Abstract
Deep sternal wound infection and bleeding are devastating complications following cardiac surgery, which may be reduced by topical application of autologous platelet gel. Systematic review identified seven comparative studies involving 4,692 patients. Meta-analysis showed significant reductions in all sternal wound infections (odds ratio 3.48 [1.08–11.23], p=0.04) and mediastinitis (odds ratio 2.69 [1.20–6.06], p=0.02) but not bleeding. No adverse events relating to the use of topical platelet-rich plasma were reported. The use of autologous platelet gel in cardiac surgery appears to provide significant reductions in serious sternal wound infections, and its use is unlikely to be associated with significant risk.
Keywords: cardiac surgery, complications, meta-analysis, platelets, sternum
Introduction
Bleeding and surgical site wound infections following cardiac surgery are both common and are each independently associated with an increase in morbidity and mortality. Efforts to reduce the incidence of re-sternotomy for bleeding and deep sternal wound infection, which may be interlinked, have helped to reduce the burden of care within the last two decades. As, however, these complications have such devastating effects on patients’ recovery and a substantial economic impact on healthcare providers, there remains incentive to reduce the risk further. The cost of deep sternal wound infection following cardiac surgery was estimated in 2008 to be $ 304,747 per patient stay in the USA1 and up to $ 500,000 in specialist centres2. Even with contemporary rates of such infections below 1%3, this represents a significant healthcare burden. Re-sternotomy for bleeding occurs in approximately 3% of patients following cardiac surgery4 and this, too, has significant effects on morbidity and mortality5.
Since the early 1990s, the use of autologous platelet-rich plasma (PRP) combined with fibrin and/or thrombin has been described to aid haemostasis and wound healing in maxillofacial, orthopaedic and plastic surgery. Derived from plasmapheresis of the patient’s own blood, the resulting plasma is rich in growth factors and cytokines, which promote tissue healing and regeneration. An experimental sheep model has shown that in sternotomy closed with PRP, trabeculated bone growth and development of haematopoietic medullary tissue were more prominent than in a control group6. This was apparent even after nine weeks, with a predominance of cartilaginous repair in the non-PRP group.
Several small, randomised and observational studies have examined the effect of platelet gel in patients undergoing cardiac surgery, but the numbers were small and the aggregate evidence has not yet been presented. Nonetheless, more than half of autologous platelet gel use in one North American survey was found to be in the setting of cardiac surgery7. We sought to identify whether, in adult patients undergoing cardiac surgery via median sternotomy, intra-operative application of platelet gel could reduce the incidence of sternal surgical site wound infections, mediastinitis or bleeding, as compared to non-treatment. To determine this, we performed a systematic review of the literature and a meta-analysis.
Materials and methods
We performed our study according to existing guidelines on the conduct of meta-analyses8. Two authors (BHK and SGJ), previously trained in the execution of systematic literature reviews, were responsible for the literature search and screening.
Literature search strategy
Electronic searches were performed using the Ovid interface to search the following databases: Medline, Embase, Pubmed, Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews. The time period was from the starting date of each database to September 2014. The search strategy for Embase was performed as follows:
exp thrombocyte rich plasma/or platelet-rich gel. mp;
cardiac surgery.mp or heart surgery/;
exp sternotomy/;
platelet gel.mp;
(1 or 4) and (2 or 3).
Modifications for the keywords and syntax of each database were made as appropriate (available on request from the corresponding author) and following removal of duplicates, references of screened papers were hand-searched for identification of other potentially relevant studies.
Selection criteria
Inclusion criteria were any study that compared the use of PRP as a topical application to the sternum intra-operatively against the standard closure technique without platelet gel. Studies on PRP infused back into the circulation were not included. Due to the dearth of evidence available, both prospective and observational studies were included as long as either post-operative sternal wound infection or bleeding was cited as an outcome. We did not exclude studies that had only been presented as conference proceedings or abstracts unless there were other exclusion criteria. We excluded studies in non-humans and studies examining the biochemical, histological or any experimental functional status of the tissues following use of PRP. Papers with no English-language title or abstract were screened out.
Data extraction and critical appraisal of evidence
Data were extracted from the article text, tables and figures. Qualitative assessment of country of origin, study period and year of publication, study design, use of a representative population, potential sources of selection or information bias, platelet gel technique and control for confounding factors were accrued. We did not apply a scoring scheme to these data. The incidence of superficial and deep sternal wound infections was collated as number of events for a given denominator but presented as risk of non-event. Information on bleeding was collected as mean total blood loss following surgery. Where published data were incomplete or ambiguous, the authors were contacted for clarification of the methods and results. Two investigators (BHK and SGJ) independently assessed and reviewed articles identified by literature searching. Any disagreement in interpretation of data was arbitrated by a third investigator (SD) and expert opinion was sought in order to help reach consensus from the consulting authors (KEM and JAH). All final decisions were reviewed by the senior author (JAH).
