Abstract
Objective
To examined whether the long-term resting heart rate (RHR) pattern can predict the risk of having arterial stiffness in a large ongoing cohort.
Approach and Results
This community-based cohort included 12,554 participants in the Kailun study, free of myocardial infraction, stroke, arrhythmia, and cancer. We used latent mixture modeling to identify RHR trajectories in 2006, 2008 and 2010. We used multivariate linear regression model to examine the association between RHR trajectory patterns and the risk of having arterial stiffness, which was assessed by brachial-ankle pulse wave velocity (baPWV) in 2010–2016. We adjusted for possible confounding factors, including social economic status, lifestyle factors, use of medications, co-morbidities, serum concentrations of lipids, glucose and high-sensitive C-reactive proteins. We identified five distinct RHR trajectory patterns based on their 2006 status and pattern of change during 2006–2010 (low-stable, moderate-stable, moderate-increasing, elevated-decreasing, and elevated-stable). We found that individuals with elevated-stable RHR trajectory pattern had the highest bpPWV value and individuals with the low-stable RHR trajectory pattern had the lowest value (adjusted mean difference = 157 cm/s, P<0.001). Adjusted odds ratio for risk of having arterial stiffness (bpPWV≥1400 cm/s) was 4.14 (95% confidence interval: 2.61–6.57) relative to these two extreme categories. Consistently, a higher average RHR, a higher annual RHR increase rate and a higher RHR variability were all associated with a higher risk of having arterial stiffness.
Conclusion
Long-term RHR pattern is a strong predictor of having arterial stiffness.
Keywords: resting heart rate, trajectory pattern, arterial stiffness, brachial-ankle pulse wave velocity, cohort study
Introduction
Resting heart rate (RHR) is a simple and useful indicator of autonomic balance and metabolic rate1. Emerging evidence has demonstrated an association between an elevated RHR and a higher risk of cardiovascular adverse events and mortality2–4. However, the underlying mechanisms are not well understood. It has been suggested that RHR may impact future cardiovascular disease risk via its association with arterial stiffness, one of the earliest detectable manifestations of adverse structural and functional changes within the vessel wall.5 An elevated RHR was associated with arterial stiffness in two large cross-sectional studies1, 6. In a small Japanese cohort, Tomiyama et al reported a synergistic role of high baseline RHR and increase in HR during a 5–6-year follow-up period in accelerating age-associated increases in arterial stiffness7. However, to the best of our knowledge, whether RHR pattern over a longer period of time can predict the arterial stiffness has not been studied in any large population.
Pulse wave velocity (PWV) is the most validated method to quantify arterial stiffness noninvasively. PWV is considered the golden standard index of arterial stiffness and a maker of atherosclerosis.5 PWV has a strong predictive value of future cardiovascular events in general population8, 9 or in patients with either hypertension10, diabetes mellitus11, or end-stage renal diseases12. Recently, brachial-ankle PWV (baPWV) measurement, which is easy and convenient to be performed and can be widely used in large-scale populations, has become available in clinical settings13, 14. baPWV values have been associated with cardiovascular events, including coronary artery disease, peripheral artery disease and stroke14–18.
Therefore we examined the association between RHR trajectory pattern over a 4-year follow-up period and the risk of having arterial stiffness, which was measured by baPWV, in a large ongoing Chinese cohort.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Five distinct RHR trajectories over a 4-year follow-up period were identified (Figure 1). During 2006–2010, 18.2% (n=2282) participants had a RHR consistently lower than 70 bpm (referred as “low-stable pattern”); 69.8% (n=8768) participants had a RHR between 70–80 bpm (referred as “moderate-stable pattern”); 6% (n=758) participants had a RHR of approximately 80 bpm at baseline in 2006 and increased to approximately 90 bmp during follow-up (referred as “moderate-increasing pattern”); 1.1% (n=135) participants had a RHR consistently over 100 bmp (referred as “elevated-stable pattern”); and 4.9% (n=611) participants had a RHR between 90–100 bpm at baseline in 2006 and decreased to approximately 80 bmp during follow-up (referred as “elevated-decreasing pattern”. The basic characteristics of the 12,554 participants were presented in Table 1.
Figure 1.
