Abstract
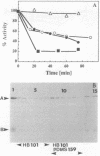
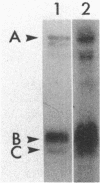
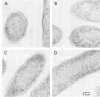
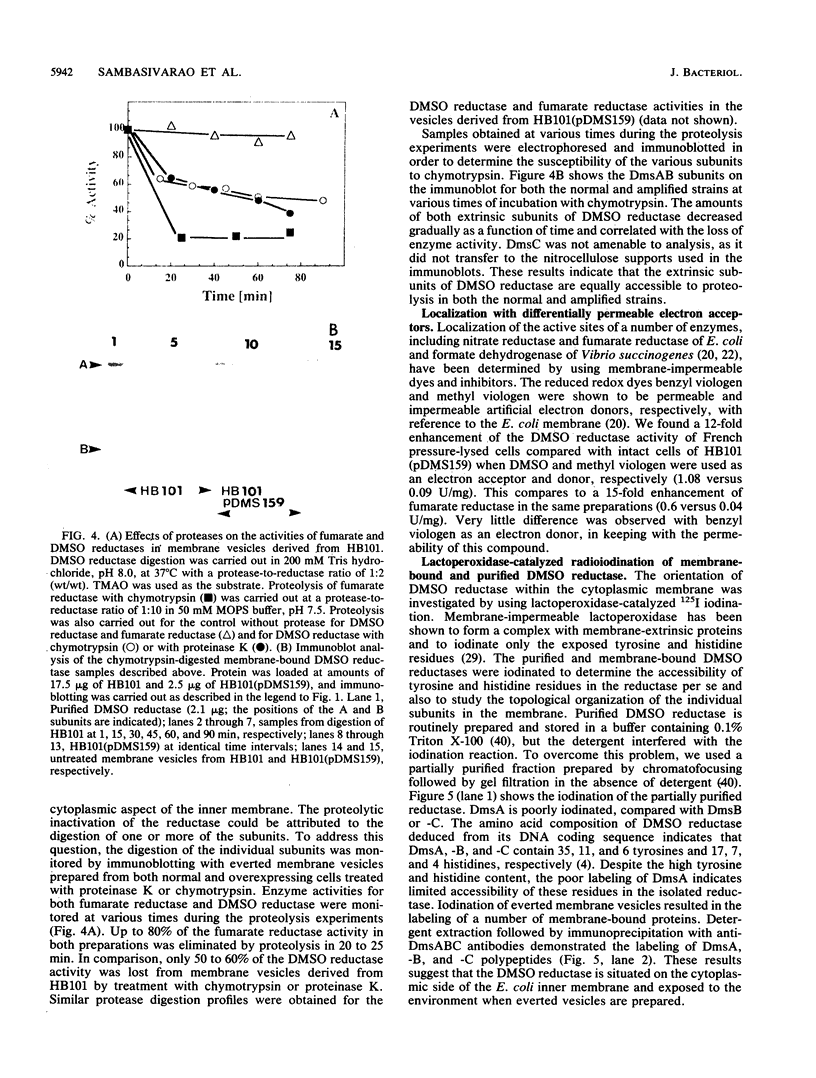
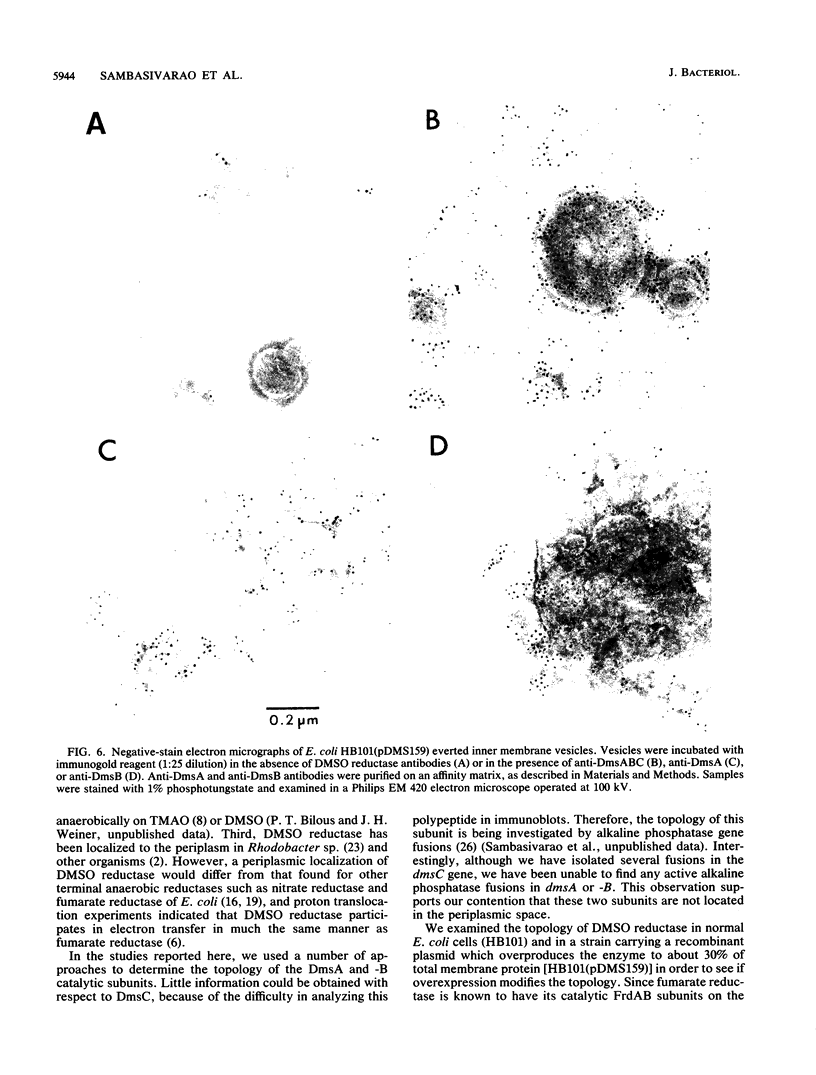
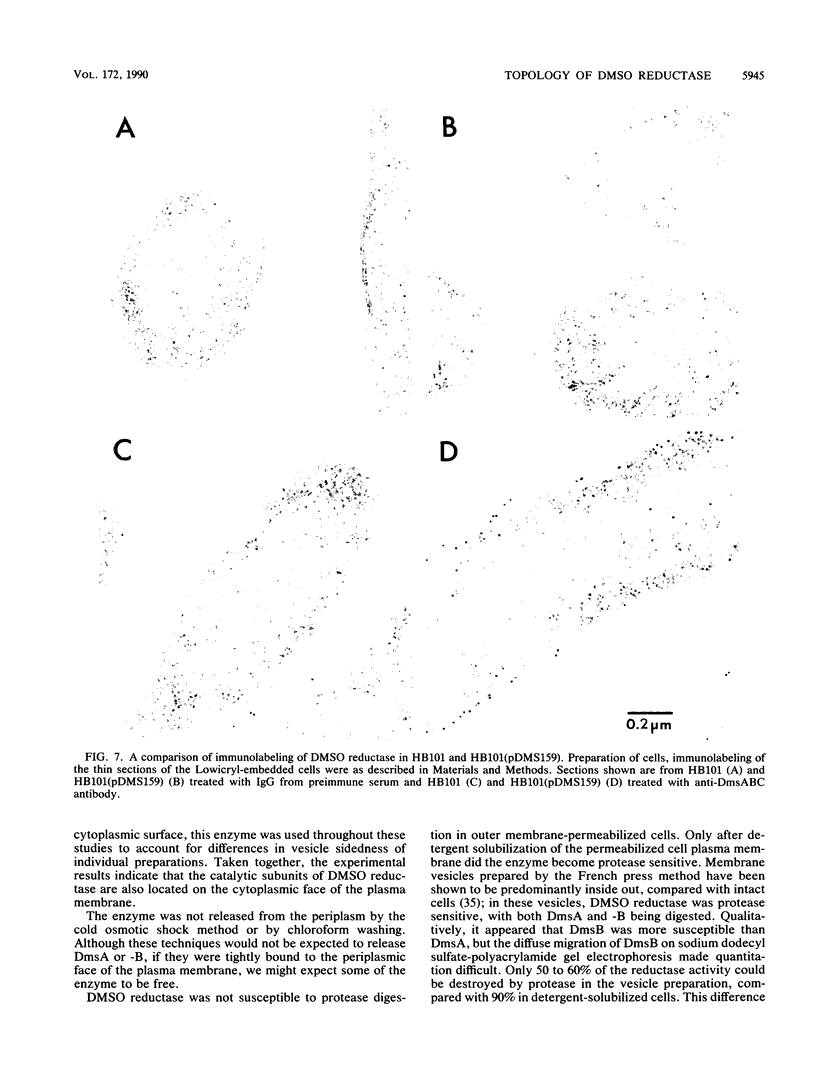
Dimethyl sulfoxide reductase is a trimeric, membrane-bound, iron-sulfur molybdoenzyme induced in Escherichia coli under anaerobic growth conditions. The enzyme catalyzes the reduction of dimethyl sulfoxide, trimethylamine N-oxide, and a variety of S- and N-oxide compounds. The topology of dimethyl sulfoxide reductase subunits was probed by a combination of techniques. Immunoblot analysis of the periplasmic proteins from the osmotic shock and chloroform wash fluids indicated that the subunits were not free in the periplasm. The reductase was susceptible to proteases in everted membrane vesicles, but the enzyme in outer membrane-permeabilized cells became protease sensitive only after detergent solubilization of the E. coli plasma membrane. Lactoperoxidase catalyzed the iodination of each of the three subunits in an everted membrane vesicle preparation. Antibodies to dimethyl sulfoxide reductase and fumarate reductase specifically agglutinated the everted membrane vesicles. No TnphoA fusions could be found in the dmsA or -B genes, indicating that these subunits were not translocated to the periplasm. Immunogold electron microscopy of everted membrane vesicles and thin sections by using antibodies to the DmsABC, DmsA, DmsB subunits resulted in specific labeling of the cytoplasmic surface of the inner membrane. These results show that the DmsA (catalytic subunit) and DmsB (electron transfer subunit) are membrane-extrinsic subunits facing the cytoplasmic side of the plasma membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Prody C., Kustu S. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J Bacteriol. 1984 Dec;160(3):1181–1183. doi: 10.1128/jb.160.3.1181-1183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. L., Kwan H. S. Bacterial reduction of trimethylamine oxide. Annu Rev Microbiol. 1985;39:131–149. doi: 10.1146/annurev.mi.39.100185.001023. [DOI] [PubMed] [Google Scholar]
