Abstract
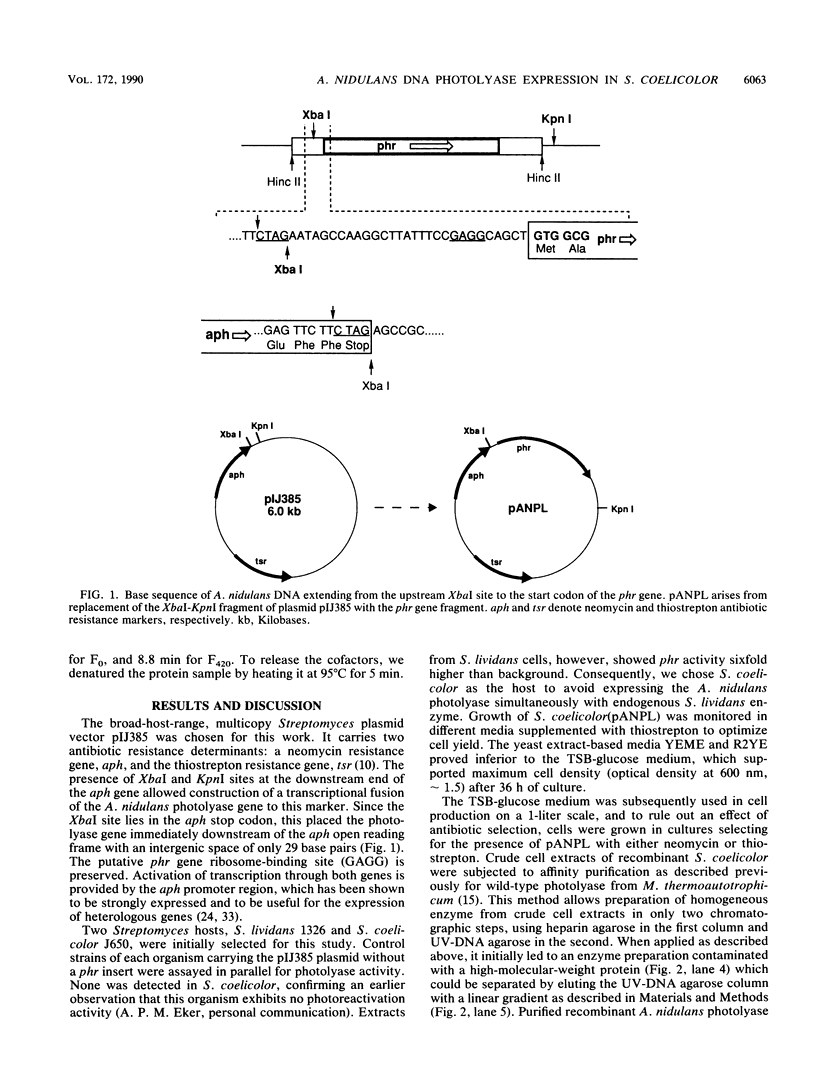
The gene encoding Anacystis nidulans 5-deazaflavin-dependent photolyase (phr) was inserted into the Streptomyces vector pIJ385 to form a transcriptional fusion with the neomycin resistance (aph) gene. The resulting plasmid, pANPL, was introduced into Streptomyces coelicolor, a host which exhibits no detectable photolyase activity and provides 5-deazaflavins. Transformants expressed functional photolyase and could be cultured at much higher cell densities than A. nidulans. A two-step affinity protocol was used to purify photolyase to homogeneity. High-pressure liquid chromatographic analysis established the presence of 5-deazaflavin cofactors in the enzyme, showing that this expression system allows heterologous production of 5-deazaflavin-class photolyases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coats J. H., Li G. P., Kuo M. S., Yurek D. A. Discovery, production, and biological assay of an unusual flavenoid cofactor involved in lincomycin biosynthesis. J Antibiot (Tokyo) 1989 Mar;42(3):472–474. doi: 10.7164/antibiotics.42.472. [DOI] [PubMed] [Google Scholar]
- Eker A. P., Dekker R. H., Berends W. Photoreactivating enzyme from Streptomyces griseus-IV. On the nature of the chromophoric cofactor in Streptomyces griseus photoreactivating enzyme. Photochem Photobiol. 1981 Jan;33(1):65–72. doi: 10.1111/j.1751-1097.1981.tb04298.x. [DOI] [PubMed] [Google Scholar]
- Eker A. P., Kooiman P., Hessels J. K., Yasui A. DNA photoreactivating enzyme from the cyanobacterium Anacystis nidulans. J Biol Chem. 1990 May 15;265(14):8009–8015. [PubMed] [Google Scholar]
- Harm W., Harm H., Rupert C. S. Analysis of photoenzymatic repair of UV lesions in DNA by single light flashes. II. In vivo studies with Escherichia coli cells and bacteriophage. Mutat Res. 1968 Nov-Dec;6(3):371–385. doi: 10.1016/0027-5107(68)90054-7. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P., Honek J. F., Walsh C. Separation of flavins and flavin analogs by high-performance liquid chromatography. Methods Enzymol. 1986;122:199–209. doi: 10.1016/0076-6879(86)22171-0. [DOI] [PubMed] [Google Scholar]
- Husain I., Sancar A. Photoreactivation in phr mutants of Escherichia coli K-12. J Bacteriol. 1987 Jun;169(6):2367–2372. doi: 10.1128/jb.169.6.2367-2372.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Hamm-Alvarez S., Payne G., Sancar G. B., Rajagopalan K. V., Sancar A. Identification of the second chromophore of Escherichia coli and yeast DNA photolyases as 5,10-methenyltetrahydrofolate. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2046–2050. doi: 10.1073/pnas.85.7.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiener A., Husain I., Sancar A., Walsh C. Purification and properties of Methanobacterium thermoautotrophicum DNA photolyase. J Biol Chem. 1989 Aug 15;264(23):13880–13887. [PubMed] [Google Scholar]
- Kobayashi T., Takao M., Oikawa A., Yasui A. Molecular characterization of a gene encoding a photolyase from Streptomyces griseus. Nucleic Acids Res. 1989 Jun 26;17(12):4731–4744. doi: 10.1093/nar/17.12.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbe C., Wolfe S., Demain A. L. Repression and inhibition of cephalosporin synthetases in Streptomyces clavuligerus by inorganic phosphate. Arch Microbiol. 1985 Jan;140(4):317–320. doi: 10.1007/BF00446970. [DOI] [PubMed] [Google Scholar]
