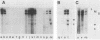Abstract
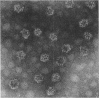
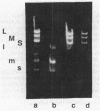
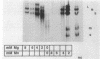
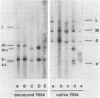
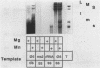
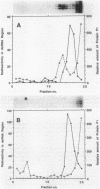
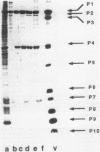
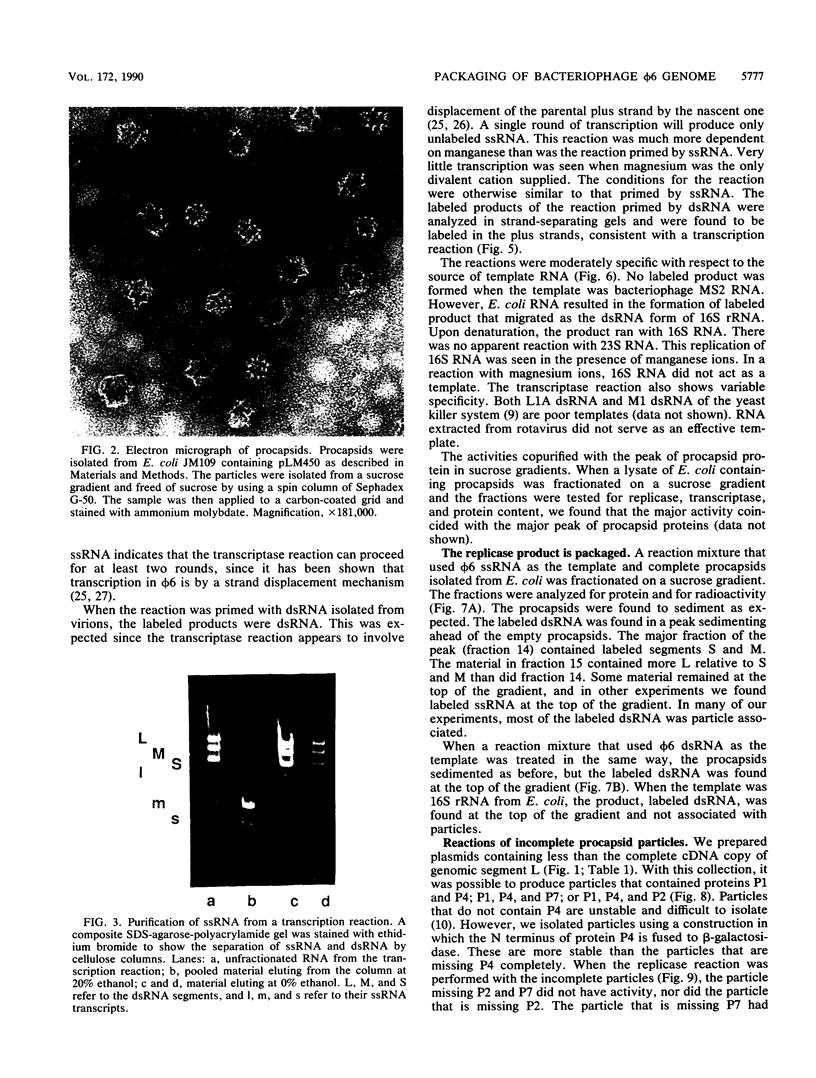
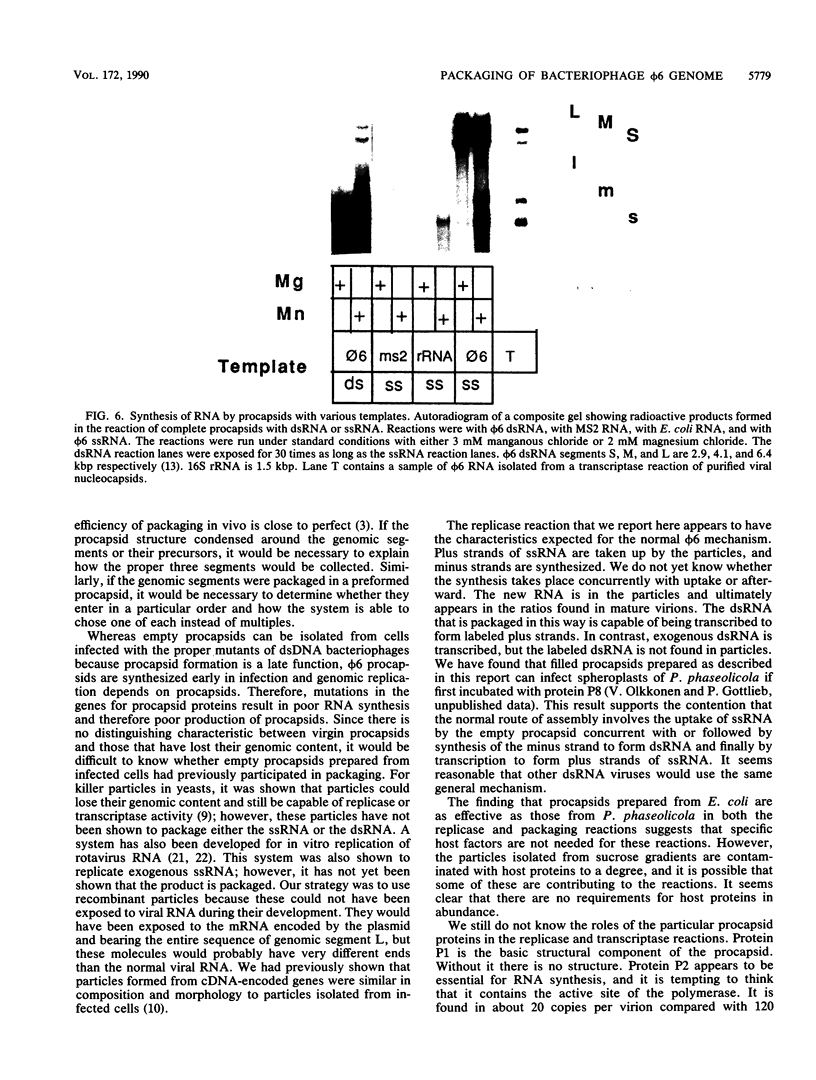
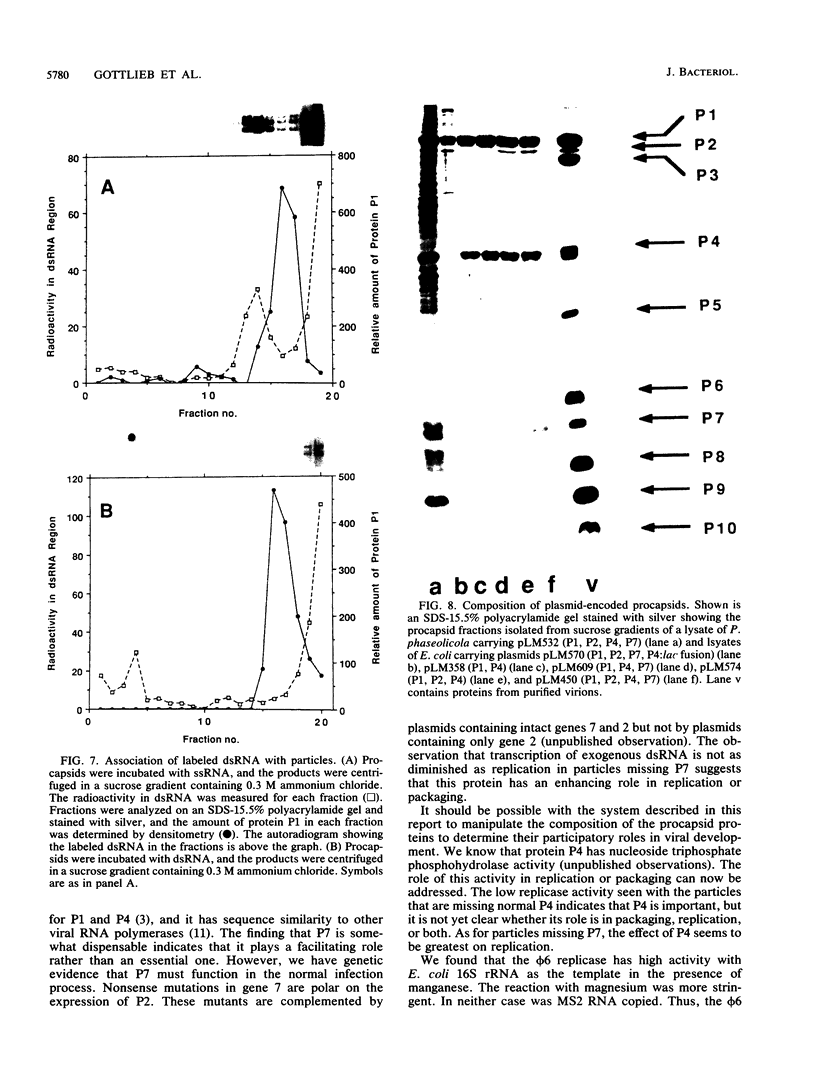
The genome of the lipid-containing bacteriophage phi 6 contains three segments of double-stranded RNA (dsRNA). We prepared cDNA copies of the viral genome and cloned this material in plasmids that replicate in Escherichia coli and Pseudomonas phaseolicola, the natural host of phi 6. These plasmids direct the formation of viral proteins and the assembly of structures similar to viral procapsids containing proteins P1, P2, P4, and P7. We found that these particles are capable of taking up viral single-stranded RNA and synthesizing the minus strands to produce dsRNA structures. Once the dsRNA is formed, it is then used as a template for the production of viral plus strands in a reaction that resembles normal transcription. The particles were also capable of directly transcribing exogenous dsRNA. The replicase reactions were specific for phi 6 RNA, were specific for procapsids, and resulted in substantial incorporation of product dsRNA into particles. These results offer strong support to a model in which genomic packaging is done by preformed procapsids.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama A., Hamatake R. K., Hayashi M. Morphogenesis of phi X174: in vitro synthesis of infectious phage from purified viral components. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7285–7289. doi: 10.1073/pnas.78.12.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day L. A., Mindich L. The molecular weight of bacteriophage phi 6 and its nucleocapsid. Virology. 1980 Jun;103(2):376–385. doi: 10.1016/0042-6822(80)90196-8. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori Y., Iba H., Okada Y. Transcriptional regulation of three double-stranded RNA segments of bacteriophage phi 6 in vitro. J Virol. 