Abstract
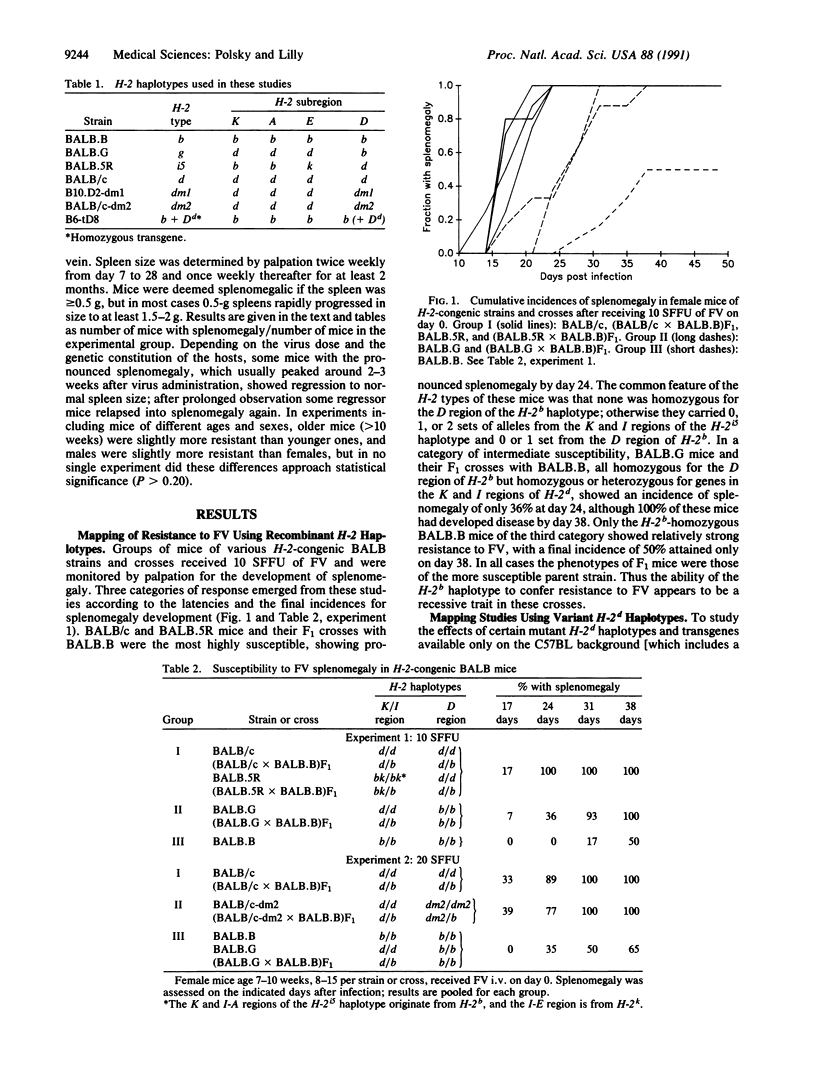
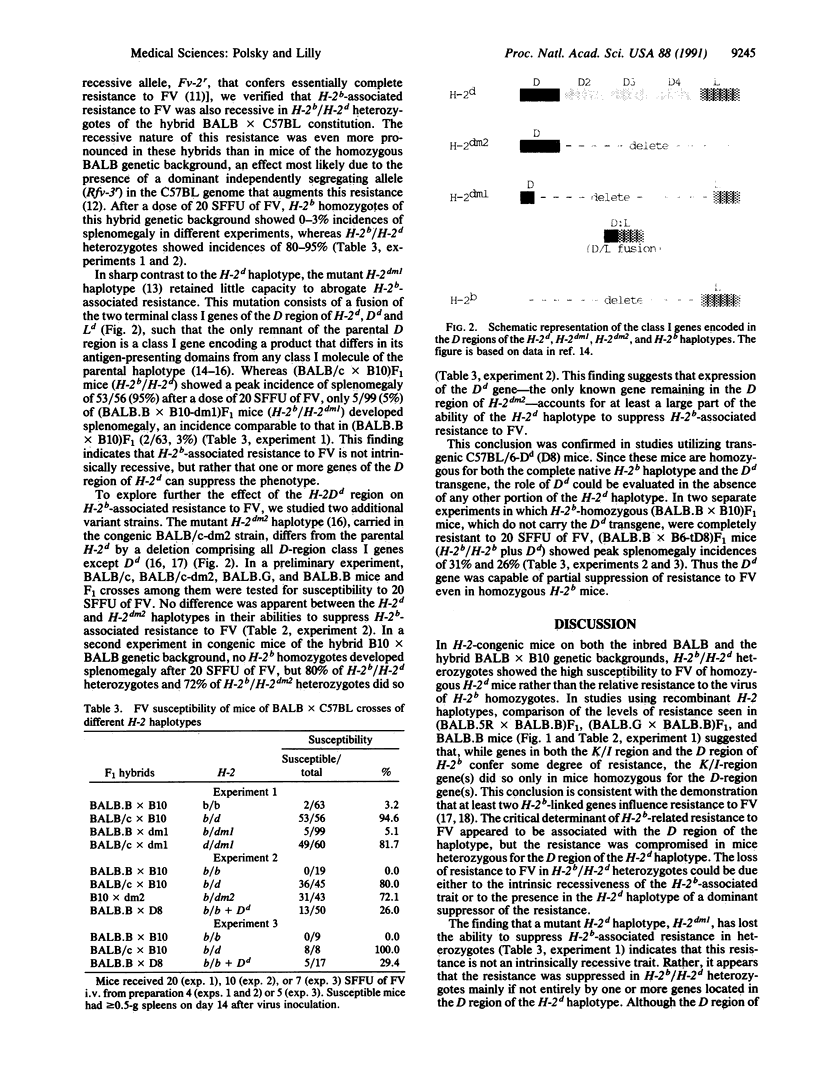
Mice homozygous for the H-2d haplotype at the major histocompatibility complex are markedly more susceptible to erythroleukemia induction by the Friend isolate of murine leukemia retrovirus (FV) than are congenic mice homozygous for the H-2b haplotype. The resistance conferred by the H-2b haplotype is recessive in this cross, since heterozygous F1 mice are as susceptible as parental strain H-2d homozygotes. However, H-2b-associated resistance is not an intrinsically recessive trait, since H-2b/H-2dm1 heterozygotes resemble H-2b homozygotes in their relative resistance to FV; the mutant H-2dm1 haplotype lacks the entire D region of the parental haplotype except for a single class I gene formed by the fusion of its terminal D-region genes to produce a class I gene differing from both parental genes, and thus this finding indicates that one or more D-region genes of the H-2d haplotype can actively suppress H-2b-associated resistance. Unlike H-2dm1, the mutant H-2dm2 haplotype, which retains only the class IDd gene in the D region of the H-2d haplotype, strongly suppresses resistance in H-2b/H-2dm2 heterozygotes, and the presence of Dd as a transgene significantly reduces the resistance of H-2b homozygotes. Since H-2b-associated resistance to FV appears to be due mainly to the capacity of Lb (also called Db), the only class I molecule encoded in the D region of the H-2b haplotype, to present viral epitopes for recognition by FV-specific cytotoxic T lymphocytes, suppression of resistance to FV by the Dd molecule implies that the presence of one class I molecule of the major histocompatibility complex can interfere with either the presentation of viral epitopes by another class I molecule or the generation of T cells that recognize viral epitopes so presented.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Bennett M. Biology and genetics of hybrid resistance. Adv Immunol. 1987;41:333–445. doi: 10.1016/s0065-2776(08)60034-6. [DOI] [PubMed] [Google Scholar]
- Bieberich C., Scangos G., Tanaka K., Jay G. Regulated expression of a murine class I gene in transgenic mice. Mol Cell Biol. 1986 Apr;6(4):1339–1342. doi: 10.1128/mcb.6.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside S. S., Hunt P., Ozato K., Sears D. W. A molecular hybrid of the H-2Dd and H-2Ld genes expressed in the dm1 mutant. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5204–5208. doi: 10.1073/pnas.81.16.