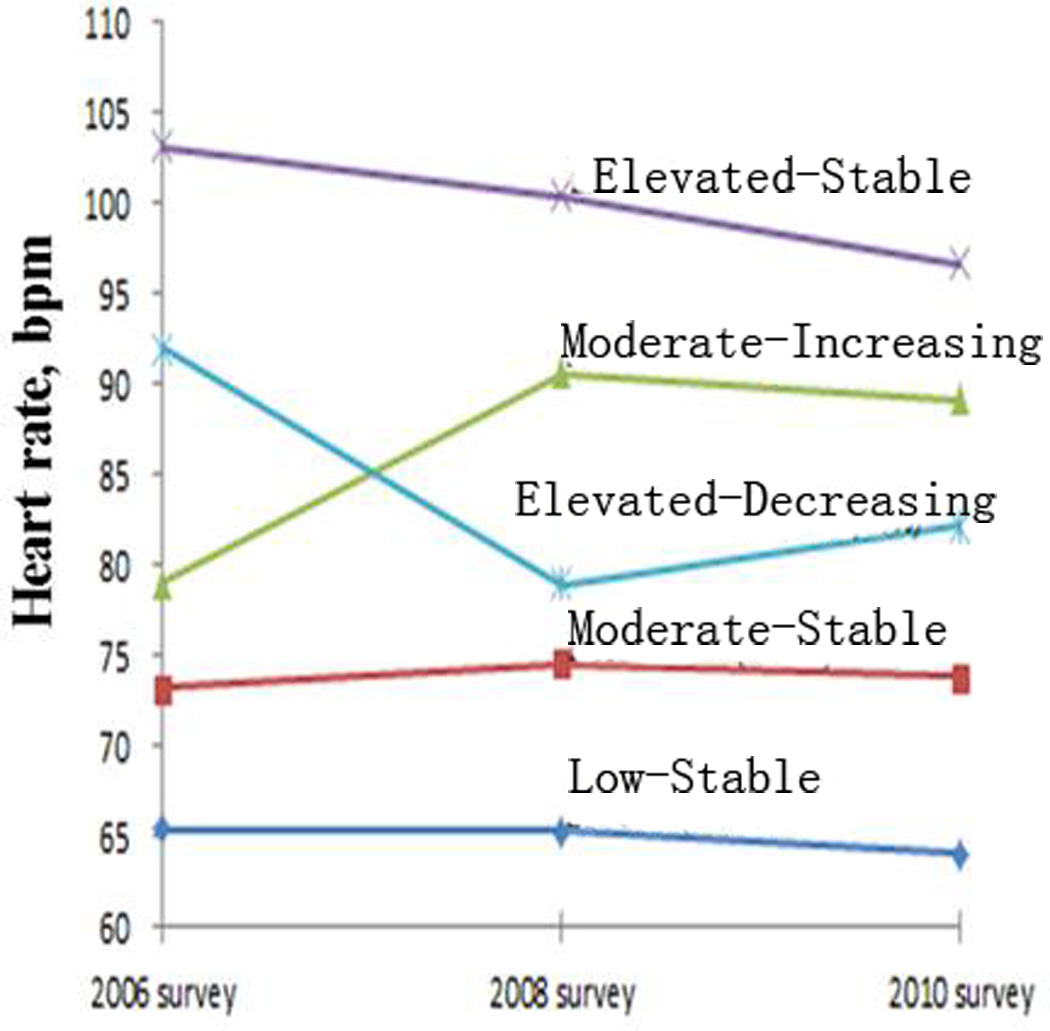
Average resting heart rate in 2006, 2008, and 2010, according to five resting heart rate trajectory patterns.
Table1.
