Abstract
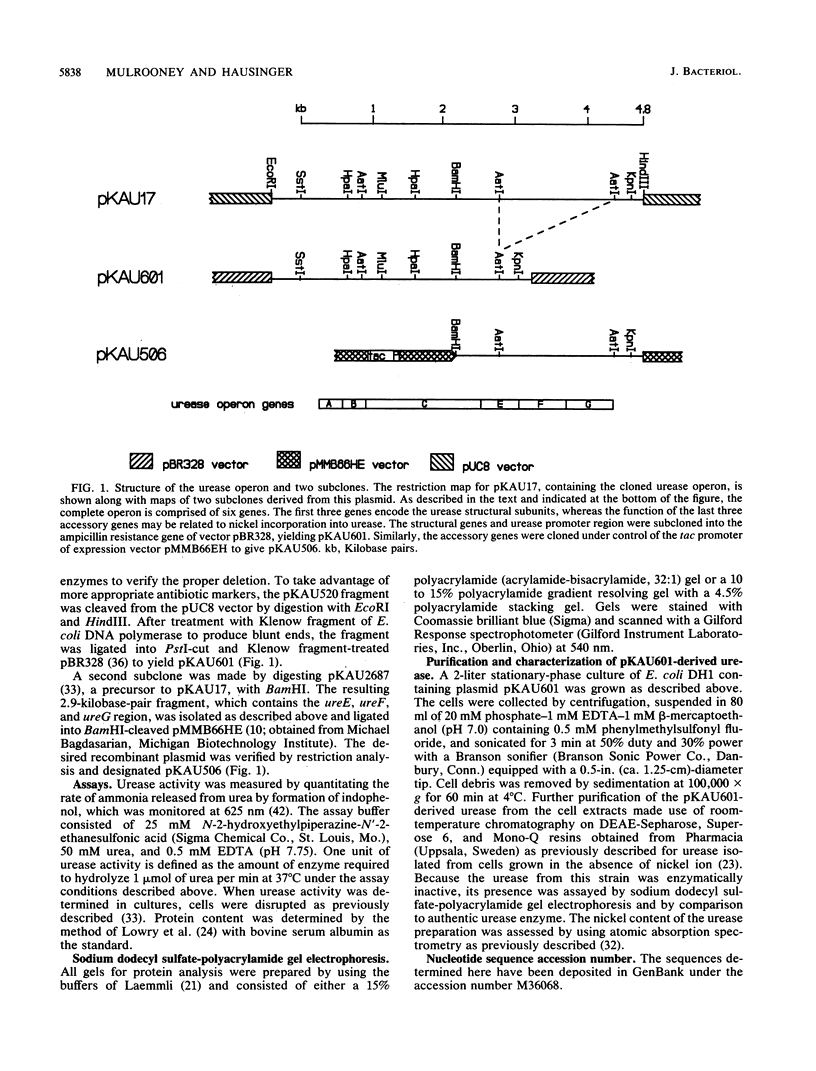
A 4.8-kilobase-pair region of cloned DNA encoding the genes of the Klebsiella aerogenes urease operon has been sequenced. Six closely spaced open reading frames were found: ureA (encoding a peptide of 11.1 kilodaltons [kDa]), ureB (11.7-kDa peptide), ureC (60.3-kDa peptide), ureE (17.6-kDa peptide), ureF (25.2-kDa peptide), and ureG (21.9-kDa peptide). Immediately after the ureG gene is a putative rho-dependent transcription terminator. The three subunits of the nickel-containing enzyme are encoded by ureA, ureB, and ureC based on protein structural studies and sequence homology to jack bean urease. Potential roles for ureE, ureF, and ureG were explored by deleting these accessory genes from the operon. The deletion mutant produced inactive urease, which was partially purified and found to have the same subunit stoichiometry and native size as the active enzyme but which contained no significant levels of nickel. The three accessory genes were able to activate apo-urease in vivo when they were cloned into a compatible expression vector and cotransformed into cells carrying the plasmid containing ureA, ureB, and ureC. Thus, one or more of the ureE, ureF, or ureG gene products are involved in nickel incorporation into urease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokranz M., Klein A., Meile L. Complete nucleotide sequence of plasmid pME2001 of Methanobacterium thermoautotrophicum (Marburg). Nucleic Acids Res. 1990 Jan 25;18(2):363–363. doi: 10.1093/nar/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. M., Falkow S. Genetic analysis of an Escherichia coli urease locus: evidence of DNA rearrangement. J Bacteriol. 1988 Mar;170(3):1041–1045. doi: 10.1128/jb.170.3.1041-1045.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon N. E., Gazzola T. C., blakeley R. L., Zermer B. Letter: Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc. 1975 Jul 9;97(14):4131–4133. doi: 10.1021/ja00847a045. [DOI] [PubMed] [Google Scholar]
- Dyke B., Hegenauer J., Saltman P., Laurs R. M. Isolation and characterization of a new zinc-binding protein from albacore tuna plasma. Biochemistry. 1987 Jun 2;26(11):3228–3234. doi: 10.1021/bi00385a044. [DOI] [PubMed] [Google Scholar]
- Ensign S. A., Campbell M. J., Ludden P. W. Activation of the nickel-deficient carbon monoxide dehydrogenase from Rhodospirillum rubrum: kinetic characterization and reductant requirement. Biochemistry. 1990 Feb 27;29(8):2162–2168. doi: 10.1021/bi00460a029. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Urease of Klebsiella aerogenes: control of its synthesis by glutamine synthetase. J Bacteriol. 1977 Aug;131(2):446–452. doi: 10.1128/jb.131.2.446-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Gatermann S., Marre R. Cloning and expression of Staphylococcus saprophyticus urease gene sequences in Staphylococcus carnosus and contribution of the enzyme to virulence. Infect Immun. 1989 Oct;57(10):2998–3002. doi: 10.1128/iai.57.10.2998-3002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hinton S. M., Dean D. Biogenesis of molybdenum cofactors. Crit Rev Microbiol. 1990;17(3):169–188. doi: 10.3109/10408419009105724. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J Bacteriol. 