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Miyazawa M., Britt W. J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Identification of a non-H-2 gene (Rfv-3) influencing recovery from viremia and leukemia induced by Friend virus complex. Proc Natl Acad Sci U S A. 1979 Jan;76(1):425–429. doi: 10.1073/pnas.76.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Rfv-1 and Rfv-2, two H-2-associated genes that influence recovery from Friend leukemia virus-induced splenomegaly. J Immunol. 1978 Apr;120(4):1081–1085. [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly: mapping of a gene within the major histocompatability complex. J Exp Med. 1974 Dec 1;140(6):1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G., Bennett M. Peculiar immunobiology of bone marrow allografts. I. Graft rejection by irradiated responder mice. J Exp Med. 1971 Jul 1;134(1):83–102. doi: 10.1084/jem.134.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L., Nunn M., Duesberg P. H., Troxler D., Scolnick E. RNAs of defective and nondefective components of Friend anemia and polycythemia virus strains identified and compared. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):823–835. doi: 10.1101/sqb.1980.044.01.087. [DOI] [PubMed] [Google Scholar]
- Hansen T. H., Cullen S. E., Melvold R., Kohn H., Flaherty L., Sachs D. H. Mutation in a new H-2-associated histocompatibility gene closely linked to H-2D. J Exp Med. 1977 Jun 1;145(6):1550–1558. doi: 10.1084/jem.145.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C. A., Osorio K., Lilly F. Friend virus-specific cytotoxic T lymphocytes recognize both gag and env gene-encoded specificities. J Exp Med. 1986 Jul 1;164(1):211–226. doi: 10.1084/jem.164.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund P., Ljunggren H. G., Ohlén C., Ahrlund-Richter L., Scangos G., Bieberich C., Jay G., Klein G., Kärre K. Natural resistance against lymphoma grafts conveyed by H-2Dd transgene to C57BL mice. J Exp Med. 1988 Oct 1;168(4):1469–1474. doi: 10.1084/jem.168.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. S., Thurlow S. M., Furmanski P. Induction of leukemia regression in mice by immunotherapeutic transfer of T-lymphocytes. Cancer Res. 1986 Jan;46(1):183–189. [PubMed] [Google Scholar]