Statistical analysis
The statistical analysis was performed using Review Manager 5.3.49 (The Cochrane Collaboration, Copenhagen, Denmark). A fixed effects model was utilised as the standard comparison. Heterogeneity was assessed using the I2 statistic and risk of publication bias interrogated using a funnel plot. Where heterogeneity was implied (I2>50% or asymmetry of the funnel plot), possible reasons for variation between studies were examined from the qualitative quality assessment and a random-effects model employed.
For randomised controlled trials, the risk of bias was assessed using the Cochrane Group methodology. For observational studies the principles outlined by the Agency for Healthcare Research and Quality10 were adopted.
Results
Literature search
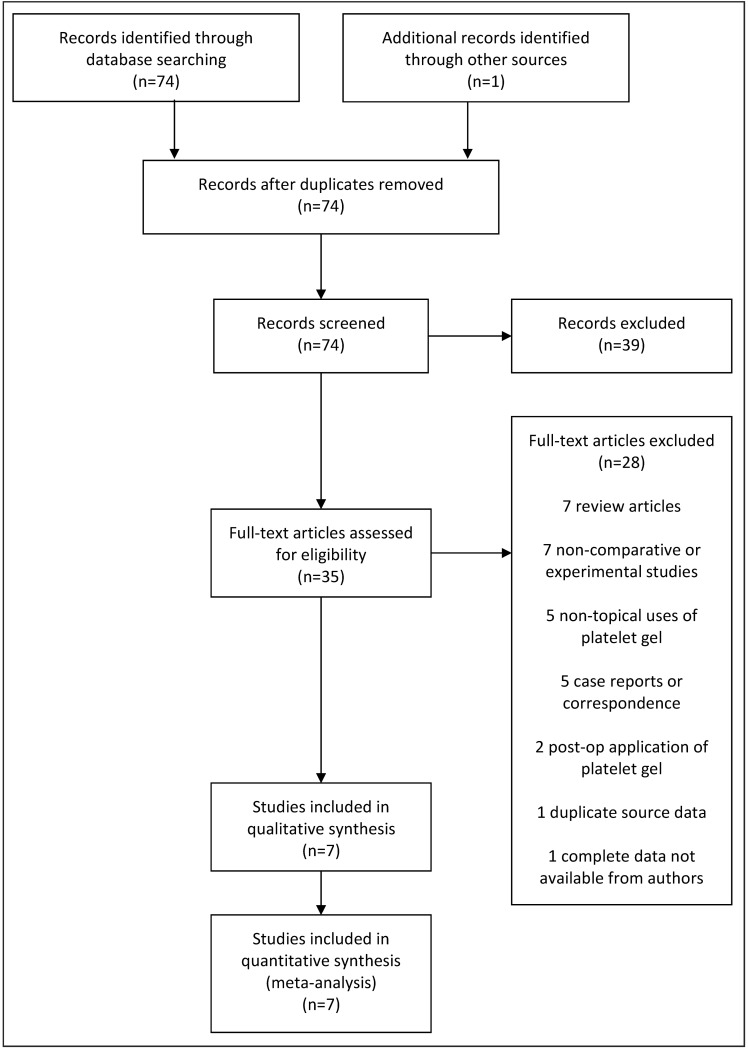
A total of 74 papers were identified using the defined search strategy on the electronic databases. One duplicate was culled and, following screening of titles, 34 papers with potentially relevant content were identified. One further unique paper was identified from hand-searching references11. Twenty-eight papers were excluded at abstract review. Seven were review articles12–18; five addressed non-topical application of PRP19–23; seven were experimental or non-comparative studies7,24–29; five were case reports or correspondence30–34 and two applied platelet gel post-operatively on non-healing wounds35,36. One comparative study was excluded because the data had been incorporated into a newer paper by the same group37. A paper presented as a conference abstract was initially screened in and the authors contacted for data to include in the meta-analysis, but finally excluded as no further information was received38.
Following screening and exclusion, therefore, seven studies in total were thought to answer the study questions: six concerning wound infection and two regarding post-operative bleeding (Figure 1). These studies involved 1,689 patients receiving platelet gel at closure of sternotomy and 3,023 patients acting as controls who did not receive PRP. Due to the small number of final papers available, meta-regression analyses were not performed. Results are presented as an odds ratio (OR) of non-event for superficial sternal infections and mediastinitis and total mean blood loss as continuous data in the forest plots. Funnel plots were not used as there were too few studies to provide sufficient power for this tool.
Figure 1.
PRISMA chart illustrating the search strategy for publications on platelet-rich plasma vs conventional treatment for cardiac surgery via sternotomy.