- Merrick M. J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976 Oct;96(2):299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- Minato S., Werbin H. Excitation and fluorescence spectra of the chromophore associated with the DNA-photoreactivating enzyme from the blue-green alga Anacystis nidulans. Photochem Photobiol. 1972 Jan;15(1):97–100. doi: 10.1111/j.1751-1097.1972.tb06227.x. [DOI] [PubMed] [Google Scholar]
- Myles G. M., Sancar A. DNA repair. Chem Res Toxicol. 1989 Jul-Aug;2(4):197–226. doi: 10.1021/tx00010a001. [DOI] [PubMed] [Google Scholar]
- Payne G., Heelis P. F., Rohrs B. R., Sancar A. The active form of Escherichia coli DNA photolyase contains a fully reduced flavin and not a flavin radical, both in vivo and in vitro. Biochemistry. 1987 Nov 3;26(22):7121–7127. doi: 10.1021/bi00396a038. [DOI] [PubMed] [Google Scholar]
- Pulido D., Jiménez A. Optimization of gene expression in Streptomyces lividans by a transcription terminator. Nucleic Acids Res. 1987 May 26;15(10):4227–4240. doi: 10.1093/nar/15.10.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. Escherichia coli DNA photolyase is a flavoprotein. J Mol Biol. 1984 Jan 15;172(2):223–227. doi: 10.1016/s0022-2836(84)80040-6. [DOI] [PubMed] [Google Scholar]
- Sancar A., Smith F. W., Sancar G. B. Purification of Escherichia coli DNA photolyase. J Biol Chem. 1984 May 10;259(9):6028–6032. [PubMed] [Google Scholar]
- Sancar G. B., Jorns M. S., Payne G., Fluke D. J., Rupert C. S., Sancar A. Action mechanism of Escherichia coli DNA photolyase. III. Photolysis of the enzyme-substrate complex and the absolute action spectrum. J Biol Chem. 1987 Jan 5;262(1):492–498. [PubMed] [Google Scholar]
- Sancar G. B., Smith F. W., Heelis P. F. Purification of the yeast PHR1 photolyase from an Escherichia coli overproducing strain and characterization of the intrinsic chromophores of the enzyme. J Biol Chem. 1987 Nov 15;262(32):15457–15465. [PubMed] [Google Scholar]
- Sancar G. B., Smith F. W., Lorence M. C., Rupert C. S., Sancar A. Sequences of the Escherichia coli photolyase gene and protein. J Biol Chem. 1984 May 10;259(9):6033–6038. [PubMed] [Google Scholar]
- Takao M., Kobayashi T., Oikawa A., Yasui A. Tandem arrangement of photolyase and superoxide dismutase genes in Halobacterium halobium. J Bacteriol. 1989 Nov;171(11):6323–6329. doi: 10.1128/jb.171.11.6323-6329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao M., Oikawa A., Eker A. P., Yasui A. Expression of an Anacystis nidulans photolyase gene in Escherichia coli; functional complementation and modified action spectrum of photoreactivation. Photochem Photobiol. 1989 Nov;50(5):633–637. doi: 10.1111/j.1751-1097.1989.tb04319.x. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Gray G. S. Nucleotide sequence of a streptomycete aminoglycoside phosphotransferase gene and its relationship to phosphotransferases encoded by resistance plasmids. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5190–5194. doi: 10.1073/pnas.80.17.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui A., Takao M., Oikawa A., Kiener A., Walsh C. T., Eker A. P. Cloning and characterization of a photolyase gene from the cyanobacterium Anacystis nidulans. Nucleic Acids Res. 1988 May 25;16(10):4447–4463. doi: 10.1093/nar/16.10.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]