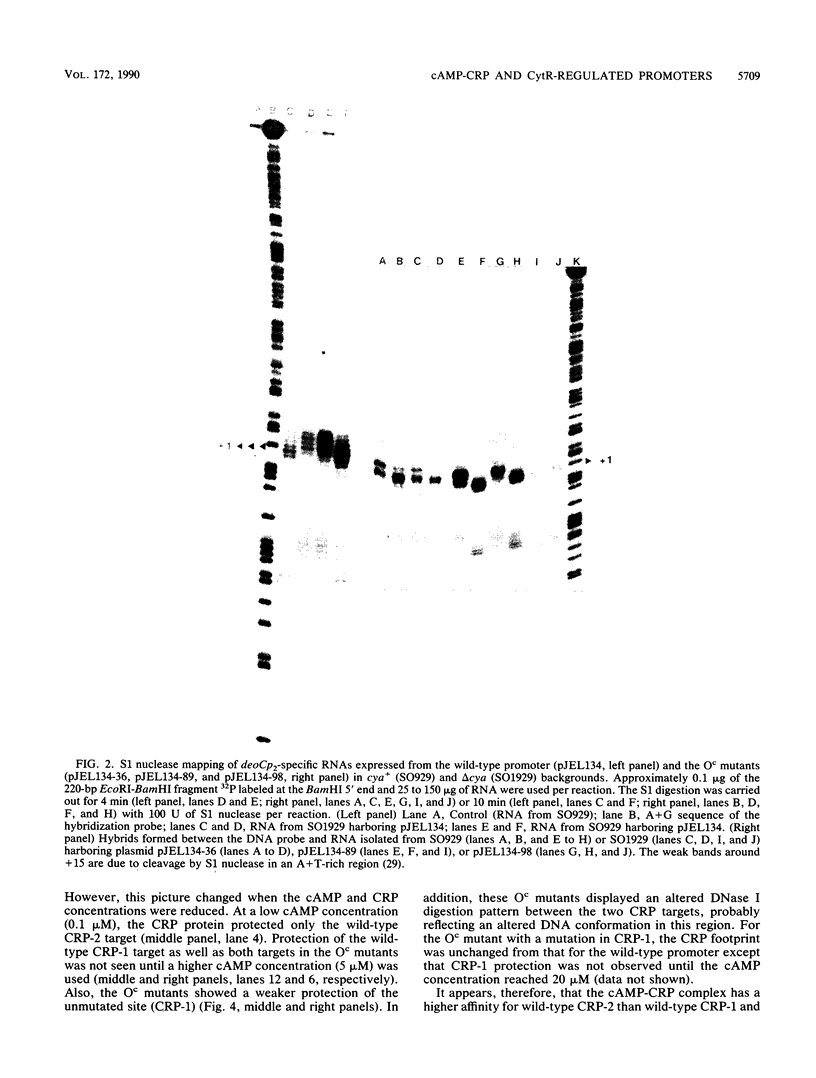
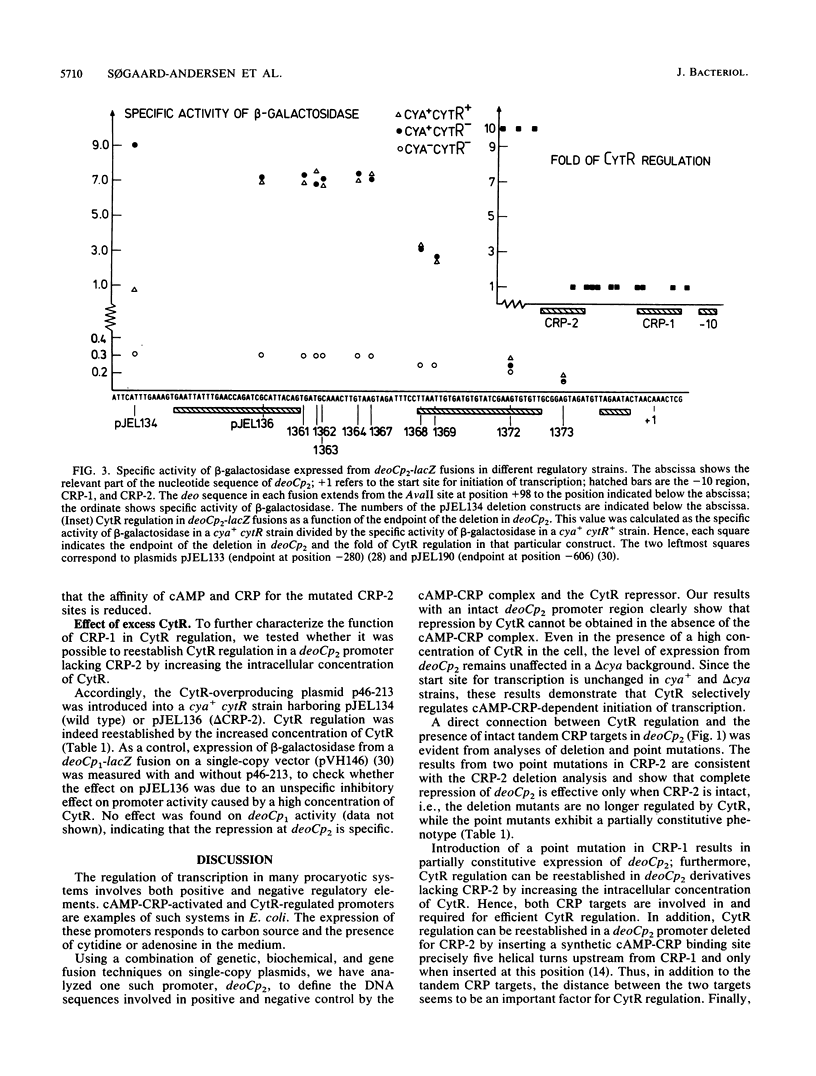
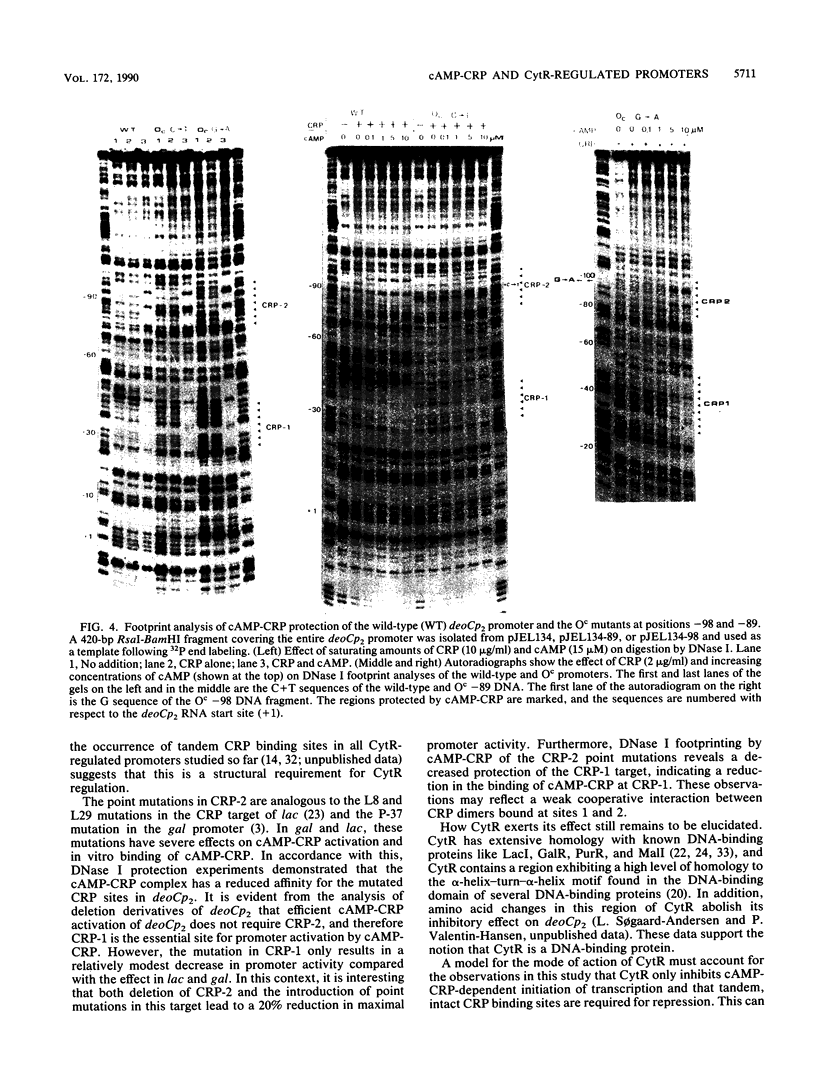
Abstract
We have investigated the regulation of the Escherichia coli deoCp2 promoter by the CytR repressor and the cyclic AMP (cAMP) receptor protein (CRP) complexed to cAMP. Promoter regions controlled by these two proteins characteristically contain tandem cAMP-CRP binding sites. Here we show that (i) CytR selectively regulated cAMP-CRP-dependent initiations, although transcription started from the same site in deoCp2 in the absence or presence of cAMP-CRP; (ii) deletion of the uppermost cAMP-CRP target (CRP-2) resulted in loss of CytR regulation, but had only a minor effect on positive control by the cAMP-CRP complex; (iii) introduction of point mutations in either CRP target resulted in loss of CytR regulation; and (iv) regulation by CytR of deletion mutants lacking CRP-2 could be specifically reestablished by increasing the intracellular concentration of CytR. These findings indicate that both CRP targets are required for efficient CytR repression of deoCp2. Models for the action of CytR are discussed in light of these findings.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. Plasmid vehicles for direct cloning of Escherichia coli promoters. J Bacteriol. 1979 Nov;140(2):400–407. doi: 10.1128/jb.140.2.400-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S., Aiba H., de Crombrugghe B. Mutations in the Escherichia coli operon that define two promoters and the binding site of the cyclic AMP receptor protein. J Mol Biol. 1982 Jan 15;154(2):211–227. doi: 10.1016/0022-2836(82)90061-4. [DOI] [PubMed] [Google Scholar]
