Abstract
Preventing haemarthroses and arthropathy is a major challenge in patients with haemophilia and inhibitors, as treatment options are limited. One potential strategy is short-term episodic prophylaxis, which extends bypassing agent therapy beyond the resolution of bleeding to include the post-bleed inflammatory phase. At the 13th Zürich Haemophilia Forum, an expert panel reviewed the rationale behind this strategy, explored its current use with recombinant activated factor VII (rFVIIa) and considered treatment monitoring and optimisation. Two protocols are currently used for short-term episodic prophylaxis, both of which stipulate on-demand rFVIIa until resolution of bleeding, followed by daily dosing for ≥3 days to prevent re-bleeds. Short-term episodic prophylaxis should be individualised to optimise outcomes, perhaps through early treatment initiation or by combining rFVIIa with other treatments (e.g. factor VIII, tranexamic acid). Encouraging treatment compliance can also improve outcomes. Additionally, there is a need to develop objective clinical outcome measures, biomarkers and imaging protocols that can monitor treatment outcomes and joint disease in patients with inhibitors. A proactive approach incorporating a systematic package of care is needed. Currently, short-term episodic prophylaxis with rFVIIa may be an alternative treatment option to on-demand treatment for patients with inhibitors.
Keywords: haemophilia, inhibitors, rFVIIa, short-term episodic prophylaxis
Introduction
Joint bleeds are the hallmark of severe haemophilia and are associated with pain, reduced mobility and, when they occur repeatedly into the same joint, irreversible damage culminating in haemophilic arthropathy1. This, in turn, lowers quality of life, increases the risk of disability and makes the need for orthopaedic intervention more likely1. Prevention of haemarthroses is therefore a treatment priority, with the aim of preventing joint damage and thus supporting the patient’s physical and social development2,3.
For patients without inhibitors, prevention of joint bleeds is achieved through prophylaxis with factor (F) VIII or FIX concentrates (in haemophilia A and haemophilia B, respectively), and this is now considered the gold standard of haemophilia treatment4. Inhibitor development, however, renders patients unresponsive to factor replacement therapy and poses a number of challenges. Patients with inhibitors face an increased risk of recurrent haemarthroses3 and treatment options are limited; as a result, target joints, arthropathy and permanent disability may ultimately develop3.
Immune tolerance induction (ITI) offers the chance of permanent eradication of inhibitors. However, ITI is not always successful or feasible; in addition, in all cases, therapy with bypassing agents (recombinant activated factor VII [rFVIIa]; NovoSeven®, Novo Nordisk, Bagsværd, Denmark) or plasma-derived activated prothrombin complex concentrates (pd-aPCC; FEIBA®, Baxter BioScience, Vienna, Austria) is required to control bleeding2,3. Although prophylaxis with bypassing agents is a potential treatment option, they are not licensed for this use in all countries, and bypassing agents are most commonly given as an on-demand treatment of acute bleeds. While on-demand therapy resolves bleeding, it may not be sufficient to prevent or delay the onset of haemophilic arthropathy5. Alternative treatment strategies for patients with inhibitors are, therefore, needed.
While continuous secondary prophylaxis, which aims to avoid repeated haemarthroses3, may be one such alternative, prophylaxis with bypassing agents is not as effective as prophylaxis with replacement factor concentrates, and as patients’ responses to such treatment are heterogeneous and need to be balanced against the additional treatment burden, secondary prophylaxis may not meet the needs of all patients with inhibitors. The cost of such treatment schedules may also be prohibitive, particularly in developing countries.
Short-term episodic prophylaxis (also described as enhanced on-demand or enhanced episodic treatment) using bypassing agents extends the treatment of haemarthroses beyond the resolution of bleeding to include the post-bleed inflammatory phase. This approach offers a treatment alternative for joint bleeds that may improve outcomes vs standard on-demand treatment alone and aims to reduce the frequency of joint bleeds. In April 2014, members of the Zürich Haemophilia Forum convened for its 13th meeting to discuss the rationale behind short-term episodic prophylaxis treatment. In particular, this expert panel explored how commonly short-term episodic prophylaxis with rFVIIa is practised in patients with inhibitors and considered strategies for optimising treatment and monitoring outcomes. This article provides a summary of the panel’s consensus and discussions.