Quality assessment
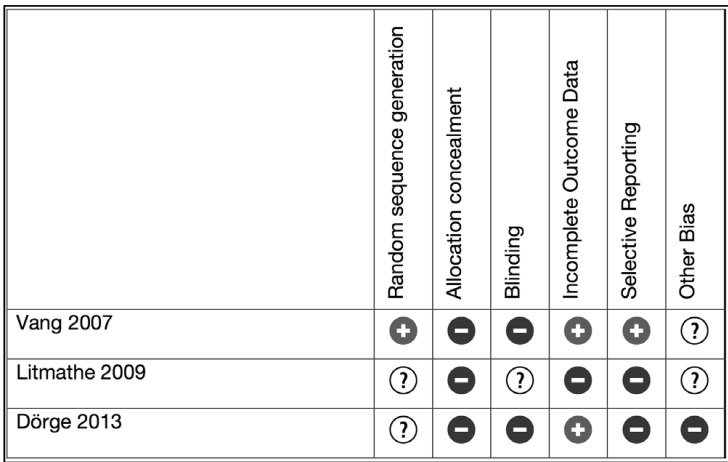
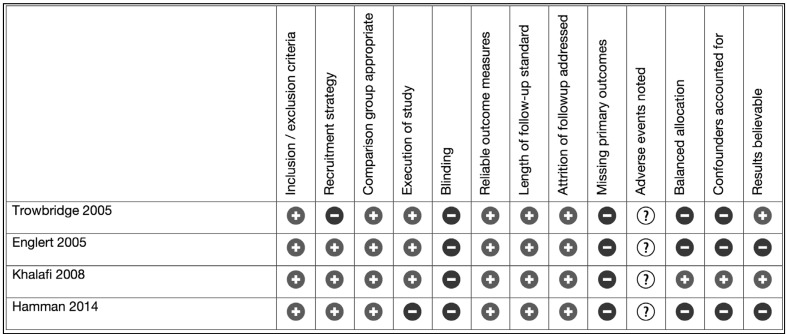
Three randomised controlled trials (n=262)39–41 and four observational studies (n=4,450)11,42–44 were identified (Table I). One paper also presented data as a propensity matched analysis11 and was included in the meta-analysis in raw data form only. None of the randomised trials included more than 100 patients and in all three cases, descriptions of randomisation sequence generation, allocation concealment and blinding were scanty. Three out of the four observational studies had more than 500 patients in each arm. The observational studies all had statistically and clinically significant differences in the demographic baselines of the patients. Four of the studies combined PRP with calcium and bovine thrombin in vitro before application to the chest. Two studies applied PRP with or without autologous thrombin sprayed on in vivo. The PRP was combined with the patient’s own thrombin in one study. Risk of bias is summarised in Figures 2 and 3.
Table I.
Studies included.
| First author | Year | Country | Study period | Study type | Control (n) | PRP (n) | (1) Platelet gel technique (2) Platelet gel quality (3) Other blood conservation or infection control intervention |
|---|---|---|---|---|---|---|---|
| Trowbridge44 | 2005 | USA | 2002–2005 | OS | 948 | 382 | (1) 120–300 mL whole blood drawn and combined 9:1 with citrate-phosphate-dextrose and spun using either a Continuous Autotransfusion System, Smart Prep II Platelet Separator or Angel Platelet Separation Device. PRP combined with 5 mL 10% calcium chloride and 5,000 U of bovine thrombin in a 10:1 ratio. Applied to chest following sternal closure, then after fascial closure, then onto skin. 2-minute pause after each application. (2) Mean platelet yield 55–68%. Mean platelet increase 3.1–4.1 times. (3) Not stated. |
| Englert42 | 2005 | USA | ns | OS | 64 | 64 | (1) Magellan Autologous Platelet Separator used to collect platelets according to “instructions for use”. PRP applied to sternum following sternotomy, prior to sternal wiring and after sternal wires closed. (2) Not stated. (3) Cefazolin 1–2 g preoperatively and variable doses postoperatively. 20 mL of platelet-poor plasma applied to subcuticular tissue prior to closure. No other blood conservation strategies stated. |
| Vang41 | 2007 | USA | ns | RCT | 15 | 5 | (1) 180 mL whole blood combined with 24 mL citrate-dextrose and spun in a Medtronic Magellan Autologous Platelet Separator to achieve 30 ml PRP. The PRP was combined with 5 mL 10% calcium chloride and 5,000 U of bovine thrombin in a 10:1 ratio. Applied to sternum following re-approximation with further pre-fascial application (2) Not stated. (3) Not stated. |
| Khalafi11 | 2008 | USA | 2000–2005 | OS | 557 | 571 | (1) 55 mL whole blood combined with 5 mL citrate-dextrose formula A. Processed in a GPS II Platelet Concentrate System to give 5–7 mL PRP. PRP combined 10:1 with 10% calcium chloride and 1,000 U/mL bovine thrombin. Applied to sternal edges and subcutaneous tissues. (2) Not stated. (3) Platelet-poor plasma applied to each layer of soft tissue closure. Antibiotics (not stated which) given 30–60 min prior to starting surgery. |
| Lithmathe40 | 2009 | Germany | ns | RCT | 22 | 22 | (1) 156 mL whole blood combined with an unspecified amount of citrate-dextrose and centrifuged in a Magellan autologous platelet separator to give up to 30mL PRP. The PRP was activated with calcium-coated glass fibres to create autologous thrombin-rich serum; mixed with more PRP and applied to sternal bone and pre-sternal soft tissue. (2) Not stated. (3) Not stated. |
| Dörge39 | 2013 | Germany | ns | RCT | 99 | 97 | (1) GPS II gravitational platelet separation system used pre-operatively. 54 mL whole blood and 6 mL calcium citrate spun to create autologous PRP. 11 mL whole blood and 1.2 mL calcium citrate spun to give autologous thrombin. Stored at 8 °C until 30 min prior to use. Injected simultaneously between sternal edges and to presternal tissue prior to wound closure. (2) Not stated. (3) 2 g cefazolin administered thrice. |
| Hamman43 | 2014 | USA | 1998–2010 | OS | 1,318 | 548 | (1) 60 mL whole blood processed using a SmartPReP 2 system to generate ~7 mL PRP. 5 mL 10% calcium chloride combined with 5,000 U bovine thrombin with 3 mL PRP and 2 g vancomycin powder to form a paste applied to the sternal edge prior to closure. (2) Not stated. (3) Antibiotics given 1 h pre-operatively and stopped 48 h after the operation. Vancomycin or ceftazidime prior to 2006, cefazolin thereafter. |
PRP: platelet-rich plasma; OS: observational study; ns: not specified; RCT: randomised controlled trial.
