Abstract
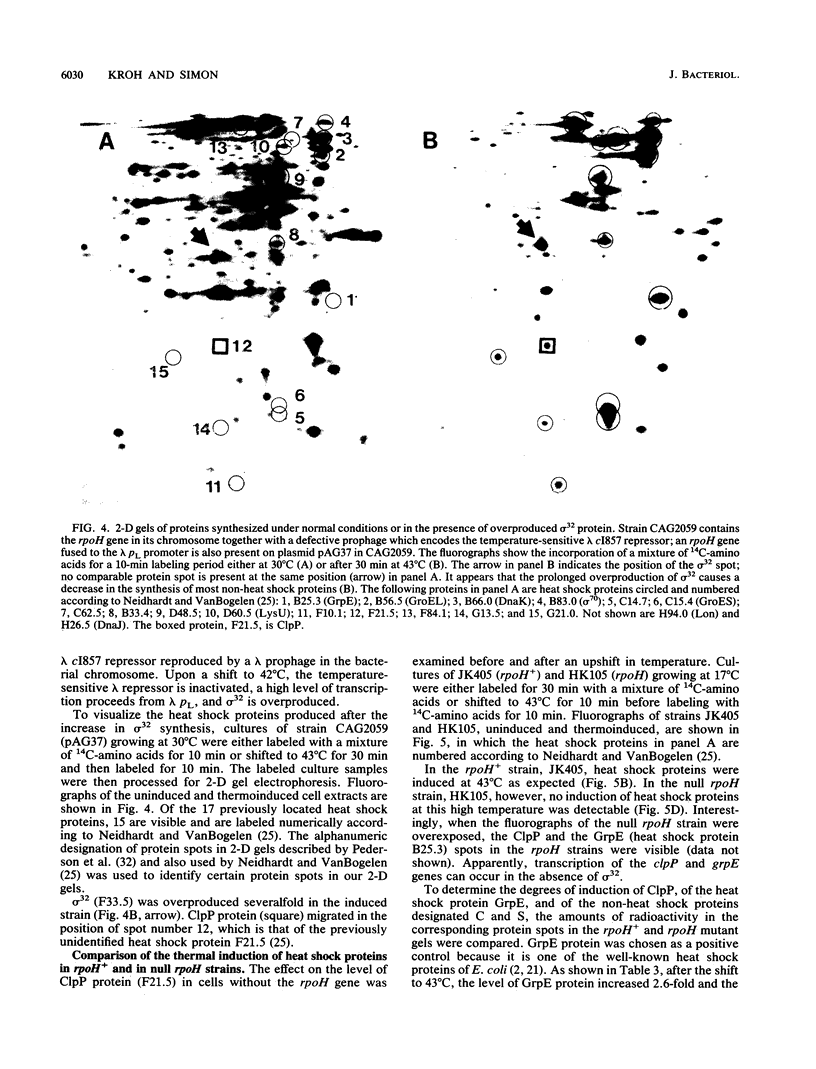
The genes that encode the subunits of the Clp protease of Escherichia coli, clpA and clpP, appear to be regulated differently from each other. The clpA gene does not seem to be under heat shock control (Y. S. Katayama, S. Gottesman, J. Pumphrey, S. Rudikoff, W. P. Clark, and M. R. Maurizi, J. Biol. Chem. 263:15226-15236, 1988). In contrast, the level of ClpP protein was increased in rpoH+ cells but not in null rpoH cells after an upshift in temperature from 17 to 43 degrees C. The level of ClpP protein in a null dnaK strain was also elevated relative to the level of ClpP protein in an otherwise isogenic dnaK+ strain. In two-dimensional gels, the ClpP protein was located in the position of the previously unidentified heat shock protein F21.5. No protein spot corresponding to F21.5 was present in two-dimensional gels of a null clpP strain. The clpP gene, therefore, appears to be a heat shock gene, expressed in a sigma 32-dependent manner and negatively regulated by DnaK; the product of clpP is the previously unidentified heat shock protein F21.5.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang D., Chandrasekhar G. N., Zylicz M., Georgopoulos C. Escherichia coli grpE gene codes for heat shock protein B25.3, essential for both lambda DNA replication at all temperatures and host growth at high temperature. J Bacteriol. 1986 Jul;167(1):25–29. doi: 10.1128/jb.167.1.25-29.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang D., Georgopoulos C. The heat-shock-regulated grpE gene of Escherichia coli is required for bacterial growth at all temperatures but is dispensable in certain mutant backgrounds. J Bacteriol. 1989 May;171(5):2748–2755. doi: 10.1128/jb.171.5.2748-2755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Grossman A. D., Gross C. A. A gene regulating the heat shock response in Escherichia coli also affects proteolysis. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6779–6783. doi: 10.1073/pnas.81.21.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Tilly K., Craig E., King J., Zylicz M., Georgopoulos C. The nucleotide sequence of the Escherichia coli K12 dnaJ+ gene. A gene that encodes a heat shock protein. J Biol Chem. 1986 Feb 5;261(4):1782–1785. [PubMed] [Google Scholar]
- Brandsma J. A., Bosch D., Backendorf C., van de Putte P. A common regulatory region shared by divergently transcribed genes of the Escherichia coli SOS system. Nature. 1983 Sep 15;305(5931):243–245. doi: 10.1038/305243a0. [DOI] [PubMed] [Google Scholar]
- Buell G., Schulz M. F., Selzer G., Chollet A., Movva N. R., Semon D., Escanez S., Kawashima E. Optimizing the expression in E. coli of a synthetic gene encoding somatomedin-C (IGF-I). Nucleic Acids Res. 1985 Mar 25;13(6):1923–1938. doi: 10.1093/nar/13.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P. R., Grossman L. Incision of damaged versus nondamaged DNA by the Escherichia coli UvrABC proteins. Nucleic Acids Res. 1988 Aug 25;16(16):7855–7865. doi: 10.1093/nar/16.16.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. T., Goff S. A., Webster T., Smith T., Goldberg A. L. Sequence of the lon gene in Escherichia coli. A heat-shock gene which encodes the ATP-dependent protease La. J Biol Chem. 1988 Aug 25;263(24):11718–11728. [PubMed] [Google Scholar]