1988 Aug;170(8):3342–3349. doi: 10.1128/jb.170.8.3342-3349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: nucleotide sequence determination and comparison with jack bean urease. J Bacteriol. 1989 Dec;171(12):6414–6422. doi: 10.1128/jb.171.12.6414-6422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Hancock R. E., Ingraham J. L. Properties of a Pseudomonas stutzeri outer membrane channel-forming protein (NosA) required for production of copper-containing N2O reductase. J Bacteriol. 1989 Apr;171(4):2096–2100. doi: 10.1128/jb.171.4.2096-2100.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. H., Mulrooney S. B., Hausinger R. P. Purification, characterization, and in vivo reconstitution of Klebsiella aerogenes urease apoenzyme. J Bacteriol. 1990 Aug;172(8):4427–4431. doi: 10.1128/jb.172.8.4427-4431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay E. M., Pateman J. A. The regulation of urease activity in Aspergillus nidulans. Biochem Genet. 1982 Aug;20(7-8):763–776. doi: 10.1007/BF00483972. [DOI] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Bothling L E, Polacco J C, Cianzio S R. Pleiotropic soybean mutants defective in both urease isozymes. Mol Gen Genet. 1987 Oct;209(3):432–438. doi: 10.1007/BF00331146. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney S. B., Lynch M. J., Mobley H. L., Hausinger R. P. Purification, characterization, and genetic organization of recombinant Providencia stuartii urease expressed by Escherichia coli. J Bacteriol. 1988 May;170(5):2202–2207. doi: 10.1128/jb.170.5.2202-2207.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney S. B., Pankratz H. S., Hausinger R. P. Regulation of gene expression and cellular localization of cloned Klebsiella aerogenes (K. pneumoniae) urease. J Gen Microbiol. 1989 Jun;135(6):1769–1776. doi: 10.1099/00221287-135-6-1769. [DOI] [PubMed] [Google Scholar]
- Mörsdorf G., Kaltwasser H. Cloning of the genes encoding urease from Proteus vulgaris and sequencing of the structural genes. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):67–73. doi: 10.1016/0378-1097(90)90260-w. [DOI] [PubMed] [Google Scholar]
- Sheen J. Y., Seed B. Electrolyte gradient gels for DNA sequencing. Biotechniques. 1988 Nov-Dec;6(10):942–944. [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Takishima K., Suga T., Mamiya G. The structure of jack bean urease. The complete amino acid sequence, limited proteolysis and reactive cysteine residues. Eur J Biochem. 1988 Jul 15;175(1):151–165. doi: 10.1111/j.1432-1033.1988.tb14177.x. [DOI] [PubMed] [Google Scholar]
- Todd M. J., Hausinger R. P. Competitive inhibitors of Klebsiella aerogenes urease. Mechanisms of interaction with the nickel active site. J Biol Chem. 1989 Sep 25;264(27):15835–15842. [PubMed] [Google Scholar]
- Todd M. J., Hausinger R. P. Purification and characterization of the nickel-containing multicomponent urease from Klebsiella aerogenes. J Biol Chem. 1987 May 5;262(13):5963–5967. [PubMed] [Google Scholar]
- Walz S. E., Wray S. K., Hull S. I., Hull R. A. Multiple proteins encoded within the urease gene complex of Proteus mirabilis. J Bacteriol. 1988 Mar;170(3):1027–1033. doi: 10.1128/jb.170.3.1027-1033.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



