Abstract
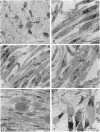
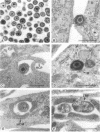
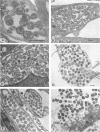
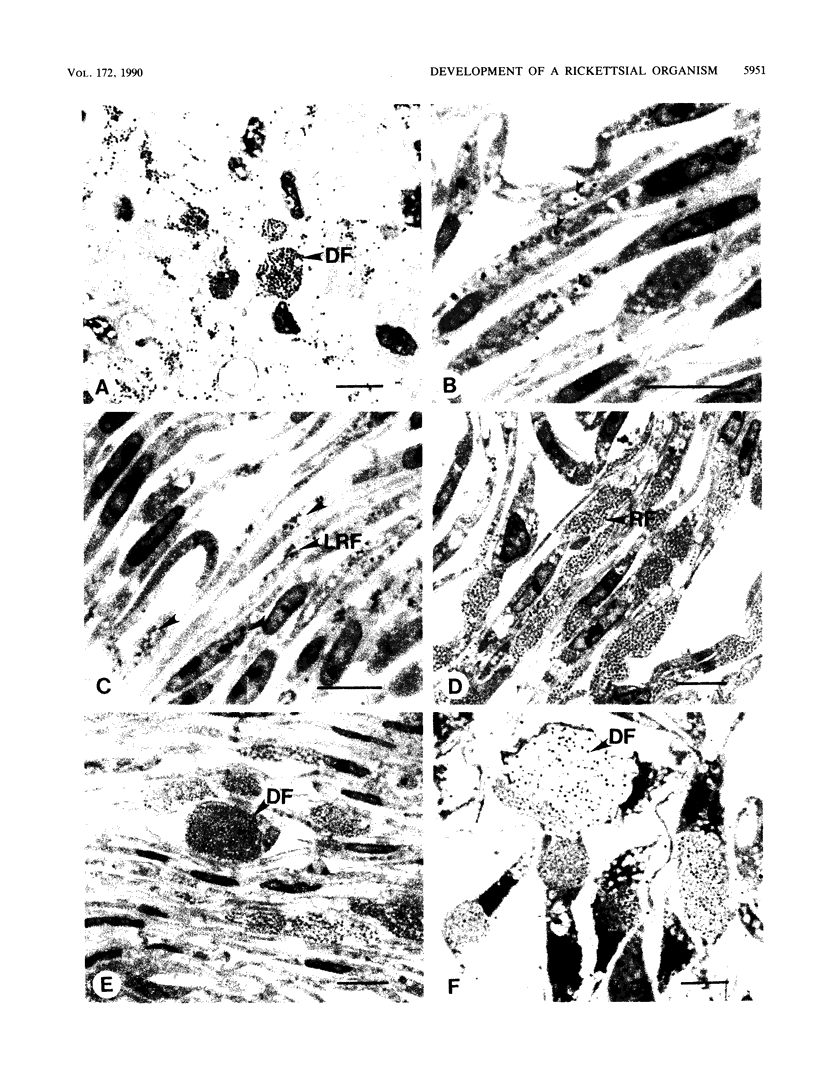
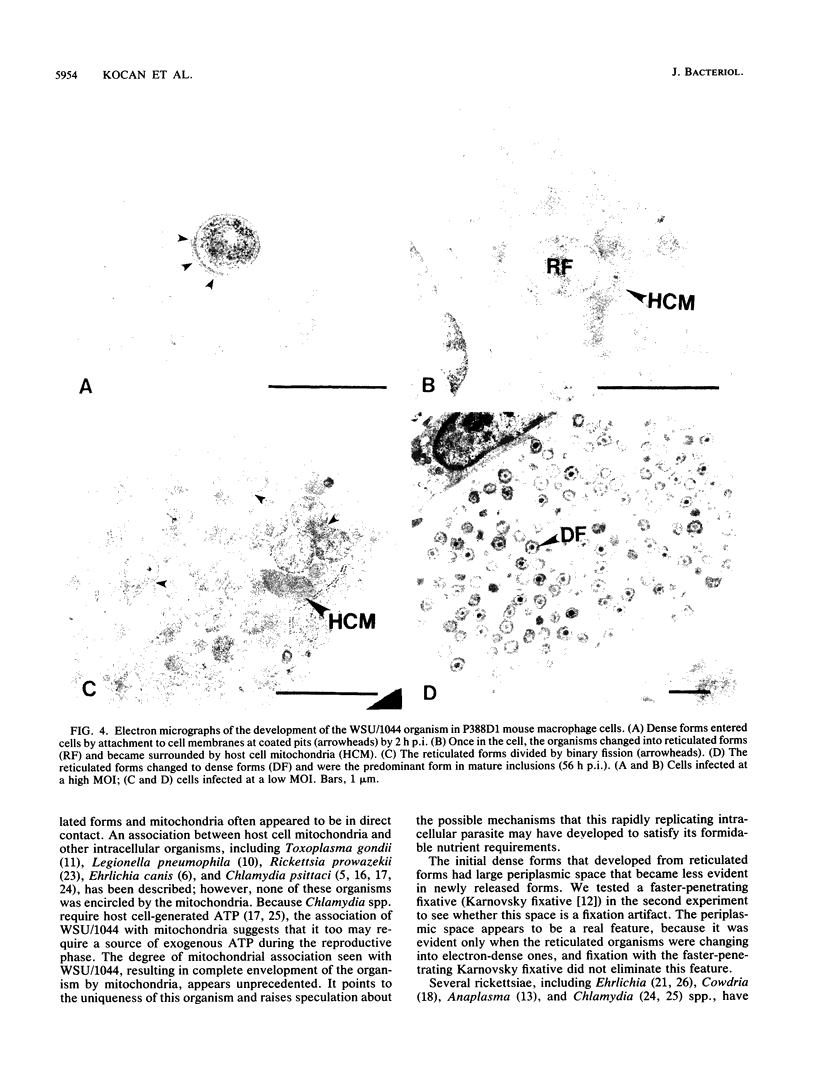
An obligate intracellular rickettsial organism isolated from an aborted bovine fetus was studied in bovine turbinate and mouse macrophage cell cultures with light and electron microscopy. Development of the organism was similar in both cell types. The organism replicated within cytoplasmic vacuoles in a developmental cycle that resembled that of both the ehrlichiae and chlamydiae. The inoculum contained only electron-dense forms, which infected cells within 2 h postinoculation by adhering to cell membranes at thickened areas that appeared to be coated pits and then being endocytosed. A striking feature occurred next as the organisms became surrounded by host cell mitochondria and, by light microscopy, appeared to have halos. During this intimate association with mitochondria, the electron-dense organisms changed into large reticulated forms that began to divide by binary fission. These large forms were often in direct contact with mitochondrial membranes. The organisms continued to divide by binary fission, and host cells contained large cytoplasmic inclusions of reticulated organisms. The reticulated organisms gradually changed into electron-dense forms that were released from degenerated host cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avakyan A. A., Popov V. L. Rickettsiaceae and Chlamydiaceae: comparative electron microscopic studies. Acta Virol. 1984 Mar;28(2):159–173. [PubMed] [Google Scholar]
- Cole A. I., Ristic M., Lewis G. E., Jr, Rapmund G. Continuous propagation of Ehrlichia sennetsu in murine macrophage cell cultures. Am J Trop Med Hyg. 1985 Jul;34(4):774–780. doi: 10.4269/ajtmh.1985.34.774. [DOI] [PubMed] [Google Scholar]
- Dilbeck P. M., Evermann J. F., Crawford T. B., Ward A. C., Leathers C. W., Holland C. J., Mebus C. A., Logan L. L., Rurangirwa F. R., McGuire T. C. Isolation of a previously undescribed rickettsia from an aborted bovine fetus. J Clin Microbiol. 1990 Apr;28(4):814–816. doi: 10.1128/jcm.28.4.814-816.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt P. K., Conroy J. D., McKee A. E., Nyindo M. B., Huxsoll D. L. Ultrastructure of Ehrlichia canis. Infect Immun. 1973 Feb;7(2):265–271. doi: 10.1128/iai.7.2.265-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Davis C. H., Choong J., Wyrick P. B. Ultrastructural study of endocytosis of Chlamydia trachomatis by McCoy cells. Infect Immun. 