- Georgopoulos C., Tilly K., Drahos D., Hendrix R. The B66.0 protein of Escherichia coli is the product of the dnaK+ gene. J Bacteriol. 1982 Mar;149(3):1175–1177. doi: 10.1128/jb.149.3.1175-1177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Casson L. P., Goldberg A. L. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6647–6651. doi: 10.1073/pnas.81.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Goldberg A. L. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985 Jun;41(2):587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Waxman L. The role of ATP hydrolysis in the breakdown of proteins and peptides by protease La from Escherichia coli. J Biol Chem. 1985 Oct 5;260(22):12029–12034. [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Bloch P. L., Van Bogelen R. A., Neidhardt F. C. Multiple forms of lysyl-transfer ribonucleic acid synthetase in Escherichia coli. J Bacteriol. 1981 Apr;146(1):345–351. doi: 10.1128/jb.146.1.345-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B. J., Park W. J., Chung C. H., Goldberg A. L. Escherichia coli contains a soluble ATP-dependent protease (Ti) distinct from protease La. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5550–5554. doi: 10.1073/pnas.84.16.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B. J., Woo K. M., Goldberg A. L., Chung C. H. Protease Ti, a new ATP-dependent protease in Escherichia coli, contains protein-activated ATPase and proteolytic functions in distinct subunits. J Biol Chem. 1988 Jun 25;263(18):8727–8734. [PubMed] [Google Scholar]
- Katayama-Fujimura Y., Gottesman S., Maurizi M. R. A multiple-component, ATP-dependent protease from Escherichia coli. J Biol Chem. 1987 Apr 5;262(10):4477–4485. [PubMed] [Google Scholar]
- Katayama Y., Gottesman S., Pumphrey J., Rudikoff S., Clark W. P., Maurizi M. R. The two-component, ATP-dependent Clp protease of Escherichia coli. Purification, cloning, and mutational analysis of the ATP-binding component. J Biol Chem. 1988 Oct 15;263(29):15226–15236. [PubMed] [Google Scholar]
- Keller J. A., Simon L. D. Divergent effects of a dnaK mutation on abnormal protein degradation in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):31–41. doi: 10.1111/j.1365-2958.1988.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipinska B., King J., Ang D., Georgopoulos C. Sequence analysis and transcriptional regulation of the Escherichia coli grpE gene, encoding a heat shock protein. Nucleic Acids Res. 1988 Aug 11;16(15):7545–7562. doi: 10.1093/nar/16.15.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M. R., Trisler P., Gottesman S. Insertional mutagenesis of the lon gene in Escherichia coli: lon is dispensable. J Bacteriol. 1985 Dec;164(3):1124–1135. doi: 10.1128/jb.164.3.1124-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa S., Gottesman S. Protein degradation in Escherichia coli: the lon gene controls the stability of sulA protein. Proc Natl Acad Sci U S A. 1983 Jan;80(2):358–362. doi: 10.1073/pnas.80.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Phillips T. A., VanBogelen R. A., Smith M. W., Georgalis Y., Subramanian A. R. Identity of the B56.5 protein, the A-protein, and the groE gene product of Escherichia coli. J Bacteriol. 1981 Jan;145(1):513–520. doi: 10.1128/jb.145.1.513-520.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. Characterization of the helicase activity of the Escherichia coli UvrAB protein complex. J Biol Chem. 1989 Jan 15;264(2):1336–1343. [PubMed] [Google Scholar]