1983 Apr;46(1):196–203. doi: 10.1128/jvi.46.1.196-203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E., Revel H. R. In vitro replication and transcription of the segmented double-stranded RNA bacteriophage phi 6. Virology. 1988 Aug;165(2):489–498. doi: 10.1016/0042-6822(88)90593-4. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Wickner R. B. Reconstitution of template-dependent in vitro transcriptase activity of a yeast double-stranded RNA virus. J Biol Chem. 1989 Jun 25;264(18):10872–10877. [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Bamford D. H., Mindich L. Production of a polyhedral particle in Escherichia coli from a cDNA copy of the large genomic segment of bacteriophage phi 6. J Virol. 1988 Jan;62(1):181–187. doi: 10.1128/jvi.62.1.181-187.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Gorbalenya A. E., Chumakov K. M. Tentative identification of RNA-dependent RNA polymerases of dsRNA viruses and their relationship to positive strand RNA viral polymerases. FEBS Lett. 1989 Jul 31;252(1-2):42–46. doi: 10.1016/0014-5793(89)80886-5. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Lebkowski J. S., Greisen K. S., Calos M. P. Specificity of mutations induced in transfected DNA by mammalian cells. EMBO J. 1984 Dec 20;3(13):3117–3121. doi: 10.1002/j.1460-2075.1984.tb02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Bacteriophage phi 6: a unique virus having a lipid-containing membrane and a genome composed of three dsRNA segments. Adv Virus Res. 1988;35:137–176. doi: 10.1016/s0065-3527(08)60710-1. [DOI] [PubMed] [Google Scholar]
- Mindich L., Bamford D., Goldthwaite C., Laverty M., Mackenzie G. Isolation of nonsense mutants of lipid-containing bacteriophage PRD1. J Virol. 1982 Dec;44(3):1013–1020. doi: 10.1128/jvi.44.3.1013-1020.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Davidoff-Abelson R. The characterization of a 120 S particle formed during phi 6 infection. Virology. 1980 Jun;103(2):386–391. doi: 10.1016/0042-6822(80)90197-x. [DOI] [PubMed] [Google Scholar]