The rationale behind short-term episodic prophylaxis
The burden of orthopaedic complications
The need for treatment alternatives for patients with inhibitors was highlighted by the European Study on Orthopaedic Status (ESOS), which found that the burden of orthopaedic complications is greater in patients with inhibitors than in their counterparts without6. In clinical assessments of arthropathy, according to the Gilbert classification (in which a lower score indicates better joint function), patients with inhibitors had a significantly worse overall mean joint score compared with patients without inhibitors for all joints (15.4 vs 5.46; p<0.05) and for all major joints (knees, ankles, elbows) (14.6 vs 5.27; p<0.05)6. Similarly, radiological evaluation of joint status using the Pettersson classification (again, a lower score indicates better function) revealed significantly worse scores in the major joints of patients with inhibitors (22.9 vs 8.0 in patients without inhibitors; p<0.05)6. Patients with inhibitors also reported significantly more pain (p<0.05) in all joints than patients without inhibitors, were significantly more likely to require wheelchairs (p=0.009) or some kind of walking aid (p=0.048), and were more frequently hospitalised or absent from work or school6.
Similar results were found in the French Statut Orthopédique des Patients Hémophiles avec Inhibiteur (SOPHI) study, when assessing the orthopaedic status and quality of life of haemophilic patients with inhibitors7. Fifty haemophilic patients aged 12–63 years with a history of high-responding inhibitors were included. Clinical assessment showed that only 12% of the patients had a zero pain score and 2% a zero Gilbert score. The mean Gilbert score was significantly higher in patients over 35 years of age than in younger patients. However, younger patients with inhibitors appeared to have a more impaired orthopaedic status than patients of similar age without inhibitors in previously published cohorts. Remarkably, older patients with inhibitors tended to have the best mental quality of life, contrasting with their highly impaired orthopaedic condition and physical quality of life.
A more recent study using the Functional Independence Score in Haemophilia (FISH) confirmed that inhibitor development significantly decreases functional independence (as measured by eight domains of activity covering self-care, transfer and mobility) in patients with haemophilia (p=0.047)8. Taken together with the results from the ESOS and SOPHI studies, these findings confirm that the failure to prevent or resolve joint bleeding leads to worse outcomes for patients with inhibitors than for those without. This also confirms the view that treatment for patients with inhibitors has been less effective than standard haemophilia therapy to date.
The consequences of inadequate therapy are particularly evident in older patients with inhibitors. In the ESOS study, while the incidence of haemarthrosis in all joints was similar between younger patients (aged 14–35 years) with or without inhibitors using an on-demand regimen during the 12-month study period (12.3 vs 11.7), older patients with inhibitors (aged 36–65 years) had an approximately 50% lower incidence of haemarthrosis in all joints (6.2) than younger patients with inhibitors. This is most likely due to the presence of fibrotic changes in association with advanced arthropathy caused by inadequate treatment in the past6.
Haemophilic arthropathy
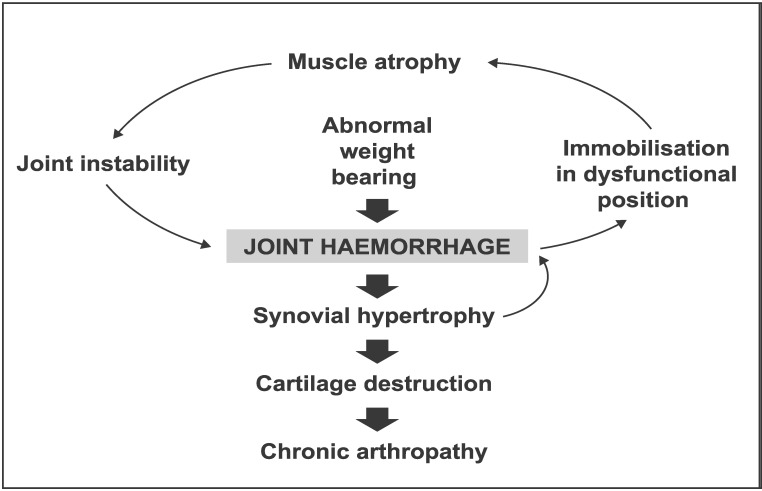
Repeated intra-articular bleeding causes damage to the joint, eventually producing deformity and joint dysfunction9 (Figure 1). The pathogenesis of haemophilic arthropathy is multifactorial and includes inflammatory synovium-mediated and degenerative cartilage-mediated components9.
Figure 1.
The cycle of haemarthrosis and haemophilic arthropathy.
Deposition of iron within the synovium from repeated intra-articular bleeds triggers synovial inflammation with infiltration of the synovial membrane by lymphocytes, monocytes and polymorphonuclear cells, resulting in increased vascularity and synovial hypertrophy9,10. In addition to its effects on the synovium, intra-articular blood has a direct, harmful effect on the cartilage9–11. Exposure of cartilage to blood inhibits turnover of the extracellular matrix in which chondrocytes are embedded, resulting in the breakdown and release of matrix components, such as proteoglycans and collagen9. This results in a loss of cartilage matrix and cartilage destruction, possibly triggered by the formation of destructive oxygen metabolites that cause chondrocyte apoptosis9. This direct cartilage damage may, in turn, induce further inflammatory responses in the synovium9.