Basic characteristics in 2006 according to the resting heart rate trajectory patterns, among 12,554 Kailuan participants
| Low- Stable |
Moderate- Stable |
Moderate- Increasing |
Elevated- Decreasing |
Elevated- Stable |
|
|---|---|---|---|---|---|
| N (%) | 2282(18.2) | 8768(69.8) | 758(6.0) | 611(4.90) | 135(1.10) |
| Age a, y | 46.8±11.8 | 44.4±11.0 | 44±11.1 | 43.9±10.5 | 45.1±11.4 |
| Men,% | 63.5 | 58.9 | 69.7 | 67.5 | 62.7 |
| Body Mass Indexa,b, kg/m2 | 24.6±3.0 | 24.7±3.2 | 24.9±3.2 | 24.7±3.4 | 24.1±3.3 |
| Smoking status, % | |||||
| Current | 30.4 | 29.5 | 53.4 | 38.7 | 28.4 |
| Past | 5.3 | 3.7 | 3.3 | 3 | 6.7 |
| Never | 64.3 | 66.8 | 43.3 | 58.4 | 64.9 |
| Alcohol intake, % | |||||
| Current | 41.1 | 37.4 | 47.3 | 42.1 | 34.3 |
| Past | 2.3 | 2 | 2.3 | 1 | 3.7 |
| Never | 56.6 | 65.6 | 50.5 | 56.9 | 61.9 |
| Physical activity, % | |||||
| Never | 8.4 | 10.8 | 17.3 | 10.3 | 12.1 |
| 1–2 times/week | 74.9 | 78 | 72.3 | 81 | 80.3 |
| 3+ times/week | 16.7 | 11.2 | 10.4 | 8.7 | 7.6 |
| Education, % | |||||
| Illiteracy or elementary school | 5.2 | 4.7 | 8.5 | 4.7 | 8.4 |
| Middle school | 78 | 82.1 | 81.4 | 83.6 | 87 |
| College /university | 16.8 | 13.2 | 10 | 11.8 | 4.6 |
| Average income, % | |||||
| <¥500/month | 23.8 | 26.6 | 36.1 | 27.2 | 35.1 |
| ¥500 to ¥2999/ month | 62.2 | 64.1 | 56 | 62.7 | 58 |
| ≥¥3000/ month | 14.1 | 9.3 | 7.9 | 10.1 | 6.9 |
| Diabetes diabetes mellitus, % | 5.2 | 8.1 | 13.6 | 12.9 | 17 |
| Hypertension status, % | 31.4 | 37.1 | 54.2 | 49.1 | 51.1 |
| Use of antihypertensive agent, % | 9.6 | 9.6 | 13.5 | 14 | 11.1 |
| TG b,c, mmol/L | 1.21 (0.91– 1.91) |
1.28 (0.91–1.91) |
1.44 (1.01–2.27) |
1.48 (1.06–2.29) |
1.33 (0.95–1.95) |
| TCa,b,mmol/L | 4.87±0.87 | 4.9±0.88 | 5.09±0.92 | 5.14±0.97 | 5.06±0.98 |
| LDL-C a,b,mmol/L | 2.42±0.67 | 2.47±0.65 | 2.67±0.68 | 2.5±0.61 | 2.5±0.67 |
| HDL-Ca,b,mmol/L | 1.56±0.34 | 1.53±0.33 | 1.57±0.30 | 1.63±0.32 | 1.62±0.36 |
| FBGa,b,mmol/L | 5.18±0.89 | 5.4±1.17 | 5.79±1.58 | 5.8±1.70 | 6.12±1.64 |
| hsCRPb,c,mmol/L | 1.0 (0.5–2.0) |
1.18 (0.61–2.3) |
1.27 (0.71–2.40) |
1.25 (0.67–2.35) |
1.47 (0.69–2.92) |
Means ± SD
Average concentrations based on three measurement in 2006, 2008, and 2010
Median and interquartile range
Abbreviations: TC, Total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Negative-log hsCRP, log-transformed serum C-reactive protein; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood-glucose; PWV, pulse ware velocity.
Then we examined whether the RHR trajectory pattern between 2006 and 2010 could predict the future risk of having arterial stiffness which was assessed by baPWV between 2010 and 2016. Relative to individuals with low-stable RHR trajectory, individuals in the other four patterns had significantly higher baPWV levels (Supplemental figure 2, and Table 2). Of note, although individuals with moderate-stable and moderate-increasing RHR patterns had similar baseline RHR (70–80 bmp), individuals with moderate-increasing RHR pattern had a significant higher baPWV (1528±13 cm/s vs 1486 ±9 cm/s), indicating RHR changes had an impact on the risk of arterial stiffness. Exclusion of participants who had hypertension (Table 2) generated similar results. After further adjustment for RHR in 2006 or in 2010, the trend remained the same and significant (data not shown). Although further adjustment for RHR at the time of baPWV assessment led to great attenuation of the association between RHR trajectories during 2006–2010 and baPWV in 2010–2016, the difference between the elevated-stable and low-stable patterns remained significant (adjusted difference= 33.8; 95%CI: 8.61, 59.0). However these results should be interpreted with caution because of concerns of over-adjustment. We observed a significant interaction between age and RHR trajectories (p-interaction <0.001) but not for sex (p-interaction=0.26). The association between heart rate and incident diabetes and IFG was stronger among individuals aged 60 years or older, relative to younger adults (Table 2).