1988 Jun;56(6):1456–1463. doi: 10.1128/iai.56.6.1456-1463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Wyrick P. B. Ultrastructural study of mode of entry of Chlamydia psittaci into L-929 cells. Infect Immun. 1986 Dec;54(3):855–863. doi: 10.1128/iai.54.3.855-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983 Oct 1;158(4):1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan K. M., Bezuidenhout J. D., Hart A. Ultrastructural features of Cowdria ruminantium in midgut epithelial cells and salivary glands of nymphal Amblyomma hebraeum. Onderstepoort J Vet Res. 1987 Mar;54(1):87–92. [PubMed] [Google Scholar]
- Kocan K. M., Bezuidenhout J. D. Morphology and development of Cowdria ruminantium in Amblyomma ticks. Onderstepoort J Vet Res. 1987 Sep;54(3):177–182. [PubMed] [Google Scholar]
- Matsumoto A. Isolation and electron microscopic observations of intracytoplasmic inclusions containing Chlamydia psittaci. J Bacteriol. 1981 Jan;145(1):605–612. doi: 10.1128/jb.145.1.605-612.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar J. G. Electron microscopy of Cowdria (Rickettsia) ruminantium (Cowdry, 1926) in the endothelial cells of the vertebrate host. Onderstepoort J Vet Res. 1970 Mar;37(1):67–78. [PubMed] [Google Scholar]
- Provost A., Bezuidenhout J. D. The historical background and global importance of heartwater. Onderstepoort J Vet Res. 1987 Sep;54(3):165–169. [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D., Cordes D. O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985 Sep;49(3):505–512. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. In vitro studies of Rickettsia-host cell interactions: ultrastructural study of Rickettsia prowazekii-infected chicken embryo fibroblasts. Infect Immun. 1980 Aug;29(2):778–790. doi: 10.1128/iai.29.2.778-790.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd W. J., Doughri A. M., Storz J. Ultrastructural changes in host cellular organelles in the course of the chlamydial developmental cycle. Zentralbl Bakteriol Orig A. 1976 Nov;236(2-3):359–373. [PubMed] [Google Scholar]
- Tuomi J., von Bonsdorff C. H. Electron microscopy of tick-borne fever agent in bovine and ovine phagocytizing leukocytes. J Bacteriol. 1966 Nov;92(5):1478–1492. doi: 10.1128/jb.92.5.1478-1492.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uilenberg G. Heartwater (Cowdria ruminantium infection): current status. Adv Vet Sci Comp Med. 1983;27:427–480. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Dobson M. E., Samuel J. E., Dasch G. A., Mallavia L. P., Baca O., Mandelco L., Sechrest J. E., Weiss E., Woese C. R. Phylogenetic diversity of the Rickettsiae. J Bacteriol. 1989 Aug;171(8):4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]