- Mindich L., MacKenzie G., Strassman J., McGraw T., Metzger S., Romantschuk M., Bamford D. cDNA cloning of portions of the bacteriophage phi 6 genome. J Bacteriol. 1985 Jun;162(3):992–999. doi: 10.1128/jb.162.3.992-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Nemhauser I., Gottlieb P., Romantschuk M., Carton J., Frucht S., Strassman J., Bamford D. H., Kalkkinen N. Nucleotide sequence of the large double-stranded RNA segment of bacteriophage phi 6: genes specifying the viral replicase and transcriptase. J Virol. 1988 Apr;62(4):1180–1185. doi: 10.1128/jvi.62.4.1180-1185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obinata M., Nasser D. S., McCarthy B. J. Synthesis of probes for RNA using Qbeta-replicase. Biochem Biophys Res Commun. 1975 May 19;64(2):640–647. doi: 10.1016/0006-291x(75)90369-1. [DOI] [PubMed] [Google Scholar]
- Pagratis N., Revel H. R. Detection of bacteriophage phi 6 minus-strand RNA and novel mRNA isoconformers synthesized in vivo and in vitro, by strand-separating agarose gels. Virology. 1990 Jul;177(1):273–280. doi: 10.1016/0042-6822(90)90480-f. [DOI] [PubMed] [Google Scholar]
- Palmenberg A., Kaesberg P. Synthesis of complementary strands of heterologous RNAs with Qbeta replicase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1371–1375. doi: 10.1073/pnas.71.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Gallegos C. O. Structure and protein composition of the rotavirus replicase particle. Virology. 1988 Oct;166(2):358–365. doi: 10.1016/0042-6822(88)90506-5. [DOI] [PubMed] [Google Scholar]
- Patton J. T. Synthesis of simian rotavirus SA11 double-stranded RNA in a cell-free system. Virus Res. 1986 Dec;6(3):217–233. doi: 10.1016/0168-1702(86)90071-7. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Brownstein B. H., Haselkorn R. Displacement of parental RNA strands during in vitro transcription by bacteriophage phi 6 nucleocapsids. Cell. 1980 Apr;19(4):855–862. doi: 10.1016/0092-8674(80)90076-8. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Cuppels D. A., Lane L. C., Vidaver A. K. Semiconservative synthesis of single-stranded RNA by bacteriophage phi 6 RNA polymerase. J Virol. 1980 Feb;33(2):769–773. doi: 10.1128/jvi.33.2.769-773.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]