These parallel processes of joint damage, which resemble the degenerative processes found in osteoarthritis and the inflammatory changes seen in rheumatoid arthritis, may ultimately result in a fibrotic and destroyed joint9. Of particular note for patients with haemophilia, however, is the finding in haemophilic mice that haemarthrosis also activates the local synovial fibrinolytic system, thus increasing functionally active plasmin. This makes the joint more vulnerable to prolonged and subsequent bleeds10. In addition, canine and murine studies have shown that blood-induced changes to the cartilage and synovium persist long beyond the episode of haemarthrosis9,12. In line with these findings, Manco-Johnson and colleagues proposed that subclinical bleeding into joints may cause further damage even in the absence of overt clinical haemarthrosis13. After a joint bleed, it is therefore crucial to restore the synovium completely and to avoid successive and subclinical bleeds.
What is short-term episodic prophylaxis?
Short-term episodic prophylaxis extends the treatment of haemarthroses beyond resolution of the bleeding to include the post-bleed inflammatory phase. It begins immediately after the initial treatment of acute joint bleeds with on-demand rFVIIa therapy, which is administered until haemostasis is achieved. Following the initial resolution of a bleed, short-term episodic prophylaxis is initiated with once-daily doses of rFVIIa for several days. The aim of this is to allow for complete healing of the haemarthrosis aftermath, prevent re-bleeding and delay the development of target joints and consequent arthropathy.
Current protocols in use
While treatment guidelines from the World Federation of Hemophilia advocate extending treatment beyond the initial bleeding event for central nervous system bleeds in patients with haemophilia14, there are no such guidelines for joint or other bleeds. Furthermore, many of the treatment guidelines and definitions used in haemophilia are not applicable to patients with inhibitors.
To the best of our knowledge, the first published report on the use of an enhanced episodic treatment protocol for acute bleeds was provided in 2007 by Manco-Johnson and colleagues in their study, which demonstrated the increased efficacy of prophylaxis vs episodic treatment in the prevention of joint bleeds and subsequent damage in patients with severe haemophilia without inhibitors13. The rationale behind enhanced episodic treatment was to decrease inflammation and prevent joint damage, and patients assigned to this treatment received 40 IU/kg FVIII at the time of the bleed, followed by 20 IU/kg at 24 and 72 hours after the first dose13.
Published data on the use of short-term episodic prophylaxis in real-world practice are scarce. However, in Spain, short-term episodic prophylaxis with rFVIIa has been used to treat bleeding episodes in patients with haemophilia and inhibitors since 1997. Two protocols are used: the Spanish Society of Thrombosis and Haemostasis (SETH) protocol for patients with at least one unaffected/minimally affected joint; and the La Paz Centre modification (Madrid) for target joints15–17, modified on the basis of published findings, and the experience of this centre, of the poorer response to treatment observed for target joints18,19. Both protocols involve: (i) a first phase of treating the bleed with a single dose of rFVIIa or repeated dosing until bleeding stops; and (ii) a second phase to prevent re-bleeds, involving once-daily rFVIIa dosing until synovial recovery is complete (≥3 days). While pd-aPCC could also be useful, the use of rFVIIa is preferred first-line, to avoid exposure to FVIII contained in pd-aPCC which may trigger anamnesis in patients who are candidates for ITI. Details of each protocol are summarised in Table I; the main treatment outcome to monitor for patients treated with these regimens is the number of re-bleeds that occur20.
Table I.
Protocols for short-term episodic prophylaxis with rFVIIa: SETH protocol and the La Paz Centre (Madrid) modification.
| SETH protocol: ≥1 unaffected/minimally affected joint | La Paz Centre (Madrid) modification: target joints | |
|---|---|---|
| To stop bleeding | Standard-dose rFVIIa (90–120 μg/kg) every 2 hours until joint pain disappears (≥3 doses) | First dose: rFVIIa 270 μg/kg Second dose at 2 hours: rFVIIa 180 μg/kg Thereafter, rFVIIa 120 μg/kg every 2 hours until joint pain is alleviated |
| To cover the synovitis phase | rFVIIa 90 μg/kg once daily until swelling and motion are completely recovered (≥3 days) | rFVIIa 120 μg/kg once daily until swelling and motion are completely recovered (≥3 days) |
rFVIIa: recombinant activated factor VII; SETH: the Spanish Society of Thrombosis and Haemostasis.
Optimising short-term episodic prophylaxis
Patient selection
While there is clearly a rationale for the use of short-term episodic prophylaxis in patients with inhibitors, it is crucial to decide how patients requiring such treatment should be identified. The selection of patients for this treatment could be determined by a high frequency of bleeding; for example, patients with ≥12 joint bleeds or at least one target joint bleed per year could be considered eligible. Ultimately, however, the question of which patients should receive short-term episodic prophylaxis may have a simple answer: if a patient does not respond to standard treatment, then intensified treatment should be administered until symptom resolution17. Thus, improving quality of life should ideally drive treatment decisions when selecting patients.