Figure 2.
Risk of bias summary for randomised controlled trials.
Figure 3.
Risk of bias summary for observational studies.
Patients’ characteristics
All studies were performed in either Germany or USA, with two out of the three randomised controlled trials performed in Germany. Adult patients undergoing cardiac surgery were considered, with two studies recruiting patients at high risk for sternal wound infection (Table II).
Table II.
Key features of included studies.
| Study | Inclusion | Exclusion | Comparable groups | Other comments |
|---|---|---|---|---|
| Trowbridge 2005 | All cardiac surgery patients over 19 years old. | None | No: differences in surgeon and procedure type; more reoperations and blood product use as well as less IMA use and fewer urgent operations in treatment group. | No |
| Englert 2005 | All CABG patients 18–90 years old. | None | No: less diabetes, COPD and smoking as well as lower weight, shorter CPB and high IMA and bone wax use in treatment group. | No |
| Vang 2007 | Patients undergoing elective CABG with EVH. | Anaemia, thrombocytopaenia, leucocytosis, recent anti- platelets/anti-fibrinolytic/anti- coagulant, pre-operative IABP. | Better ejection fraction and platelet count in treatment group. | Eight patients dropped out because of peri-operative MI and CVA or conversion from EVH to open harvest. |
| Khalafi 2008 | All CABG by two surgeons at six centres. | Any patients with severe morbidity outside the scope of the procedure, e.g. cancer. | No: more antibiotic prophylaxis, elective surgery, off-pump surgery, mammary vein harvest and blood product use in treatment group. | Propensity matched analysis also performed. |
| Litmathe 2009 | Obese, diabetic patients undergoing cardiac surgery. | Emergency operation, active endocarditis, lung infections or pneumonia, IV drug abuse, HIV or thrombopenia. | Yes | Staphylococcus aureus culprit organism in all cases (MRSA in one case). |
| Dörge 2013 | All consecutive patients undergoing cardiac surgery with CPB and cardioplegic cardiac arrest with at least one risk factor for deep sternal wound infection. Risk factors considered were diabetes mellitus, COPD, chronic dialysis, BMI >30 kg/m2, ejection fraction <35%, age >80 years, use of bilateral IMA, chronic systemic corticosteroid use. | Emergency operation and acute infection, surgery requiring chest to be left open or re-thoracotomy for any reason except deep sternal wound infection. | Yes | No |
| Hamman 2014 | All consecutive patients of one surgeon undergoing first time cardiac surgery. | None | No: more Caucasian and Hispanic patients, more hypertension, less PVD, more non-smokers, more heart failure, more pacemakers, more left main disease, less elective surgery, less pre-operative IABP in treatment group. Control group was historical. | Change in antibiotic prophylaxis during course of study. Study sponsored by industry partner. |
IMA: internal mammary artery; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CPB: cardiopulmonary bypass; EVH: endoscopic vein harvest; IABP: intra-aortic balloon pump; MI: myocardial infarction; CVA: cerebrovascular accident; MRSA: methicillin resistant Staphylococcus aureus; BMI: body mass index; PVD: peripheral vascular disease.