- Bernadac A., Bolla J. M., Lazdunski C., Inouye M., Pages J. M. Precise localization of an overproduced periplasmic protein in Escherichia coli: use of double immuno-gold labelling. Biol Cell. 1987;61(3):141–147. doi: 10.1111/j.1768-322x.1987.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Bilous P. T., Cole S. T., Anderson W. F., Weiner J. H. Nucleotide sequence of the dmsABC operon encoding the anaerobic dimethylsulphoxide reductase of Escherichia coli. Mol Microbiol. 1988 Nov;2(6):785–795. doi: 10.1111/j.1365-2958.1988.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Bilous P. T., Weiner J. H. Dimethyl sulfoxide reductase activity by anaerobically grown Escherichia coli HB101. J Bacteriol. 1985 Jun;162(3):1151–1155. doi: 10.1128/jb.162.3.1151-1155.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilous P. T., Weiner J. H. Molecular cloning and expression of the Escherichia coli dimethyl sulfoxide reductase operon. J Bacteriol. 1988 Apr;170(4):1511–1518. doi: 10.1128/jb.170.4.1511-1518.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilous P. T., Weiner J. H. Proton translocation coupled to dimethyl sulfoxide reduction in anaerobically grown Escherichia coli HB101. J Bacteriol. 1985 Jul;163(1):369–375. doi: 10.1128/jb.163.1.369-375.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hackett N. R. Cytochromes of the trimethylamine N-oxide anaerobic respiratory pathway of Escherichia coli. Biochim Biophys Acta. 1983 Oct 31;725(1):168–177. doi: 10.1016/0005-2728(83)90237-2. [DOI] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Immunological screening method to detect specific translation products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2746–2749. doi: 10.1073/pnas.75.6.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T., Condon C., Lemire B. D., Weiner J. H. Molecular biology, biochemistry and bioenergetics of fumarate reductase, a complex membrane-bound iron-sulfur flavoenzyme of Escherichia coli. Biochim Biophys Acta. 1985 Dec;811(4):381–403. doi: 10.1016/0304-4173(85)90008-4. [DOI] [PubMed] [Google Scholar]