Individualising short-term episodic prophylaxis
As for all treatment options in haemophilia, short-term episodic prophylaxis should be individualised to optimise outcomes. Several options for treatment individualisation currently exist.
The timing of rFVIIa dosing may significantly influence treatment outcome. Several studies have shown that early (vs late) treatment is more effective at controlling bleeds21–25. In a retrospective review of data from the HemoRec registry in the Czech Republic, for example, the incidence of re-bleeding was more than twice as high in patients who received rFVIIa >2 hours after the onset of bleeding (13.7%) compared with patients who received rFVIIa ≤2 hours after bleed onset (5.2%)24. Early treatment initiation also reduces the number of doses required to control a bleed21–23,25. On-demand treatment with an initial high dose of rFVIIa (≤270 μg/kg) also improves outcomes over standard dosing, as shown by greater bleed cessation rates, requirement for fewer doses and reduced overall rFVIIa consumption19,26,27.
Other methods of treatment individualisation include sequential or combined therapy with rFVIIa and pd-aPCC. Concomitant infusion of rFVIIa and pd-aPCC may exert an additive or synergistic effect on thrombin generation28,29 and was shown to produce good haemostatic efficacy in patients whose bleeds were refractory to monotherapy with either agent28,29. However, extreme caution is required when considering this approach as combined therapy with rFVIIa and pd-aPCC is not licensed, and there remains a potential risk of thrombosis with parallel treatment30 and only very few data are available to support this strategy.
Combining other therapies also offers an alternative treatment option for short-term episodic prophylaxis. For example, MC710 (a mixture of plasma-derived FVIIa and FX) has provided favourable ex vivo results in blood from patients with inhibitors, with significant improvements in coagulation activity and thrombin production vs the effects achieved by rFVIIa and pd-aPCC31. While this suggests improved bypassing activity, no clinical efficacy data are yet available. Additionally, rFVIIa therapy can be supplemented by adding FVIII, which has been shown to potentiate thrombin generation and may therefore optimise haemostatic efficacy32. Adjunctive tranexamic acid is commonly used with rFVIIa therapy as it is inexpensive and does not carry a risk of side effects, although it is difficult to assess its impact on outcomes33. Finally, in our opinion, combining cyclo-oxygenase-2 inhibitors with rFVIIa treatment has produced better outcomes than adjunctive FVIII when used in synovectomy.
As a final note regarding treatment individualisation, we believe that the treatment of patients with inhibitors is often too passive and a more proactive approach would be beneficial. Other techniques (such as physiotherapy, joint aspiration, synoviorthesis, synovectomy and joint replacement) could be considered as a means of improving and preserving joint function. A systematic package of care needs to be developed.
Encouraging treatment compliance
Compliance to preventive treatment regimens in haemophilia is low across many age groups34. Barriers to treatment compliance may be physical, such as pain/discomfort with injections35, poor recognition of (or fluctuation in/disappearance of) symptoms of bleeding35–38 and difficulties with venous access38. There are also many potential psychosocial barriers to compliance, including feelings of sadness, helplessness or anger39; needle phobia and other treatment-related fears35,38; lack of self-efficacy35; denial of problems38; traumatic childhood memories; lack of cooperation from paediatric patients; and forgetfulness36,37. Finally, practical barriers include a busy lifestyle and family/work commitments35,38, time commitments necessitated by treatment35–38, lack of privacy for patients in shared accommodation, unmanageable treatment regimens and financial concerns35,38.
The challenge of overcoming barriers to compliance requires a comprehensive care team, which should include a social worker and/or a psychologist14. Physical barriers can often be overcome by ensuring good venous access (e.g. through insertion of a central venous access device), and providing training to help patients and parents recognise bleeds, manage home treatment regimens and administer treatment38. Proper training can also ensure correct maintenance of venous access lines and reduce the risk of infection and other complications38. Finally, discomfort associated with venipuncture can be minimised by pre-treatment application of a topical anaesthetic cream36.
It is essential that health-care providers also address the psychosocial barriers to compliance36,38. A key area for psychosocial support is the provision of psycho-educational intervention, which can help to address underlying issues (such as denial and needle phobia) and build both parents’ and patients’ trust in the medical team36,38. Increasing a patient’s self-efficacy is crucial as well, especially during adolescence and the transition to adulthood38. Peer support and encouragement is also important, as it can remove feelings of isolation, build self-esteem36 and increase self-efficacy. Patient groups and associations provide valuable peer support, and inhibitor patients should be encouraged to attend patient/parent conferences. However, patient groups and associations that do not have members with inhibitors may be unaware of the associated challenges; it is, therefore, crucial that patient groups increase their understanding of the specific issues, problems and challenges faced by patients with inhibitors.