All surgical site infections
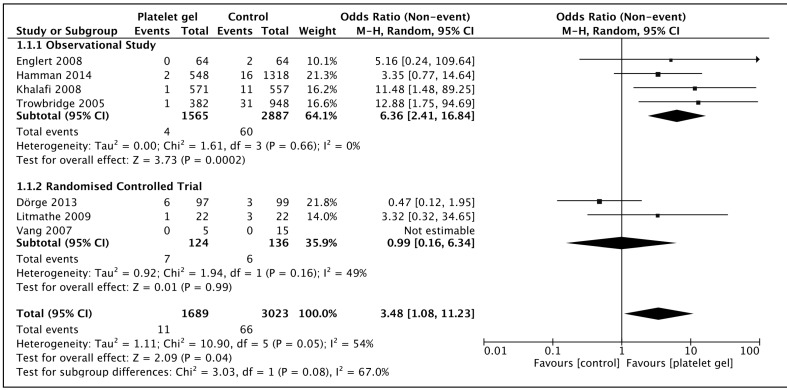
There was significant heterogeneity in the studies (I2 54%) and a random effects model was utilised. Observational studies were more likely to demonstrate a protective effect than randomised trials, but the net effect was of significant reduction in all sternal wound infections with the use of platelet gel (OR 3.48 [1.08–11.23], p=0.04), as demonstrated in Figure 4. The 95% confidence intervals of the two randomised trials39,40 both crossed over the line of equipoise and the subgroup effect of both these trials was of no treatment effect (OR 0.99 [0.16–6.34], p=0.99). The net effect including the much larger observational studies was, however, of an overall benefit.
Figure 4.
Sternal wound infection in patients receiving topical platelet gel at closure of median sternotomy vs those managed with the standard closure regime.
Squares denote odds ratio, with size proportional to the weight assigned to the study. Horizontal bars indicate 95% confidence intervals (CI) for each study. The black diamond represents the aggregate effect, with the width representing the 95% confidence interval of the total effect. M-H: Mantel-Haenszel; SD: standard deviation.
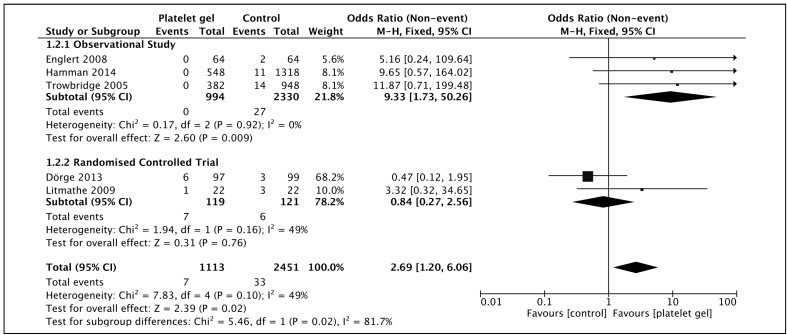
Deep sternal wound infections
Two randomised controlled trials and three observational studies, with low heterogeneity (I2 49%), demonstrated a significant reduction in mediastinitis with platelet gel (OR 2.69 [1.20–6.06], p=0.02) when compared with a fixed effects model (Figure 5). The 95% confidence intervals of the individual studies all crossed over the line of zero-effect, including the combined effect of the randomised controlled trial subgroup, but the aggregate of all studies was statistically significant.
Figure 5.
Deep sternal wound infection in patients receiving topical platelet gel at closure of median sternotomy vs those managed with the standard closure regime.
Squares denote odds ratio, with size proportional to the weight assigned to the study. Horizontal bars indicate 95% confidence intervals (CI) for each study. The black diamond represents the aggregate effect, with the width representing the 95% confidence interval of the total effect. M-H: Mantel-Haenszel; SD: standard deviation.
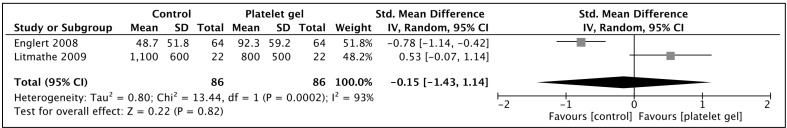
Post-operative bleeding
Only two studies presented bleeding data in a format suitable for aggregation by meta-analysis40,42 (Figure 6). Mean blood loss varied quite significantly between the two studies, with average blood loss of <100 mL in the observational study by Englert et al. and nearly 1,000 mL in Litmathe’s study. A standardised mean difference was therefore used. The I2 was high at 93%, inferring heterogeneity, and a random effects model was utilised. No significant difference in the post-operative bleeding rates was demonstrated (p=0.82).
Figure 6.
Post-operative blood loss in patients receiving topical platelet gel at closure of median sternotomy vs those managed with the standard closure regime.
Squares denote odds ratio, with size proportional to the weight assigned to the study. Horizontal bars indicate 95% confidence intervals (CI) for each study. The black diamond represents the aggregate effect, with the width representing the 95% confidence interval of the total effect. SD: standard deviation; IV: inverse variance.
Due to the small number of studies available, sensitivity testing did not provide meaningful results.