- Condon C., Weiner J. H. Fumarate reductase of Escherichia coli: an investigation of function and assembly using in vivo complementation. Mol Microbiol. 1988 Jan;2(1):43–52. doi: 10.1111/j.1365-2958.1988.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Elmes M. L., Scraba D. G., Weiner J. H. Isolation and characterization of the tubular organelles induced by fumarate reductase overproduction in Escherichia coli. J Gen Microbiol. 1986 Jun;132(6):1429–1439. doi: 10.1099/00221287-132-6-1429. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Owens K., Pietri R., Peterkofsky A. Localization to the inner surface of the cytoplasmic membrane by immunoelectron microscopy of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):849–853. doi: 10.1073/pnas.86.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. The organization of formate dehydrogenase in the cytoplasmic membrane of Escherichia coli. Biochem J. 1981 Jun 1;195(3):627–637. doi: 10.1042/bj1950627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977 Apr 15;164(1):199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A., Dorrer E., Winkler E. The orientation of the substrate sites of formate dehydrogenase and fumarate reductase in the membrane of Vibrio succinogenes. Biochim Biophys Acta. 1980 Jan 4;589(1):118–136. doi: 10.1016/0005-2728(80)90136-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemire B. D., Robinson J. J., Bradley R. D., Scraba D. G., Weiner J. H. Structure of fumarate reductase on the cytoplasmic membrane of Escherichia coli. J Bacteriol. 1983 Jul;155(1):391–397. doi: 10.1128/jb.155.1.391-397.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Morrison M. The determination of the exposed proteins on membranes by the use of lactoperoxidase. Methods Enzymol. 1974;32:103–109. doi: 10.1016/0076-6879(74)32013-7. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Pascal M. C., Burini J. F., Chippaux M. Regulation of the trimethylamine N-oxide (TMAO) reductase in Escherichia coli: analysis of tor::Mud1 operon fusion. Mol Gen Genet. 1984;195(1-2):351–355. doi: 10.1007/BF00332770. [DOI] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., Tsuchiya T. Preparation of everted membrane vesicles from Escherichia coli for the measurement of calcium transport. Methods Enzymol. 1979;56:233–241. doi: 10.1016/0076-6879(79)56026-1. [DOI] [PubMed] [Google Scholar]