- Dandanell G., Hammer K. Two operator sites separated by 599 base pairs are required for deoR repression of the deo operon of Escherichia coli. EMBO J. 1985 Dec 1;4(12):3333–3338. doi: 10.1002/j.1460-2075.1985.tb04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandanell G., Valentin-Hansen P., Larsen J. E., Hammer K. Long-range cooperativity between gene regulatory sequences in a prokaryote. 1987 Feb 26-Mar 4Nature. 325(6107):823–826. doi: 10.1038/325823a0. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosaini L. R., Brown A. M., Sturtevant J. M. Scanning calorimetric study of the thermal unfolding of catabolite activator protein from Escherichia coli in the absence and presence of cyclic mononucleotides. Biochemistry. 1988 Jul 12;27(14):5257–5261. doi: 10.1021/bi00414a046. [DOI] [PubMed] [Google Scholar]
- Larsen J. E., Gerdes K., Light J., Molin S. Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene. 1984 Apr;28(1):45–54. doi: 10.1016/0378-1119(84)90086-6. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Minton N. P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984 Nov;31(1-3):269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- Mironov A. S., Nechaeva G. D., Sukhodolets V. V. Vzaimodeistvie élementov negativnoi (CytR) i pozitivnoi (cAMP-CRP) reguliatsii v promotornoi oblasti uridinfosforilaznogo (udp) gena Escherichia coli K-12). Genetika. 1989 Mar;25(3):438–447. [PubMed] [Google Scholar]
- Mortensen L., Dandanell G., Hammer K. Purification and characterization of the deoR repressor of Escherichia coli. EMBO J. 1989 Jan;8(1):325–331. doi: 10.1002/j.1460-2075.1989.tb03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Reidl J., Römisch K., Ehrmann M., Boos W. MalI, a novel protein involved in regulation of the maltose system of Escherichia coli, is highly homologous to the repressor proteins GalR, CytR, and LacI. J Bacteriol. 1989 Sep;171(9):4888–4899. doi: 10.1128/jb.171.9.4888-4899.