- Orren D. K., Sancar A. The (A)BC excinuclease of Escherichia coli has only the UvrB and UvrC subunits in the incision complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5237–5241. doi: 10.1073/pnas.86.14.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek K. H., Walker G. C. Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987 Jan;169(1):283–290. doi: 10.1128/jb.169.1.283-290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell D. A., Sauer R. T. Induction of a heat shock-like response by unfolded protein in Escherichia coli: dependence on protein level not protein degradation. Genes Dev. 1989 Aug;3(8):1226–1232. doi: 10.1101/gad.3.8.1226. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Phillips T. A., VanBogelen R. A., Neidhardt F. C. lon gene product of Escherichia coli is a heat-shock protein. J Bacteriol. 1984 Jul;159(1):283–287. doi: 10.1128/jb.159.1.283-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. Escherichia coli heat shock gene mutants are defective in proteolysis. Genes Dev. 1988 Dec;2(12B):1851–1858. doi: 10.1101/gad.2.12b.1851. [DOI] [PubMed] [Google Scholar]
- Swamy K. H., Goldberg A. L. E. coli contains eight soluble proteolytic activities, one being ATP dependent. Nature. 1981 Aug 13;292(5824):652–654. doi: 10.1038/292652a0. [DOI] [PubMed] [Google Scholar]
- Taylor W. E., Straus D. B., Grossman A. D., Burton Z. F., Gross C. A., Burgess R. R. Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell. 1984 Sep;38(2):371–381. doi: 10.1016/0092-8674(84)90492-6. [DOI] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Tilly K., VanBogelen R. A., Georgopoulos C., Neidhardt F. C. Identification of the heat-inducible protein C15.4 as the groES gene product in Escherichia coli. J Bacteriol. 1983 Jun;154(3):1505–1507. doi: 10.1128/jb.154.3.1505-1507.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Acton M. A., Neidhardt F. C. Induction of the heat shock regulon does not produce thermotolerance in Escherichia coli. Genes Dev. 1987 Aug;1(6):525–531. doi: 10.1101/gad.1.6.525. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnbauer B. A., Foley E. C., Henderson G. W., Markovitz A. Identification and purification of the Lon+ (capR+) gene product, a DNA-binding protein. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2043–2047. doi: 10.1073/pnas.78.4.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. N., Kusukawa N., Erickson J. W., Gross C. A., Yura T. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor sigma 32. J Bacteriol. 1988 Aug;170(8):3640–3649. doi: 10.1128/jb.170.8.3640-3649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sluis C. A., Moolenaar G. F., Backendorf C. Regulation of the uvrC gene of Escherichia coli K12: localization and characterization of a damage-inducible promoter. EMBO J. 1983;2(12):2313–2318. doi: 10.1002/j.1460-2075.1983.tb01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E., Zwetsloot J., Noordermeer I., Pannekoek H., Dekker B., Dijkema R., van Ormondt H. The structure and function of the regulatory elements of the Escherichia coli uvrB gene. Nucleic Acids Res. 1981 Nov 11;9(21):5623–5643. doi: 10.1093/nar/9.21.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]