Discussion
The present meta-analysis suggests that platelet gel applied at the time of sternotomy closure may reduce the odds of developing sternal wound infections, including mediastinitis. There is no indication from the current evidence that the use of platelet gel reduces bleeding complications. The quality of the data used to make this assertion, however, is poor. Three randomised controlled trials, with a paucity of information regarding the randomisation or blinding methods, all involved fewer than 100 patients. This necessitated the inclusion in this meta-analysis of observational studies that were themselves at high risk of bias. We acknowledge the inclusion of non-randomised data has significant limitations, but may also be of value where there is a paucity of clinical trials45.
There was some variation in the techniques of preparation and application of platelet gel, which may have had some effect on the outcomes of the intervention. Larger platelets are thought to be more effective30 and different plasmapheresis equipment may harvest platelets more or less effectively31. Accounting for real-world variability in equipment and surgical techniques, however, we felt that such heterogeneity offers this study external validity.
Similarly, the distribution of index procedures varied between studies with some looking at elective coronary artery bypass grafts alone and others taking any patient undergoing cardiac surgery. Details of the surgical technique were not outlined in these studies, and may have included bilateral mammary harvest or skeletonisation of the mammary artery, which may also have had an impact on the outcomes of interest. In addition, the variation in closure methods and antibiotic prophylaxis were not well described in any of the included studies and may have further contributed to differences in sternal site infection or bleeding rates. Within randomised studies, such differences should have been equally distributed between the test and control groups, but such differences are likely to have been amplified in the observational studies.
As such the patient populations were significantly heterogeneous, with some studies recruiting from the whole surgical population and others selecting patients with a high risk of sternal wound infection. One retrospective study, by Hamman et al., utilised historical controls over a 12-year period and may, therefore, have had a multifactorial explanation for the improvement in sternal wound infections. Khalafi and colleagues presented contemporaneous data from six centres, comprising 1,446 patients, and demonstrated a significant advantage of platelet gel. Even following propensity matched analysis, the reductions in sternal infection and post-operative bleeding were estimated at 93% and 96%, respectively. Trowbridge’s retrospective study was conducted over 3 years and included two control groups: one concurrent and one historical. The latter was discounted for the purposes of this study, in order to reduce the impact of other factors in the intervening period.
One study, randomised prospectively, but with fewer than 100 patients in each arm39, accounted for over half the weight of the total effect for deep sternal wound infections. The rate of mediastinitis requiring surgical revision in this study was 3.0% in the control group and 6.2% in the treatment group. This difference was not statistically significant, but the higher rate of deep infection in general may have reflected the inclusion criteria of high-risk patients only. In contrast, in the two largest studies the incidence was 0.8–1.5% which is in keeping with the findings of contemporaneous studies looking at un-selected patients.
None of the studies included cost-benefit analyses, so it would be difficult to assess the overall economic effect of prophylactic platelet gel application. An early study examining the use of platelet gel in cardiac surgery estimated costs at approximately $ 1,000 per patient12. Our contemporaneous costs for platelet gel in the UK are approximately $ 600 (£ 400). Although none of the studies examined provided robust cost-analysis, we calculated, from the odds of developing sternal wound infection, that the number of patients who would need to be treated with PRP to prevent one case of mediastinitis is 140 (95% confidence interval: 73.1–1,506.2). With an overall cost of deep sternal wound infections presumed to be the more conservative of the two quoted figures at $ 300,0001,2, the cost of platelet gel for 140 patients ($ 84,000) would still favour the prophylactic use of PRP.
Conclusions
If the treatment effect is as great as found in this meta-analysis for protection against mediastinitis, then platelet gel therapy can be a cost-effective measure in the prevention of sternal wound infections following cardiac surgery. A large, well-designed, randomised controlled trial with measures to reduce introduced bias is required to fully elucidate the effects of platelet gel on prevention of sternal wound infection.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Center for Medicare and Medicaid Services Ruling (CMS-1533-FC) [Accessed on 30/11/2014]. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/downloads/CMS-1533-FC.pdf.
