Abstract
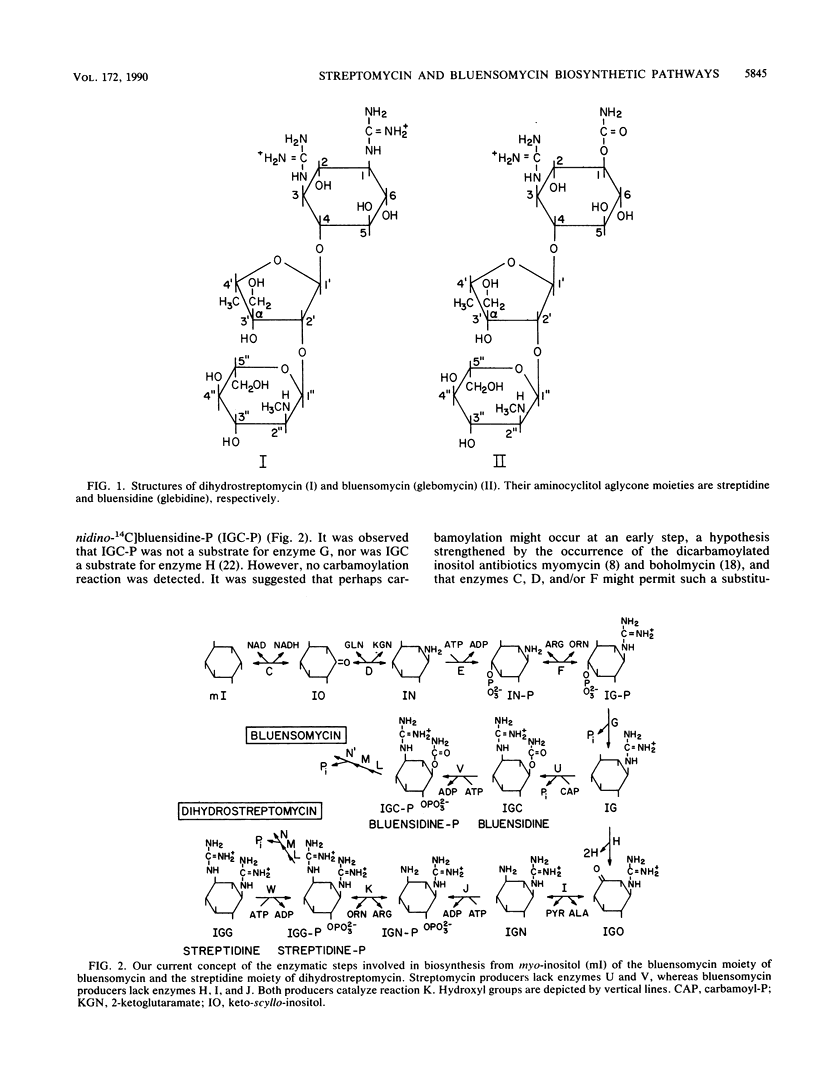
Bluensomycin (glebomycin) is an aminocyclitol antibiotic that differs structurally from dihydrostreptomycin in having bluensidine (1D-1-O-carbamoyl-3-guanidinodeoxy-scyllo-inositol) rather than streptidine (1,3-diguanidino-1,3-dideoxy-scyllo-inositol) as its aminocyclitol moiety. Extracts of the bluensomycin producer Streptomyces hygroscopicus form glebosus ATCC 14607 (S. glebosus) were found to have aminodeoxy-scyllo-inositol kinase activity but to lack 1D-1-guanidino-3-amino-1,3-dideoxy-scyllo-inositol kinase activity, showing for the first time that these two reactions in streptomycin producers must be catalyzed by different enzymes. S. glebosus extracts therefore possess the same five enzymes required for synthesis of guanidinodeoxy-scyllo-inositol from myo-inositol that are found in streptomycin producers but lack the next three of the four enzymes found in streptomycin producers that are required to synthesize the second guanidino group of streptidine-P. In place of a second guanidino group, S. glebosus extracts were found to catalyze a Mg2(+)-dependent carbamoylation of guanidinodeoxy-scyllo-inositol to form bluensidine, followed by a phosphorylation to form bluensidine-P. The novel carbamoyl-P:guanidinodeoxy-scyllo-inositol O-carbamoyltransferase and ATP:bluensidine phosphotransferase activities were not detected in streptomycin producers or in S. glebosus during its early rapid growth phase. Free bluensidine appears to be a normal intermediate in bluensomycin biosynthesis, in contrast to the case of streptomycin biosynthesis; in the latter, although exogenous streptidine can enter the pathway via streptidine-P, free streptidine is not an intermediate in the endogenous biosynthetic pathway. Comparison of the streptomycin and bluensomycin biosynthetic pathways provides a unique opportunity to evaluate those proposed mechanisms for the evolutionary acquisition of new biosynthetic capabilities that involve gene duplication and subsequent mutational changes in one member of the pair. In this model, there are at least five pairs of enzymes catalyzing analogous reactions that can be analyzed for homology at both the protein and DNA levels, including two putative pairs of inositol kinases detected in this study.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer S. J., Taylor P. M., Turner M. K. An adenosine triphosphate-dependent carbamoylphosphate--3-hydroxymethylcephem O-carbamoyltransferase from Streptomyces clavuligerus. Biochem J. 1980 Mar 1;185(3):555–564. doi: 10.1042/bj1850555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cundliffe E. How antibiotic-producing organisms avoid suicide. Annu Rev Microbiol. 1989;43:207–233. doi: 10.1146/annurev.mi.43.100189.001231. [DOI] [PubMed] [Google Scholar]