- Roth J. Post-embedding cytochemistry with gold-labelled reagents: a review. J Microsc. 1986 Aug;143(Pt 2):125–137. doi: 10.1111/j.1365-2818.1986.tb02771.x. [DOI] [PubMed] [Google Scholar]
- Shimokawa O., Ishimoto M. Purification and some properties of inducible tertiary amine N-oxide reductase from Escherichia coli. J Biochem. 1979 Dec;86(6):1709–1717. doi: 10.1093/oxfordjournals.jbchem.a132691. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., Lemire B. D., Elmes M. L., Bradley R. D., Scraba D. G. Overproduction of fumarate reductase in Escherichia coli induces a novel intracellular lipid-protein organelle. J Bacteriol. 1984 May;158(2):590–596. doi: 10.1128/jb.158.2.590-596.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., MacIsaac D. P., Bishop R. E., Bilous P. T. Purification and properties of Escherichia coli dimethyl sulfoxide reductase, an iron-sulfur molybdoenzyme with broad substrate specificity. J Bacteriol. 1988 Apr;170(4):1505–1510. doi: 10.1128/jb.170.4.1505-1510.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W., Goodman J. M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983 Oct 10;258(19):12073–12080. [PubMed] [Google Scholar]
- Yamamoto I., Okubo N., Ishimoto M. Further characterization of trimethylamine N-oxide reductase from Escherichia coli, a molybdoprotein. J Biochem. 1986 Jun;99(6):1773–1779. doi: 10.1093/oxfordjournals.jbchem.a135655. [DOI] [PubMed] [Google Scholar]
- Yamato I., Anraku Y., Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem. 1975 Apr;77(4):705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Wickner W., Dalbey R. E. The cytoplasmic domain of Escherichia coli leader peptidase is a "translocation poison" sequence. Proc Natl Acad Sci U S A. 1988 May;85(10):3363–3366. doi: 10.1073/pnas.85.10.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]