Table 2.
Mean difference and 95% confidence interval in baPWV according to the resting heart rate trajectory patterns, among 12,554 Kailuan participants
| Low-Stable | Moderate- Stable | Moderate- Increasing | Elevated- Decreasing | Elevated- Stable | |
|---|---|---|---|---|---|
| N (%) | 2282(18.2) | 8768(69.8) | 758(6.0) | 611(4.90) | 135(1.10) |
| Age and sex-adjusted | 0(ref.) | 85.0(72.6–97.5) | 197(176–220) | 192(168–216) | 278(232–326) |
| Multivariate adjusted model 11 |
0(ref.) | 84.0(71.5–96.9) | 193(171–216) | 190(166–215) | 279(232–327) |
| Multivariate adjusted model 22 |
0(ref.) | 44.3(32.5–56.0) | 95.1(77.1–116) | 97.4(74.8–120) | 157(114–201) |
| Excluding participants with hypertension2 |
0(ref.) | 29.6(17.6–41.6) | 62.3(37.7–86.9) | 69.0(43.3–94.6) | 157(105–209) |
| Age < 60 y2 | 0(ref.) | 31.5(20.1–42.9) | 69.6(49.4–89.7) | 80.0(58.4–101) | 130(87.9–172) |
| Age ≥ 60 y2 | 0(ref.) | 105(51.3–106) | 210(107–314) | 192(69.7–314) | 372(150–395) |
Adjusted for age (y), sex, smoking (current, past or never), alcohol intake (current, past or never), education (illiteracy or elementary school, middle school, or college/university), physical activity (never, sometimes or active), and average monthly income of each family member (<500, 500–3000, or≥3000¥).
adjusted for age (y), sex, smoking (current, past or never), alcohol intake (current, past or never), education (illiteracy or elementary school, middle school, or college/university), physical activity (never, sometimes or active), average monthly income of each family member (<500, 500–3000, or≥3000¥), body mass index, use of antihypertensive agents (yes/no for each), hypertension status (yes/no), diabetes mellitus(yes/no), and average serum concentrations of triglycerides, high-density lipoprotein cholesterol, total cholesterol, and high sensitive C-reactive protein during 2006–2010.
P-interaction for age (y) and resting heart rate trajectory patterns was <0.001
The RHR trajectory pattern was a strong predictor of having arterial stiffness (bpPWV≥1400 cm/s) -- adjusted odds ratio was 4.14 (95% confidence interval: 2.61–6.57) for the elevated-stable vs low-stable RHR patterns. (Figure 2).
Figure 2.
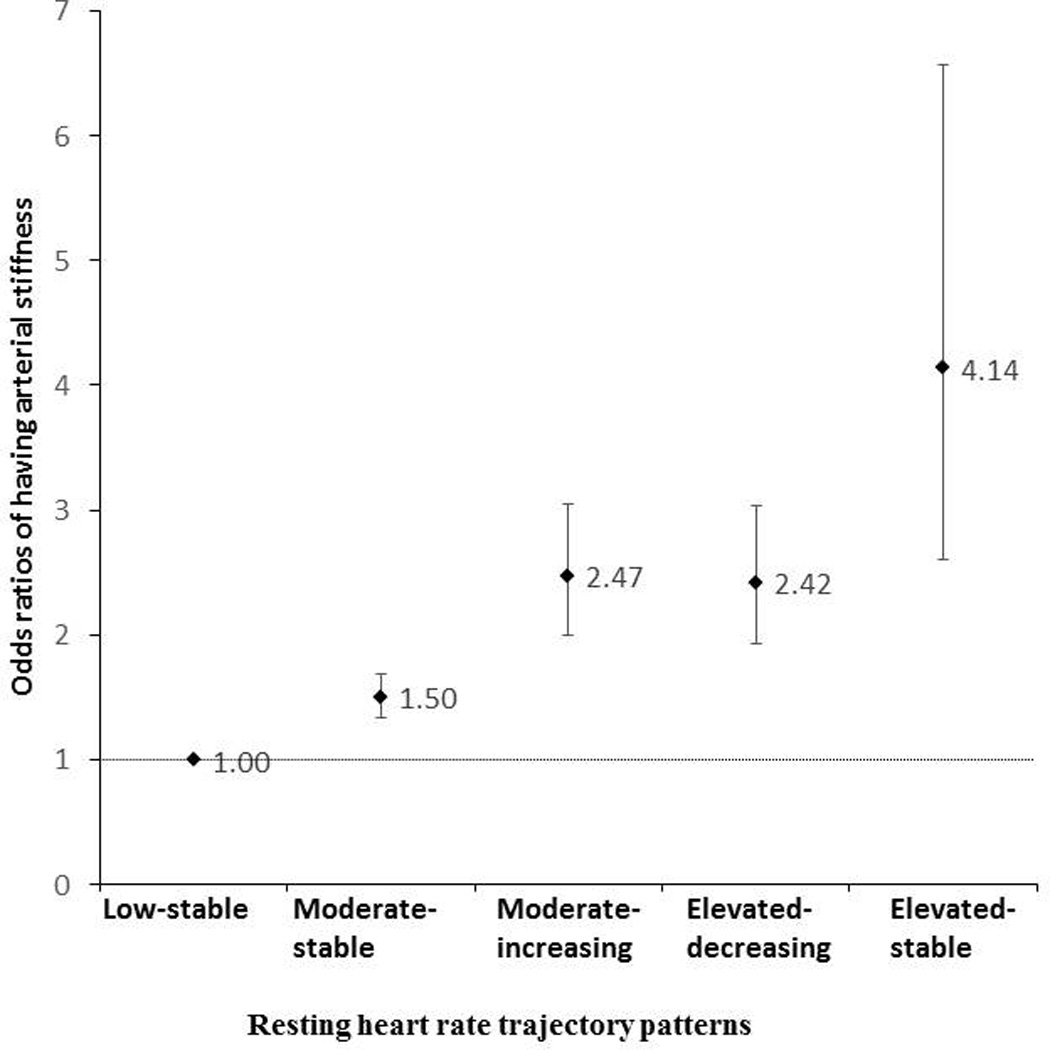