- Distler J., Ebert A., Mansouri K., Pissowotzki K., Stockmann M., Piepersberg W. Gene cluster for streptomycin biosynthesis in Streptomyces griseus: nucleotide sequence of three genes and analysis of transcriptional activity. Nucleic Acids Res. 1987 Oct 12;15(19):8041–8056. doi: 10.1093/nar/15.19.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J. C., Bartz Q. R., Dion H. W. Myomycin, a new antibiotic. J Antibiot (Tokyo) 1973 May;26(5):272–283. doi: 10.7164/antibiotics.26.272. [DOI] [PubMed] [Google Scholar]
- Lucher L. A., Chen Y. M., Walker J. B. Reactions catalyzed by purified L-glutamine: keto-scyllo-inositol aminotransferase, an enzyme required for biosynthesis of aminocyclitol antibiotics. Antimicrob Agents Chemother. 1989 Apr;33(4):452–459. doi: 10.1128/aac.33.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín M. F., Liras P. Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites. Annu Rev Microbiol. 1989;43:173–206. doi: 10.1146/annurev.mi.43.100189.001133. [DOI] [PubMed] [Google Scholar]
- Murphy P. J., Heycke N., Banfalvi Z., Tate M. E., de Bruijn F., Kondorosi A., Tempé J., Schell J. Genes for the catabolism and synthesis of an opine-like compound in Rhizobium meliloti are closely linked and on the Sym plasmid. Proc Natl Acad Sci U S A. 1987 Jan;84(2):493–497. doi: 10.1073/pnas.84.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka K., Demain A. L. Mutational biosynthesis of a new antibiotic, streptomutin A, by an idiotroph of Streptomyces griseus. J Antibiot (Tokyo) 1975 Sep;28(9):627–635. doi: 10.7164/antibiotics.28.627. [DOI] [PubMed] [Google Scholar]
- Ohnuki T., Imanaka T., Aiba S. Isolation of streptomycin-nonproducing mutants deficient in biosynthesis of the streptidine moiety or linkage between streptidine 6-phosphate and dihydrostreptose. Antimicrob Agents Chemother. 1985 Mar;27(3):367–374. doi: 10.1128/aac.27.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuki T., Imanaka T., Aiba S. Self-cloning in Streptomyces griseus of an str gene cluster for streptomycin biosynthesis and streptomycin resistance. J Bacteriol. 1985 Oct;164(1):85–94. doi: 10.1128/jb.164.1.85-94.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh K., Tsunakawa M., Tomita K., Miyaki T., Konishi M., Kawaguchi H. Boholmycin, a new aminoglycoside antibiotic. I. Production, isolation and properties. J Antibiot (Tokyo) 1988 Jul;41(7):855–861. doi: 10.7164/antibiotics.41.855. [DOI] [PubMed] [Google Scholar]