- 2.Lee JC, Raman J, Song DH. Primary sternal closure with titanium plate fixation: plastic surgery effecting a paradigm shift. Plast Reconstr Surg. 2010;125:1720–4. doi: 10.1097/PRS.0b013e3181d51292. [DOI] [PubMed] [Google Scholar]
- 3.Kirmani BH, Mazhar K, Saleh HZ, et al. External validity of the Society of Thoracic Surgeons risk stratification tool for deep sternal wound infection after cardiac surgery in a UK population. Interact Cardiovasc Thorac Surg. 2013;17:479–84. doi: 10.1093/icvts/ivt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Čanádyová J, Zmeko D, Mokráček A. Re-exploration for bleeding or tamponade after cardiac operation. Interact Cardiovasc Thorac Surg. 2012;14:704–7. doi: 10.1093/icvts/ivs087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unsworth-White MJ, Herriot A, Valencia O, et al. Resternotomy for bleeding after cardiac operation: a marker for increased morbidity and mortality. Ann Thorac Surg. 1995;59:664–7. doi: 10.1016/0003-4975(94)00995-3. [DOI] [PubMed] [Google Scholar]
- 6.Gallo I, Sáenz A, Artiñano E, Esquide J. Autologous platelet-rich plasma: effect on sternal healing in the sheep model. Interact Cardiovasc Thorac Surg. 2010;11:223–5. doi: 10.1510/icvts.2010.237776. [DOI] [PubMed] [Google Scholar]
- 7.Raval JS, Dyga RM, Harm SK, Waters JH. Autologous platelet gel (APG) use in a multihospital health care system. Transfusion (Paris) 2013;53:62A–3A. [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.The Cochrane Collaboration. Review Manager (RevMan) Copenhagen: The Nordic Cochrane Centre; 2014. [Google Scholar]
- 10.Viswanathan M, Berkman ND, Dryden DM, Hartling L. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. [Accessed on 24/11/2014]. Available at: http://www.ncbi.nlm.nih.gov/books/NBK154461/ [PubMed] [Google Scholar]
- 11.Khalafi RS, Bradford DW, Wilson MG. Topical application of autologous blood products during surgical closure following a coronary artery bypass graft. Eur J Cardiothorac Surg. 2008;34:360–4. doi: 10.1016/j.ejcts.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Gravlee GP. Autologous platelet-rich plasma in cardiac surgery: aesthetics versus virtue. J Cardiothorac Vasc Anesth. 1993;7:1–3. doi: 10.1016/1053-0770(93)90109-x. [DOI] [PubMed] [Google Scholar]
- 13.Rubens FD, Fergusson D, Wells PS, et al. Platelet-rich plasmapheresis in cardiac surgery: a meta-analysis of the effect on transfusion requirements. J Thorac Cardiovasc Surg. 1998;116:641–7. doi: 10.1016/s0022-5223(98)70172-2. [DOI] [PubMed] [Google Scholar]
- 14.Everts PA, Overdevest EP, Jakimowicz JJ, et al. The use of autologous platelet-leukocyte gels to enhance the healing process in surgery, a review. Surg Endosc Interv Tech. 2007;21:2063–8. doi: 10.1007/s00464-007-9293-x. [DOI] [PubMed] [Google Scholar]
- 15.Gunaydin S. Clinical impact and biomaterial evaluation of bone marrow aspirate and platelet gel in cardiac surgery. IUBMB Life. 2009;61:295. doi: 10.1177/0267659108097783. [DOI] [PubMed] [Google Scholar]
- 16.Cove ME, Spelman DW, MacLaren G. Infectious complications of cardiac surgery: a clinical review. J Cardiothorac Vasc Anesth. 2012;26:1094–100. doi: 10.1053/j.jvca.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everts PA, Hoogbergen MM, Weber TA, et al. Is the use of autologous platelet-rich plasma gels in gynecologic, cardiac, and general, reconstructive surgery beneficial? Curr Pharm Biotechnol. 2012;13:1163–72. doi: 10.2174/138920112800624346. [DOI] [PubMed] [Google Scholar]
- 18.Lubkowska A, Dolegowska B, Banfi G. Growth factor in PRP-general approaches-a review of recent developments. Am J Hematol. 2012;87:E110. [Google Scholar]
- 19.Stammers AH, Rasmussen C, Kratz JM. The effects of platelet-rich-plasma on post-cardiopulmonary bypass fibrinolysis. J Extra Corpor Technol. 1993;25:122–32. [Google Scholar]
- 20.Li S, Ji H, Lin J, et al. Combination of acute preoperative plateletpheresis, cell salvage, and aprotinin minimizes blood loss and requirement during cardiac surgery. J Extra Corpor Technol. 2005;37:9–14. [PMC free article] [PubMed] [Google Scholar]
- 21.Alberts M, Bandarenko N, Hill SE, et al. Pilot program using trima accel to reduce allogenic platelet usage during cardiac surgery. J Clin Apheresis. 2011;26:81. [Google Scholar]
- 22.Agarwal S, Rai S. Efficacy of platelet rich plasma vs. buffy coat method platelet concentrate use in cardiac surgery patients. Transfusion (Paris) 2012;52:191A. [Google Scholar]
- 23.Zhou S-F, Estrera AL, Miller CC, III, et al. Analysis of autologous platelet-rich plasma during ascending and transverse aortic arch surgery. Ann Thorac Surg. 2013;95:1525–31. doi: 10.1016/j.athoracsur.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 24.Fried DW, Leo JJ, Weber FP, et al. Quantitative and qualitative analysis of platelet-rich plasma collection using the haemonetics cell saver 5 in open heart surgery. J Extra Corpor Technol. 2006;38:235–40. [PMC free article] [PubMed] [Google Scholar]