Multivariable adjusted odds ratios (ORs) and 95% confidence intervals (95%Cis) of arterial stiffness (baPWV ≥1400 cm/s), based on trajectories of resting heart rate, after adjustment for age, sex, smoking (current, past or never), alcohol intake (current, past, never), education (illiteracy or elementary school, middle school, or college/university), physical activity (never, sometimes or active), average monthly income of each family member (<500, 500–3000, or≧3000¥), body mass index, use of antihypertensive agents (yes/no), hypertension status (yes/no), diabetes mellitus(yes/no), and average serum concentrations of triglycerides, high-density lipoprotein cholesterol, total cholesterol, and high sensitive C-reactive protein during 2006–2010.
We further found that a higher average RHR, a higher annual RHR increase rate, and a higher RHR variability between 2006 and 2010 were all associated with a significantly higher baPWV, a marker of arterial stiffness (Table 3).
Table 3.
Mean difference and 95% confidence interval in baPWV according to the resting heart rate in cumulative average, increase rate, and variability of HR from 2006 to 2010.
| Resting heart rate | Ptrend | |||||
|---|---|---|---|---|---|---|
| Average resting heart rate during 2006–2010 | ||||||
| Range, bpm | <60 | 61–70 | 71–80 | 81–90 | >90 | |
| Mean different | 0(ref.) | 47.8(18.1–77.5) | 91.8(61–123) | 139(103–174) | 217(171–264) | <0.001 |
| Annual increasing rate of resting heart rate during 2006–2010 | ||||||
| Range, bpm per year |
<−2.24 | −2.25–−0.49 | −0.5–0.74 | 0.5–2.5 | >2. 5 | |
| Mean different | 0(ref.) | 32.1(16.7–47.6) | 48.9(32.9–64.9) | 64.3(48.1–80.5) | 111(94.4–128) | <0.001 |
| Variability of resting heart rate during 2006–2010 | ||||||
| Range, bpm | <2.30 | 2.31–4.23 | 4.24–6.10 | 6.11–9.18 | >9.19 | |
| mean different of baPWV |
0(ref.) | 14(−1.08–29.2) | 6.32(−8.82–21.5) | 9.27(−6.06–24.6) | 30.5(15.1–45.8) | <0.001 |
Adjusted for age, sex, smoking (current, past or never), alcohol intake (current, past, never), education (illiteracy or elementary school, middle school, or college/university), physical activity (never, sometimes or active), average monthly income of each family member (<500, 500–3000, or≥3000¥), body mass index, use of antihypertensive agents (yes/no), hypertension status (yes/no), diabetes mellitus(yes/no), and average serum concentrations of triglycerides, high-density lipoprotein cholesterol, total cholesterol, and high sensitive C-reactive protein during 2006–2010.