Finally, reviewing the patient’s treatment regimen can ensure ease of use and thus reduce some of the practical barriers to compliance. Providing home, family and nursing support can help patients and parents with the practical aspects of treatment administration, support them through any associated relationship issues and help them to maintain their daily routine despite the time requirements imposed by treatment. Any financial concerns should also be addressed (e.g. assistance with grant applications and insurance forms). Another key tool in enabling patients with inhibitors to manage their treatment better and become more autonomous is education of patients and caregivers; patient-friendly education can be particularly helpful in increasing patients’ understanding of the need for treatment intensification even in the absence of symptoms37.
Monitoring treatment and clinical outcomes
There is a need for clearly defined outcome measures that can guide treatment in patients with haemophilia and inhibitors. In these patients, clinical outcomes are closely related to the patients’ characteristics (e.g. bleeding phenotype, joint status, age, lifestyle, previous response to bypassing agents) and the treatment interventions used (e.g. bypassing agent dose, other haemostatic agents used, physiotherapy).
Clinical outcome measures
It is essential to develop an objective clinical outcome measure that is able to detect and monitor joint disease. In developing such a measure, it will be crucial to regularly assess the joints to determine a baseline measure of joint status so that clinicians can know a joint’s usual range of motion, discern whether a particular joint is swollen and judge when on-demand treatment can be discontinued. Review clinics should be conducted regularly for patients with inhibitors to enable the patients to be advised appropriately if their joint status has deteriorated.
Treatment intensity and the duration of short-term episodic prophylaxis can be tailored according to pre-bleed joint status (e.g. target joint or well-preserved joint). The intensity and duration of on-demand therapy and short-term episodic prophylaxis may need to be greater in children than in adults to prevent the development of target joints.
Biomarkers to detect and monitor joint disease in haemophilia
A major goal in haemophilia research is the discovery of a biomarker to guide treatment or to be used as an objective measure to indicate a pathogenic process, or the pharmacological response to treatment. Several potential biomarkers have been explored to date in haemophilia, including enzymes, cytokines and chemokines, growth factors and cellular constituents. Some data suggest that a combination of commonly used serum and urinary biomarkers correlates better with the severity of joint damage in haemophilia than individual biomarkers alone40. These results also demonstrate that haemophilic arthropathy might be useful for the screening of newly developed biomarkers of joint damage40.
When considering biomarkers of joint disease in haemophilia, it is important to remember that markers may not be specific to cartilage or the affected joint; a change in expression related to the development of joint disease may be too dilute to provide an alert signal because of its greater overall expression in other tissues. However, collagenous biomarkers have shown promise in haemophilia, particularly type II collagen, as this is relatively specific for articular collagen, makes up only 1% of all collagen in the body and has a low turnover. Finally, as there is considerable overlap between candidate biomarkers for osteoarthritis and haemophilic joint disease (e.g. tumour necrosis factor-alpha), it will be important to monitor the progress of candidate biomarkers for osteoarthritis in order not to miss any important developments and potential new biomarkers that may also be relevant to haemophilia.
Monitoring treatment using ultrasound
Imaging can be used in haemophilia to help achieve the correct balance between under- and over-treatment of joint bleeds. Ultrasonography is particularly useful in this regard because, when compared with other imaging techniques, it is relatively accessible, repeatable and affordable. However, its disadvantages include a limited field of view, inability to examine intra-articular structures and lack of standardisation. Ultrasonography is also highly operator-dependent, with a long learning curve, and can be difficult for physicians to interpret.
A simplified ultrasound scanning procedure and additive scoring method, known as Haemophilia Arthropathy Detection with Ultrasound (HEAD-US), has been developed to evaluate the joints of patients with haemophilic arthropathy41. HEAD-US aims to detect early signs of joint damage (in elbows, ankles, and knees), while keeping the procedure quick and easy to perform, and it may provide an objective assessment tool to provide helpful joint status information to complement that obtained using physical examination. It may go some way towards meeting the need for a more objective measure for use in clinical practice to detect early joint disease, evaluate disease progression and monitor treatment. Ultrasound examination may also be useful in guiding orthopaedic procedures (e.g. joint aspiration and synoviorthesis); however, its use in these areas requires validation.
As ultrasound can be used frequently (weeks), it may be the preferred imaging option to use for regular treatment follow-up and monitoring the progress/regress of a target joint in patients with inhibitors, but has to be individualised to the specific joint and other circumstances. Magnetic resonance imaging can be a valuable tool for the longer term (years) follow-up of such a joint.