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes R. J., Zalkin H. Escherichia coli gene purR encoding a repressor protein for purine nucleotide synthesis. Cloning, nucleotide sequence, and interaction with the purF operator. J Biol Chem. 1988 Dec 25;263(36):19653–19661. [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Aiba H., Schümperli D. The structure of tandem regulatory regions in the deo operon of Escherichia coli K12. EMBO J. 1982;1(3):317–322. doi: 10.1002/j.1460-2075.1982.tb01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Albrechtsen B., Løve Larsen J. E. DNA-protein recognition: demonstration of three genetically separated operator elements that are required for repression of the Escherichia coli deoCABD promoters by the DeoR repressor. EMBO J. 1986 Aug;5(8):2015–2021. doi: 10.1002/j.1460-2075.1986.tb04458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Hammer-Jespersen K., Boetius F., Svendsen I. Structure and function of the intercistronic regulatory deoC-deoA element of Escherichia coli K-12. EMBO J. 1984 Jan;3(1):179–183. doi: 10.1002/j.1460-2075.1984.tb01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Holst B., Josephsen J., Hammer K., Albrechtsen B. CRP/cAMP- and CytR-regulated promoters in Escherichia coli K12: the cdd promoter. Mol Microbiol. 1989 Oct;3(10):1385–1390. doi: 10.1111/j.1365-2958.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Larsen J. E., Højrup P., Short S. A., Barbier C. S. Nucleotide sequence of the CytR regulatory gene of E. coli K-12. Nucleic Acids Res. 1986 Mar 11;14(5):2215–2228. doi: 10.1093/nar/14.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Svenningsen B. A., Munch-Petersen A., Hammer-Jespersen K. Regulation of the deo operon in Escherichia coli: the double negative control of the deo operon by the cytR and deoR repressors in a DNA directed in vitro system. Mol Gen Genet. 1978 Feb 16;159(2):191–202. doi: 10.1007/BF00270893. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P. Tandem CRP binding sites in the deo operon of Escherichia coli K-12. EMBO J. 1982;1(9):1049–1054. doi: 10.1002/j.1460-2075.1982.tb01295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]