Discussions
In the current study, we observed heterogeneous RHR trajectory patterns in a large cohort of 12,554 participants over a 4-year follow-up. We identified five unique RHR trajectory patterns, individuals with elevated-stable RHR trajectory pattern (RHR 90–100 bmp) had the highest risk of having arterial stiffness, which was assessed by baPWV, and individuals with low-stable RHR trajectory pattern (RHR 60–70 bmp) had the lowest risk after adjustment for potential confounding factors, including socioeconomic status, lifestyle factors, use of medications, comorbidities, serum lipids, glucose, hs-CRP concentrations. When baseline RHR was further adjusted, RHR trajectories were still independently associated with the risk of having arterial stiffness.
A large body of epidemiologic evidences has shown that RHR is associated with cardiovascular morbidity and mortality2–4, 19, 20. As a consequence, increased RHR has emerged as an independent risk factor both in primary prevention and in patients with hypertension, coronary artery disease, and myocardial infarction21. It was estimated that for each 15 bmp increase in RHR, the risk of cardiovascular disease can be increased by 24% in men and 32% in women19. Despite the compelling evidences from epidemiologic studies, it is challengeable to determine the actual role of RHR in cardiovascular events because the complex interaction among the various risk factors22. There have been several potential mechanisms proposed, including increased sympathetic tones, increased vascular sheer stress and endothelial damage by increased heart rate, and accelerated atherosclerosis. In animal studies, accelerated heart rate is associated with cellular signaling events leading to vascular oxidative stress, endothelial dysfunction, and acceleration of artherogenesis. Mangoni et al provided experimental evidence that progressive increases in heart rate caused by atrial pacing in rats led to marked reductions in carotid artery compliance23. In a 6-year follow-up of patients who were treated with hypertension, high RHR was associated with an accelerated progression of arterial stiffness, as estimated by carotid/femoral pulse wave velocity24. Our results also support that not only a single RHR measurement or an average RHR value, but also the trend of RHR change over time, are import predictors of arterial stiffness.
Studies in different mouse models and different vascular beds consistently have shown that mild heart rate reduction (13% to 17%) protects endothelial-dependent vaso-relaxation21, although no human studies have been done to investigate whether there is any benefit of slowing RHR as a primary prevention strategy of cardiovascular disease. Our analysis adjusted for the potential confounding factors, including medications that can affect RHR and PWV, physical activity that can modify RHR and comorbidities, and RHR and its change over time remain independent risk factors for accelerated arterial stiffness. It remains to be seen that whether this association translates to a higher incidence of cardiovascular events and longer follow-up will be needed for these events to occur.
It is important to note that whether heart rate patterns represent a cause or consequence of arterial stiffness is a worthy question to be considered, and one that our cross-sectional methodology cannot definitely disentangle. On one hand, our results suggested that higher RHR and increasing heart rate trajectories over time were associated with higher arterial stiffness measured via baPWV. On the other hand, previous studies have shown that the assessment of arterial stiffness itself can be positively confounded by higher heart rate. 25, 26; thereby suggesting that the real-time measurement of baPWV may be influenced by the heart rate at the time of assessment, or that greater arterial stiffness in itself induces higher heart rates. The idea that greater arterial stiffness causes higher heart rate makes physiologic sense; and therefore, the directionality or bidirectionality of our predictor and outcomes may be much more complex than our analyses suggest. Our study methodologies may be limited in that they cannot discern these potential interpretations; however, regardless of the direction or type of the interpretation, the observation that higher heart rate and increasing heart rate over time associate with greater arterial stiffness is robust in our current study, and in many smaller studies prior to ours. 1, 6, 7, 24
Although RHR trajectories were significantly associated with altered baPWV levels in age-stratified analyses, the association appeared to be more pronounced in elderly participants, relative to those with younger age. Previous studies regarding whether age modified the association between RHR and cardiovascular events or risk factors generated inconsistent results. For example, two previous studies reported that the associations between faster RHR and risk of hypertension 27 and diabetes28 were stronger in participants with younger age. In contrast, in another study regarding total and cardiovascular disease mortality, the interaction between age and RHR was not significant.29 In this context, further studies are warranted to examine whether the observed significant interaction between age and RHR trajectories in relation to arterial stiffness reflected true biological effects or was due to chance.
