Abstract
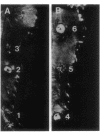
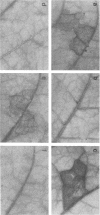
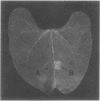
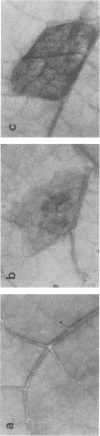
Injection into tobacco leaves of biotype 1 Agrobacterium tumefaciens or of Pseudomonas savastanoi inhibited the development of a visible hypersensitive response to the subsequent injection at the same site of Pseudomonas syringae pv. phaseolicola. This interference with the hypersensitive response was not seen with injection of bacterial growth medium or Escherichia coli cells. Live A. tumefaciens cells were required for the inhibitory effect. Various mutants and strains of A. tumefaciens were examined to determine the genes involved. Known chromosomal mutations generally had no effect on the ability of A. tumefaciens to inhibit the hypersensitive response, except for chvB mutants which showed a reduced (but still significant) inhibition of the hypersensitive response. Ti plasmid genes appeared to be required for the inhibition of the hypersensitive response. The bacteria did not need to be virulent in order to inhibit the hypersensitive response. Deletion of the vir region from pTi had no effect on the inhibition. However, the T region of the Ti plasmid was required for inhibition. Studies of transposon mutants suggested that the tms but not tmr or ocs genes were required. These genes were not acting after transfer to plant cells since they were effective in strains lacking vir genes and thus unable to transfer DNA to plant cells. The results suggest that the expression of the tms genes in the bacteria may inhibit the development of the hypersensitive response by the plant. An examination of the genes required in P. savastanoi for the inhibition of the hypersensitive response suggested that bacterial production of auxin was also required for the inhibition of the hypersensitive response by these bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Douglas C. J., Halperin W., Nester E. W. Agrobacterium tumefaciens mutants affected in attachment to plant cells. J Bacteriol. 1982 Dec;152(3):1265–1275. doi: 10.1128/jb.152.3.1265-1275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin S. B., Gordon M. P., Nester E. W., Aronson A. I. Transcription of the Agrobacterium Ti plasmid in the bacterium and in crown gall tumors. Plasmid. 1981 Jul;6(1):17–29. doi: 10.1016/0147-619x(81)90051-2. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G. Characterization of nonattaching mutants of Agrobacterium tumefaciens. J Bacteriol. 1987 Jan;169(1):313–323. doi: 10.1128/jb.169.1.313-323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G. Effect of Plasmid pSa and of Auxin on Attachment of Agrobacterium tumefaciens to Carrot Cells. Appl Environ Microbiol. 1987 Oct;53(10):2574–2582. doi: 10.1128/aem.53.10.2574-2582.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol. 1983 May;154(2):906–915. doi: 10.1128/jb.154.2.906-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder G., Klipp W., Hillebrand A., Ehring R., Koncz C., Schröder J. The conserved part of the T-region in Ti-plasmids expresses four proteins in bacteria. EMBO J. 1983;2(3):403–409. doi: 10.1002/j.1460-2075.1983.tb01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Sykes L. C., Matthysse A. G. Time required for tumor induction by Agrobacterium tumefaciens. Appl Environ Microbiol. 1986 Sep;52(3):597–598. doi: 10.1128/aem.52.3.597-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virts E. L., Gelvin S. B. Analysis of transfer of tumor-inducing plasmids from Agrobacterium tumefaciens to Petunia protoplasts. J Bacteriol. 1985 Jun;162(3):1030–1038. doi: 10.1128/jb.162.3.1030-1038.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]