Final considerations
A key goal in haemophilia treatment is to ensure that patients with inhibitors have access to the same treatment options as patients without inhibitors. However, prophylaxis for patients with inhibitors is not available in all countries and is currently less effective than prophylaxis in patients without inhibitors. At present, therefore, short-term episodic prophylaxis may offer the best (and indeed only) treatment option for patients with inhibitors. This is not a “one size fits all” approach: as with any haemophilia treatment, therapy must be individualised to ensure the best outcome, and optimal methods to monitor treatment and outcomes must be determined. There also remains a need for a bypassing agent that is more easily administered and faster-acting, with a prolonged half-life to allow prophylaxis for those patients in whom ITI fails to eradicate the inhibitor.
Acknowledgements
Novo Nordisk provided financial support for the 13th Zürich Haemophilia Forum and for medical writing assistance, provided by Sharon Eastwood of PAREXEL, in compliance with international guidelines for good publication practice.
Footnotes
Disclosure of conflicts of interest
GA has received reimbursement for attending symposia/congresses, and/or honoraria for speaking and/or consulting, and/or funds for research from Baxter, Bayer, CSL Behring, Grifols, Novo Nordisk, Biotest and Pfizer. GD has received honoraria from Novo Nordisk for speaking and participating on advisory boards. AD will receive an honorarium payment from Novo Nordisk for reviewing research grant applications this year. CH has acted as a consultant and been a board member for Bayer, Baxter, Pfizer, Sobi, Biogen, CSL Behring, LFB, Octapharma, Novo Nordisk and CAF-DCF, and has received grants from Bayer, Baxter and Pfizer. VJ-Y has received reimbursement for attending symposia/congresses, and/or honoraria for speaking and/or consulting, and/or funds for research from Baxter, Bayer, CSL Behring, Grifols, Novo Nordisk, Octapharma and Pfizer. RL has received consultancy or speaker fees from Bayer, Baxter, Novo Nordisk, Biogen and Octapharma during the past 5 years. MM has acted as a paid consultant to Bayer, Baxter and Novo Nordisk and has served as a consultant on Pfizer advisory boards; he has received speaker fees from CSL Behring, Octapharma, Bayer and Novo Nordisk, and unrestricted research grants from Bayer, Pfizer and Baxter. ES has acted as a paid consultant to Bayer, Baxter, Novo Nordisk, Pfizer, Roche, Biogen Idec, Sobi, CSL Behring and Grifols; she has received speaker fees from Octapharma, Kedrion and Biotest, and has received unrestricted research grants from Pfizer. TL has been an expert consultant for national and European advisory boards, and/or a clinical investigator for Baxter, Bayer, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Sobi and Roche Laboratories.
SZS and GB have no conflicts of interest to declare.
References
- 1.Gringeri A, Ewenstein B, Reininger A. The burden of bleeding in haemophilia: is one bleed too many? Haemophilia. 2014;20:459–63. doi: 10.1111/hae.12375. [DOI] [PubMed] [Google Scholar]
- 2.Blanchette VS, Manco-Johnson MJ. Meeting unmet needs in inhibitor patients. Haemophilia. 2010;16(Suppl 3):46–51. doi: 10.1111/j.1365-2516.2010.02260.x. [DOI] [PubMed] [Google Scholar]
- 3.Santagostino E, Morfini M, Auerswald GK, et al. Paediatric haemophilia with inhibitors: existing management options, treatment gaps and unmet needs. Haemophilia. 2009;15:983–9. doi: 10.1111/j.1365-2516.2009.01999.x. [DOI] [PubMed] [Google Scholar]
- 4.Fischer K, Konkle B, Broderick C, Kessler CM. Prophylaxis in real life scenarios. Haemophilia. 2014;20(Suppl 4):106–13. doi: 10.1111/hae.12425. [DOI] [PubMed] [Google Scholar]
- 5.Aznar JA, Marco A, Jiménez-Yuste V, et al. Is on-demand treatment effective in patients with severe haemophilia? Haemophilia. 2012;18:738–42. doi: 10.1111/j.1365-2516.2012.02806.x. [DOI] [PubMed] [Google Scholar]
- 6.Morfini M, Haya S, Tagariello G, et al. European study on orthopaedic status of haemophilia patients with inhibitors. Haemophilia. 2007;13:606–12. doi: 10.1111/j.1365-2516.2007.01518.x. [DOI] [PubMed] [Google Scholar]