The advantages of our study are the large number of participants and repeated RHR measurements over a long period of time. Our study has a few limitations. Our study population only included individuals from the Kailun cohort, Tangshan community. More studies in the general population with different ethnic, education, and cultural background may be needed to make the study results more generalizable. Our study should be considered as a cross-sectional analysis because we did not assess baPWV repeatedly. The direction of the observed association thus cannot be inferred. Further, because the baPWV assessments were completed in all participants in 2016, it remains to be seen whether the RHR pattern and its potential effect on baPWV can predict future risks of cardiovascular events. Longer follow-up is needed.
In conclusion, in this large community-based cohort, we found that RHR trajectory pattern is an independent risk factor for accelerated arteriosclerosis. Further longitudinal studies are warranted to examine whether RHR is associated with subsequent changes in arteriosclerosis status.
Supplementary Material
Highlights.
In this large community-based cohort included 12,554 Chinese participants, free of cardiovascular diseases and cancer, we identified five distinct resting heart rate (RHR) trajectory patterns based on their 2006 status and pattern of change during 2006–2010.
We found that individuals with elevated-stable RHR trajectory pattern were approximately 3 times more likely to have arterial stiffness in 2010–2016, as assessed by brachial-ankle pulse wave velocity (baPWV) ≥1400 cm/s, relative to individuals with the low-stable RHR trajectory pattern.
We also observed that a higher average RHR, a higher annual RHR increase rate and a higher RHR variability were all associated with a higher risk of having arterial stiffness.
Acknowledgments
Anand Vaidya was supported by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number R01 DK107407, by Grant 2015085 from the Doris Duke Charitable Foundation, and by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL111771.
Abbreviations
- baPWV
brachial-ankle pulse wave velocity
- RHR
resting heart rate
- Bpm
beats per minute
Footnotes
Disclosures
All authors read and approved the final manuscript. The authors do not have conflicts of interest to disclose.
References
- 1.Park BJ, Lee HR, Shim JY, Lee JH, Jung DH, Lee YJ. Association between resting heart rate and arterial stiffness in Korean adults. Arch Cardiovasc Dis. 2010;103:246–252. doi: 10.1016/j.acvd.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. Am Heart J. 1991;121:172–177. doi: 10.1016/0002-8703(91)90970-s. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 4.Ho JE, Larson MG, Ghorbani A, Cheng S, Coglianese EE, Vasan RS, Wang TJ. Long-term cardiovascular risks associated with an elevated heart rate: the Framingham Heart Study. J Am Heart Assoc. 2014;3:e000668. doi: 10.1161/JAHA.113.000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Whelton SP, Blankstein R, Al-Mallah MH, Lima JA, Bluemke DA, Hundley WG, Polak JF, Blumenthal RS, Nasir K, Blaha MJ. Association of resting heart rate with carotid and aortic arterial stiffness: multi-ethnic study of atherosclerosis. Hypertension. 2013;62:477–484. doi: 10.1161/HYPERTENSIONAHA.113.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomiyama H, Hashimoto H, Tanaka H, Matsumoto C, Odaira M, Yamada J, Yoshida M, Shiina K, Nagata M, Yamashina A, ba PWVcCG. Synergistic relationship between changes in the pulse wave velocity and changes in the heart rate in middle-aged Japanese adults: a prospective study. J Hypertens. 2010;28:687–694. doi: 10.1097/HJH.0b013e3283369fe8. [DOI] [PubMed] [Google Scholar]