- Tohyama H., Okami Y., Umezawa H. Nucleotide sequence of the streptomycinphosphotransferase and amidinotransferase genes from Streptomyces griseus. Nucleic Acids Res. 1987 Feb 25;15(4):1819–1833. doi: 10.1093/nar/15.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER J. B., HNILICA V. S. DEVELOPMENTAL CHANGES IN ARGININE: X AMIDINOTRANSFERASE ACTIVITY IN STREPTOMYCIN-PRODUCING STRAINS OF STREPTOMYCES. Biochim Biophys Acta. 1964 Sep 18;89:473–482. doi: 10.1016/0926-6569(64)90073-2. [DOI] [PubMed] [Google Scholar]
- Walker J. B. Biosynthesis of the monoguanidinated inositol moiety of bluensomycin, a possible evolutionary precursor of streptomycin. J Biol Chem. 1974 Apr 25;249(8):2397–2404. [PubMed] [Google Scholar]
- Walker J. B. Enzymatic reactions involved in streptomycin biosynthesis and metabolism. Lloydia. 1971 Dec;34(4):363–371. [PubMed] [Google Scholar]
- Walker J. B. On the development of enzymic pathways for the biosynthesis of aminocyclitol antibiotics and other idiolites. Folia Microbiol (Praha) 1979;24(3):286–291. doi: 10.1007/BF02926462. [DOI] [PubMed] [Google Scholar]
- Walker J. B., Skorvaga M. Phosphorylation of streptomycin and dihydrostreptomycin by Streptomyces. Enzymatic synthesis of different diphosphorylated derivatives. J Biol Chem. 1973 Apr 10;248(7):2435–2440. [PubMed] [Google Scholar]
- Walker J. B. Streptomycin biosynthesis: enzymatic synthesis of inosamine and inosadiamine derivatives in the biosynthesis of streptidine. Ann N Y Acad Sci. 1969 Oct 17;165(2):646–654. [PubMed] [Google Scholar]
- Walker J. B., Walker M. S. Enzymatic synthesis of streptidine from scyllo-inosamine. Biochemistry. 1967 Dec;6(12):3821–3829. doi: 10.1021/bi00864a028. [DOI] [PubMed] [Google Scholar]
- Walker J. B., Walker M. S. Streptomycin biosynthesis. Enzymatic synthesis of O-phosphorylstreptidine from streptidine and adenosinetriphosphate. Biochim Biophys Acta. 1967 Nov 28;148(2):335–341. doi: 10.1016/0304-4165(67)90128-6. [DOI] [PubMed] [Google Scholar]
- Walker J. B., Walker M. S. Streptomycin biosynthesis. Transamination reactions involving inosamines and inosadiamines. Biochemistry. 1969 Mar;8(3):763–770. doi: 10.1021/bi00831a003. [DOI] [PubMed] [Google Scholar]
- Walker J. B., Walker M. S. Streptomycin biosynthesis: participation of a phosphatase, aminating enzyme, and kinase in cell-free synthesis of streptidine-P from inosamine-P. Biochem Biophys Res Commun. 1967 Feb 8;26(3):278–283. doi: 10.1016/0006-291x(67)90118-0. [DOI] [PubMed] [Google Scholar]
- Walker M. S., Walker J. B. Streptomycin biosynthesis. Separation and substrate specificities of phosphatases acting on guanidinodeoxy-scyllo-inositol phosphate and streptomycin-(streptidino)phosphate. J Biol Chem. 1971 Nov 25;246(22):7034–7040. [PubMed] [Google Scholar]
- Williams D. H., Stone M. J., Hauck P. R., Rahman S. K. Why are secondary metabolites (natural products) biosynthesized? J Nat Prod. 1989 Nov-Dec;52(6):1189–1208. doi: 10.1021/np50066a001. [DOI] [PubMed] [Google Scholar]