- 7.Stieltjes N, Torchet MF, Misrahi L, et al. Epidemiological survey of haemophiliacs with inhibitors in France: orthopaedic status, quality of life and cost--the ‘Statut Orthopedique des Patients Hemophiles’ avec Inhibiteur study. Blood Coagul Fibrinolysis. 2009;20:4–11. doi: 10.1097/mbc.0b013e328313fc8e. [DOI] [PubMed] [Google Scholar]
- 8.Kachooei AR, Badiei Z, Zandinezhad ME, et al. Influencing factors on the functional level of haemophilic patients assessed by FISH. Haemophilia. 2014;20:185–9. doi: 10.1111/hae.12273. [DOI] [PubMed] [Google Scholar]
- 9.Roosendaal G, Lafeber FP. Pathogenesis of haemophilic arthropathy. Haemophilia. 2006;12(Suppl 3):117–21. doi: 10.1111/j.1365-2516.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwenhuizen L, Roosendaal G, Coeleveld K, et al. Haemarthrosis stimulates the synovial fibrinolytic system in haemophilic mice. Thromb Haemost. 2013;110:173–83. doi: 10.1160/TH13-01-0080. [DOI] [PubMed] [Google Scholar]
- 11.Jansen NW, Roosendaal G, Lafeber FP. Understanding haemophilic arthropathy: an exploration of current open issues. Br J Haematol. 2008;143:632–40. doi: 10.1111/j.1365-2141.2008.07386.x. [DOI] [PubMed] [Google Scholar]
- 12.Hakobyan N, Kazarian T, Jabbar AA, et al. Pathobiology of hemophilic synovitis I: overexpression of mdm2 oncogene. Blood. 2004;104:2060–4. doi: 10.1182/blood-2003-12-4231. [DOI] [PubMed] [Google Scholar]
- 13.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 15.Aznar JA, Perez-Alenda S, Abad-Franch L, et al. Intensive treatment of haemarthrosis in haemophilia: clincial and ultrasound managment [abstract] Haemophilia. 2010;16(Suppl 4):21. [Google Scholar]
- 16.Querol F, Cortina V, Cid AR, et al. Clinical and echographical control protocol of haemarthrosis in haemophilia patients with inhibitors: evaluation of the efficacy of recombinant factor VIIa in the evolution process (EFFISEVEN protocol) Haemophilia. 2008;14(Suppl 6):36–44. doi: 10.1111/j.1365-2516.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Merchan EC, Jiménez-Yuste V, Aznar JA, et al. Joint protection in haemophilia. Haemophilia. 2011;17(Suppl 2):1–23. doi: 10.1111/j.1365-2516.2011.02615.x. [DOI] [PubMed] [Google Scholar]
- 18.Kenet G, Lubetsky A, Luboshitz J, Martinowitz U. A new approach to treatment of bleeding episodes in young hemophilia patients: a single bolus megadose of recombinant activated factor VII (NovoSeven) J Thromb Haemost. 2003;1:450–5. doi: 10.1046/j.1538-7836.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 19.Santagostino E, Mancuso ME, Rocino A, et al. A prospective randomized trial of high and standard dosages of recombinant factor VIIa for treatment of hemarthroses in hemophiliacs with inhibitors. J Thromb Haemost. 2006;4:367–71. doi: 10.1111/j.1538-7836.2006.01772.x. [DOI] [PubMed] [Google Scholar]
- 20.Amby LK, Seremetis S, Obergfell A, Bjerre J. Challenges of defining reliable clinical surrogate end points in haemophilia trials: a critical review. Blood Coagul Fibrinolysis. 2009;20:488–93. doi: 10.1097/MBC.0b013e32832c8803. [DOI] [PubMed] [Google Scholar]
- 21.Kavakli K, Yesilipek A, Antmen B, et al. The value of early treatment in patients with haemophilia and inhibitors. Haemophilia. 2010;16:487–94. doi: 10.1111/j.1365-2516.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- 22.Lusher JM. Early treatment with recombinant factor VIIa results in greater efficacy with less product. Eur J Haematol Suppl. 1998;63:7–10. doi: 10.1111/j.1600-0609.1998.tb01103.x. [DOI] [PubMed] [Google Scholar]
- 23.Lusher JM. Acute hemarthroses: the benefits of early versus late treatment with recombinant activated factor VII. Blood Coagul Fibrinolysis. 2000;11(Suppl 1):S45–9. doi: 10.1097/00001721-200004001-00010. [DOI] [PubMed] [Google Scholar]
- 24.Salaj P, Brabec P, Penka M, et al. Effect of rFVIIa dose and time to treatment on patients with haemophilia and inhibitors: analysis of HemoRec registry data from the Czech Republic. Haemophilia. 2009;15:752–9. doi: 10.1111/j.1365-2516.2009.02007.x. [DOI] [PubMed] [Google Scholar]
- 25.Santagostino E, Gringeri A, Mannucci PM. Home treatment with recombinant activated factor VII in patients with factor VIII inhibitors: the advantages of early intervention. Br J Haematol. 1999;104:22–6. doi: 10.1046/j.1365-2141.1999.01128.x. [DOI] [PubMed] [Google Scholar]