- 8.Shokawa T, Imazu M, Yamamoto H, Toyofuku M, Tasaki N, Okimoto T, Yamane K, Kohno N. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J. 2005;69:259–264. doi: 10.1253/circj.69.259. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 11.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 12.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 13.Tomiyama H, Koji Y, Yambe M, Shiina K, Motobe K, Yamada J, Shido N, Tanaka N, Chikamori T, Yamashina A. Brachial -- ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J. 2005;69:815–822. doi: 10.1253/circj.69.815. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Wu Y, Li J, Ma W, Guo X, Luo Y, Hu D. The predictive value of brachial-ankle pulse wave velocity in coronary atherosclerosis and peripheral artery diseases in urban Chinese patients. Hypertens Res. 2008;31:1079–1085. doi: 10.1291/hypres.31.1079. [DOI] [PubMed] [Google Scholar]
- 15.Ninomiya T, Kojima I, Doi Y, Fukuhara M, Hirakawa Y, Hata J, Kitazono T, Kiyohara Y. Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general Japanese population: the Hisayama Study. J Hypertens. 2013;31:477–483. doi: 10.1097/HJH.0b013e32835c5c23. discussion 483. [DOI] [PubMed] [Google Scholar]
- 16.Takashima N, Turin TC, Matsui K, Rumana N, Nakamura Y, Kadota A, Saito Y, Sugihara H, Morita Y, Ichikawa M, Hirose K, Kawakani K, Hamajima N, Miura K, Ueshima H, Kita Y. The relationship of brachial-ankle pulse wave velocity to future cardiovascular disease events in the general Japanese population: the Takashima Study. J Hum Hypertens. 2014;28:323–327. doi: 10.1038/jhh.2013.103. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Song TJ, Song D, Lee KJ, Kim EH, Lee HS, Nam CM, Nam HS, Kim YD, Heo JH. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension. 2014;64:240–246. doi: 10.1161/HYPERTENSIONAHA.114.03304. [DOI] [PubMed] [Google Scholar]
- 18.Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, Yamamoto Y, Hori S. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–622. doi: 10.1291/hypres.26.615. [DOI] [PubMed] [Google Scholar]
- 19.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619. e3. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26:967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 21.Custodis F, Schirmer SH, Baumhakel M, Heusch G, Bohm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56:1973–1983. doi: 10.1016/j.jacc.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Palatini P, Parati G. Persistently elevated heart rate accelerates the progression of arterial stiffness. J Hypertens. 2010;28:653–656. doi: 10.1097/HJH.0b013e3283389e3d. [DOI] [PubMed] [Google Scholar]
- 23.Mangoni AA, Mircoli L, Giannattasio C, Ferrari AU, Mancia G. Heart rate-dependence of arterial distensibility in vivo. J Hypertens. 1996;14:897–901. doi: 10.1097/00004872-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 25.Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. doi: 10.1161/01.hyp.0000019132.41066.95. [DOI] [PubMed] [Google Scholar]
- 26.Su HM, Lee KT, Chu CS, Lee MY, Lin TH, Voon WC, Sheu SH, Lai WT. Effects of heart rate on brachial-ankle pulse wave velocity and ankle-brachial pressure index in patients without significant organic heart disease. Angiology. 2007;58:67–74. doi: 10.1177/0003319706295481. [DOI] [PubMed] [Google Scholar]
- 27.Aladin AI, Al Rifai M, Rasool SH, Keteyian SJ, Brawner CA, Michos ED, Blaha MJ, Al-Mallah MH, McEvoy JW. The Association of Resting Heart Rate and Incident Hypertension: The Henry Ford Hospital Exercise Testing (FIT) Project. American journal of hypertension. 2016;29:251–257. doi: 10.1093/ajh/hpv095. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Cui L, Wang Y, Vaidya A, Chen S, Zhang C, Zhu Y, Li D, Hu FB, Wu S, Gao X. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: the Kailuan prospective study. International journal of epidemiology. 2015;44:689–699. doi: 10.1093/ije/dyv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legeai C, Jouven X, Tafflet M, Dartigues JF, Helmer C, Ritchie K, Amouyel P, Tzourio C, Ducimetiere P, Empana JP. Resting heart rate, mortality and future coronary heart disease in the elderly: the 3C Study. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2011;18:488–497. doi: 10.1177/1741826710389365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