- 25.Smith CW, Binford RS, Holt DW, Webb DP. Quality assessment of platelet rich plasma during anti-platelet therapy. Perfusion. 2007;22:41–50. doi: 10.1177/0267659107077950. [DOI] [PubMed] [Google Scholar]
- 26.Gunaydin S, McCusker K, Sari T, et al. Clinical impact and biomaterial evaluation of autologous platelet gel in cardiac surgery. Perfusion. 2008;23:179–86. doi: 10.1177/0267659108097783. [DOI] [PubMed] [Google Scholar]
- 27.Gallo I, Saenz A, Arevalo A, et al. Effect of autologous platelet-rich plasma on heart infarction in sheep. Arch Cardiol Mex. 2013;83:154–8. doi: 10.1016/j.acmx.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Wei HY, Ding ZN, Shi HW, et al. [Qualitative analysis of platelet rich plasma prepared by acute plateletpheresis in patients undergoing heart surgery]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014;22:521–4. doi: 10.7534/j.issn.1009-2137.2014.02.047. [In Chinese.] [DOI] [PubMed] [Google Scholar]
- 29.Trowbridge CC, Stammers AH, Wood GC, et al. Improved outcomes during cardiac surgery: a multifactorial enhancement of cardiopulmonary bypass techniques. J Extra Corpor Technol. 2005;37:165–72. [PMC free article] [PubMed] [Google Scholar]
- 30.Whitten CW, Allison PM. Why is acute preoperative plasmapheresis not uniformly effective at decreasing bleeding following cardiac surgery? J Cardiothorac Vasc Anesth. 1993;7:766. doi: 10.1016/1053-0770(93)90083-w. [DOI] [PubMed] [Google Scholar]
- 31.Stover EP, Siegel LC, Hofer BO, et al. Platelet-rich plasmapheresis in cardiac surgery: efficacy may yet be demonstrated. J Thorac Cardiovasc Surg. 1994;108:1148–9. [PubMed] [Google Scholar]
- 32.Lusini M, Di Martino A, Spadaccio C, et al. Resynthesis of sternal dehiscence with autologous bone graft and autologous platelet gel. J Wound Care. 2012;21:74–7. doi: 10.12968/jowc.2012.21.2.74. [DOI] [PubMed] [Google Scholar]
- 33.Jiritano F, Serraino GF, Rossi M, et al. Ventricular assist device driveline infection: treatment with platelet-rich plasma. Ann Thorac Surg. 2013;96:e37–8. doi: 10.1016/j.athoracsur.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 34.Formica F, Perseghin P, Ciro A, Paolini G. Late driveline left ventricular assist device infection treated with frozen-and-thawed allogeneic platelet gel. Interact Cardiovasc Thorac Surg. 2014;19:523–5. doi: 10.1093/icvts/ivu195. [DOI] [PubMed] [Google Scholar]
- 35.Mazzucco L, Medici D, Serra M, et al. The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion (Paris) 2004;44:1013–8. doi: 10.1111/j.1537-2995.2004.03366.x. [DOI] [PubMed] [Google Scholar]
- 36.Centella T, Oliva E, Garcia JC, et al. [Treatment of infected wounds after cardiac surgery with the use of platelet-rich plasma-growth factors. Preliminary report]. An Cirugia Card Vasc. 2005;11:208–13. [In Spanish.] [Google Scholar]
- 37.Englert SJ, Estep TH, Ellis-Stoll CC. Autologous platelet gel applications during cardiovascular surgery: effect on wound healing. J Extra Corpor Technol. 2005;37:148–52. [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitto J, Reiprich A, Drescher A, et al. Intraoperative application of gravitational separated, autologous platelets reduces wound infection in diabetes mellitus patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2009;9:S106–7. [Google Scholar]
- 39.Dorge H, Sellin C, Bury M-C, et al. Incidence of deep sternal wound infection is not reduced with autologous platelet rich plasma in high-risk cardiac surgery patients. Thorac Cardiovasc Surg. 2013;61:180–4. doi: 10.1055/s-0032-1304537. [DOI] [PubMed] [Google Scholar]
- 40.Litmathe J, Philipp C, Kurt M, et al. The use of autologous platelet gel (APG) for high-risk patients in cardiac surgery - Is it beneficial? Perfusion. 2009;24:381–7. doi: 10.1177/0267659109358283. [DOI] [PubMed] [Google Scholar]
- 41.Vang SN, Brady CP, Christensen KA, et al. Autologous platelet gel in coronary artery bypass grafting: Effects on surgical wound healing. J Extra Corpor Technol. 2007;39:31–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Englert SJ, Estep TH, Ellis-Stoll CC. Postoperative surgical chest and leg incision sites using platelet gel: a retrospective study. J Extra Corpor Technol. 2008;40:225–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Hamman BL, Stout LY, Theologes TT, et al. Relation between topical application of platelet-rich plasma and vancomycin and severe deep sternal wound infections after a first median sternotomy. Am J Cardiol. 2014;113:1415–9. doi: 10.1016/j.amjcard.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 44.Trowbridge CC, Stammers AH, Woods E, et al. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381–6. [PMC free article] [PubMed] [Google Scholar]
- 45.Shrier I, Boivin J-F, Steele RJ, et al. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol. 2007;166:1203–9. doi: 10.1093/aje/kwm189. [DOI] [PubMed] [Google Scholar]