- 26.Parameswaran R, Shapiro AD, Gill JC, Kessler CM. Dose effect and efficacy of rFVIIa in the treatment of haemophilia patients with inhibitors: analysis from the Hemophilia and Thrombosis Research Society Registry. Haemophilia. 2005;11:100–6. doi: 10.1111/j.1365-2516.2005.01075.x. [DOI] [PubMed] [Google Scholar]
- 27.Young G, Shapiro AD, Walsh CE, et al. Patient/caregiver-reported recombinant factor VIIa (rFVIIa) dosing: home treatment of acute bleeds in the Dosing Observational Study in Hemophilia (DOSE) Haemophilia. 2012;18:392–9. doi: 10.1111/j.1365-2516.2011.02704.x. [DOI] [PubMed] [Google Scholar]
- 28.Martinowitz U, Livnat T, Zivelin A, Kenet G. Concomitant infusion of low doses of rFVIIa and FEIBA in haemophilia patients with inhibitors. Haemophilia. 2009;15:904–10. doi: 10.1111/j.1365-2516.2009.02028.x. [DOI] [PubMed] [Google Scholar]
- 29.Schneiderman J, Rubin E, Nugent DJ, Young G. Sequential therapy with activated prothrombin complex concentrates and recombinant FVIIa in patients with severe haemophilia and inhibitors: update of our previous experience. Haemophilia. 2007;13:244–8. doi: 10.1111/j.1365-2516.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 30.Ingerslev J, Sorensen B. Parallel use of by-passing agents in haemophilia with inhibitors: a critical review. Br J Haematol. 2011;155:256–62. doi: 10.1111/j.1365-2141.2011.08854.x. [DOI] [PubMed] [Google Scholar]
- 31.Shirahata A, Fukutake K, Mimaya J, et al. Results of clot waveform analysis and thrombin generation test for a plasma-derived factor VIIa and X mixture (MC710) in haemophilia patients with inhibitors--phase I trial: 2nd report. Haemophilia. 2013;19:330–7. doi: 10.1111/hae.12024. [DOI] [PubMed] [Google Scholar]
- 32.Klintman J, Astermark J, Berntorp E. Combination of FVIII and by-passing agent potentiates in vitro thrombin production in haemophilia A inhibitor plasma. Br J Haematol. 2010;151:381–6. doi: 10.1111/j.1365-2141.2010.08378.x. [DOI] [PubMed] [Google Scholar]
- 33.Tran HT, Sørensen B, Rea CJ, et al. Tranexamic acid as adjunct therapy to bypassing agents in haemophilia A patients with inhibitors. Haemophilia. 2014;20:369–75. doi: 10.1111/hae.12318. [DOI] [PubMed] [Google Scholar]
- 34.Berntorp E. Joint outcomes in patients with haemophilia: the importance of adherence to preventive regimens. Haemophilia. 2009;15:1219–27. doi: 10.1111/j.1365-2516.2009.02077.x. [DOI] [PubMed] [Google Scholar]
- 35.Auerswald G, Šalek SZ, Benson G, et al. Beyond patient benefit: clinical development in hemophilia. Hematology. 2012;17:1–8. doi: 10.1179/102453312X13221316477372. [DOI] [PubMed] [Google Scholar]
- 36.Novo Nordisk. Psychosocial aspects of life with haemophilia (review by Kantar Health) 2014. [Accessed on 16/11/2015]. Available at http://www.herostudy.org/content/dam/hero-study/AFFILIATE/www-herostudy-com/Media-Publication/Documents/hero-lit-review-feb-2010.pdf.
- 37.Niv H. Prophylaxis: the challenges of sticking to treatment. Haemophilia Today 2012. 2014. [Accessed on 15/11/2015]. Available at https://www.haemophilia.ie/content.php?id=5&article_id=231&level3_id=594.
- 38.Saxena K. Barriers and perceived limitations to early treatment of hemophilia. J Blood Med. 2013;4:49–56. doi: 10.2147/JBM.S43734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benson G, Auerswald G, Elezovic I, et al. Immune tolerance induction in patients with severe hemophilia with inhibitors: expert panel views and recommendations for clinical practice. Eur J Haematol. 2012;88:371–9. doi: 10.1111/j.1600-0609.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- 40.Jansen NW, Roosendaal G, Lundin B, et al. The combination of the biomarkers urinary C-terminal telopeptide of type II collagen, serum cartilage oligomeric matrix protein, and serum chondroitin sulfate 846 reflects cartilage damage in hemophilic arthropathy. Arthritis Rheum. 2009;60:290–8. doi: 10.1002/art.24184. [DOI] [PubMed] [Google Scholar]
- 41.Martinoli C, Della Casa AO, Di Minno G, et al. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) Thromb Haemost. 2013;109:1170–9. doi: 10.1160/TH12-11-0874. [DOI] [PubMed] [Google Scholar]