- LILLY F., BOYSE E. A., OLD L. J. GENETIC BASIS OF SUSCEPTIBILITY TO VIRAL LEUKAEMOGENESIS. Lancet. 1964 Dec 5;2(7371):1207–1209. doi: 10.1016/s0140-6736(64)91043-8. [DOI] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Lilly F. The effect of histocompatibility-2 type on response to friend leukemia virus in mice. J Exp Med. 1968 Mar 1;127(3):465–473. doi: 10.1084/jem.127.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino M., Morse H. C., 3rd, Fredrickson T. N., Hartley J. W. H-2-associated and background genes influence the development of a murine retrovirus-induced immunodeficiency syndrome. J Immunol. 1990 Jun 1;144(11):4347–4355. [PubMed] [Google Scholar]
- McDevitt H. O., Sela M. Genetic control of the antibody response. I. Demonstration of determinant-specific differences in response to synthetic polypeptide antigens in two strains of inbred mice. J Exp Med. 1965 Sep 1;122(3):517–531. doi: 10.1084/jem.122.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers I., Rajewsky K., Shreffler D. C. Ir-LDHB: map position and functional analysis. Eur J Immunol. 1973 Dec;3(12):754–761. doi: 10.1002/eji.1830031204. [DOI] [PubMed] [Google Scholar]
- Ohlén C., Kling G., Höglund P., Hansson M., Scangos G., Bieberich C., Jay G., Kärre K. Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science. 1989 Nov 3;246(4930):666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- Plata F., Lilly F. Viral specificity of H-2-restricted T killer cells directed against syngeneic tumors induced by Gross, Friend, or Rauscher leukemia virus. J Exp Med. 1979 Nov 1;150(5):1174–1186. doi: 10.1084/jem.150.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan K. S., Lilly F. Identification of an epitope encoded in the env gene of Friend murine leukemia virus recognized by anti-Friend virus cytotoxic T lymphocytes. Virology. 1991 Mar;181(1):91–100. doi: 10.1016/0042-6822(91)90473-o. [DOI] [PubMed] [Google Scholar]
- Stephan D., Sun H., Lindahl K. F., Meyer E., Hämmerling G., Hood L., Steinmetz M. Organization and evolution of D region class I genes in the mouse major histocompatibility complex. J Exp Med. 1986 May 1;163(5):1227–1244. doi: 10.1084/jem.163.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. H., Goodenow R. S., Hood L. Molecular basis of the dm1 mutation in the major histocompatibility complex of the mouse: a D/L hybrid gene. J Exp Med. 1985 Nov 1;162(5):1588–1602. doi: 10.1084/jem.162.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Scolnick E. M. Rapid leukemia induced by cloned friend strain of replicating murine type-C virus. Association with induction of xenotropic-related RNA sequences contained in spleen focus-forming virus. Virology. 1978 Mar;85(1):17–27. doi: 10.1016/0042-6822(78)90408-7. [DOI] [PubMed] [Google Scholar]




