Abstract
OBJECTIVE
The aim of this study was to better delineate the complex interrelationship among insulin resistance (IR), secretion rate (ISR) and clearance rate (ICR) to increase plasma insulin concentrations in obesity.
METHODS
Healthy volunteers (92 non-diabetic individuals) had an insulin suppression test (IST) to measure IR, and graded-glucose infusion test (GGIT) to measure ISR and ICR. Obesity was defined as a body mass index (BMI) ≥ 30 kg/m2, and IR was defined as steady-state plasma glucose (SSPG) ≥ 10 mmol/L during the IST. We compared plasma glucose and insulin concentrations, ISR, and ICR in 3 groups: insulin sensitive/overweight (IS-OW); insulin sensitive/obese (IS-OB); and insulin resistant/obese (IR-OB).
RESULTS
Compared with IS-OW, IS-OB had significantly higher insulin area under the curve (AUC) and ISR AUC during the GGIT (P<0.001). Glucose AUC and ICR were similar. IR-OB had higher insulin AUC and ISR AUC compared with IS-OB but also had higher glucose AUC and decreased ICR (P<0.01). In multivariate analysis, both BMI and SSPG were significantly associated with ISR.
CONCLUSIONS
Plasma insulin concentration and ISR are increased in individuals with obesity, irrespective of degree of IR, but a decrease in ICR is confined to the subset of individuals who are IR.
Keywords: Insulin clearance, insulin resistance, obesity, hyperinsulinemia
INTRODUCTION
In a recent study, we provided evidence that obesity, per se, was an independent predictor of fasting hyperinsulinemia (1). Previous studies have shown that hyperinsulinemia in individuals with obesity is related to increases in both insulin secretion and decreases in insulin clearance (2–4). However, the prevalence of insulin resistance is increased in individuals with obesity (5), and insulin resistance is also associated with an increase in insulin secretion and decrease in insulin clearance (6–8). Furthermore, when we compared individuals with and without obesity, matched for insulin resistance, we could not discern any difference in insulin clearance associated with obesity, per se (1).
Based on these findings we hypothesized that hyperinsulinemia associated with insulin resistance is secondary to an increase in insulin secretion and a decrease in insulin clearance. On the other hand, in the absence of insulin resistance, obesity-related hyperinsulinemia was secondary to an increase in insulin secretion, without any change in insulin clearance. Although this formulation was consistent with our results, we had not quantified insulin secretion rate in the previous study (1). Consequently, we initiated the current analysis in which we compared plasma glucose and insulin concentrations, insulin secretion, and insulin clearance in 3 groups of individuals: insulin sensitive/overweight (IS-OW); insulin sensitive/obese (IS-OB); and insulin resistant/obese (IR-OB). By so doing, we had the opportunity to define the respective roles of increased insulin secretion vs. decreased insulin clearance in the hyperinsulinemia associated with obesity and insulin resistance.
RESEARCH DESIGN AND METHODS
Subjects
The subjects included 92 individuals without diabetes, who were previously recruited for studies related to insulin resistance (9–10) through newspaper or radio advertisements. They were required to have completed the following 3 tests prior to any intervention: 75-gram oral glucose tolerance test (OGTT) to evaluate glucose tolerance status, insulin suppression test (IST) to quantify insulin-mediated glucose disposal (insulin resistance) and the graded glucose infusion test (GGIT) to determine insulin secretion rate. Subjects ranged from 19–69 years of age, were overweight or obese according to body mass index ([BMI]: 25–40 kg/m2), and were of a non-Hispanic white race/ethnicity. Non-diabetic status was confirmed by OGTT (fasting glucose < 7 mmol/L, 2-hr glucose < 11 mmol/L), and no use of antidiabetic medications. Exclusion criteria also included previous history of coronary artery, kidney or liver disease, or anemia. All subjects provided written informed consent approved by the Stanford Intuitional Review Board prior to participating in any experimental procedures.
Measurements
All tests were conducted in the Stanford Clinical and Translational Research Unit. Individuals were instructed to fast for 12 hours prior to each study. The median time interval between the OGTT and IST was 35 days (interquartile range, 14–48 days).The median time interval between the IST and GGIT was 12 days (7–21 days).
Oral Glucose Tolerance Test (OGTT)
Glucose was measured at fasting and 120 minutes after 75-gram glucose challenge. Normal glucose tolerance was defined as fasting glucose < 5.6 mmol/L and 2-hr glucose < 7.8 mmol/L, isolated impaired fasting glucose (IFG) was defined as fasting glucose of 5.6 to 6.9 mmol/L and 2-hr glucose < 7.8 mmol/L, and isolated impaired glucose tolerance (IGT) was defined as fasting glucose < 5.6 mmol/L and 2-hr glucose of 7.8 to 11.0 mmol/L.
Insulin Suppression Test
Peripheral insulin resistance was measured using a modified version of the insulin suppression test (11). Individuals were infused with octreotide (0.27mcg·m−2·min−1), insulin (32mU·m−2·min−1), and glucose (267mg·m−2·min−1) for 180 minutes. Blood was drawn for glucose and insulin at 10-minute intervals between 150–180 minutes to calculate the steady-state plasma glucose (SSPG) and insulin (SSPI) concentrations. The SSPG concentration provides a direct measure of insulin-mediated glucose disposal; the higher the SSPG concentration, the greater the degree of insulin resistance. SSPG concentration is highly correlated (r~ 0.9) with the measure of insulin sensitivity obtained with the hyperinsulinemic euglycemic clamp (12–13).
Graded-glucose Infusion Test (GGIT)
Subjects received a graded intravenous infusion of glucose for 240 minutes. Glucose infusion rates were increased every 40 minutes, starting with 1 mg/kg/min followed by 2, 3, 4, 6, and 8 mg/kg/min. Glucose, insulin and c-peptide concentrations were measured at fasting and 30 and 40 minutes into each glucose infusion period. The last two values at the end of each infusion period were averaged and used as the mean for that infusion. Insulin secretion rate (ISR) were derived by deconvolution of peripheral plasma C-peptide concentrations, using a two-compartment model of C-peptide kinetics and standard parameters for C-peptide clearance estimated for each subject based on body surface area and age (14). The total area-under-curve (AUC) was calculated for glucose, insulin and ISR using the trapezoidal method. The change in ISR per molar increase in plasma glucose (slope) during the GGIT represented the pancreatic beta cell sensitivity to glucose (pmol/min per mmol/l). The ISR and insulin AUC were also adjusted for BSA to avoid overestimating the differences between individuals based on obesity status.
Calculations
Insulin clearance rate (ICR) was calculated in 2 different ways, using measurements obtained during the IST and the GGIT. During the IST, ICR was calculated by dividing the insulin infusion rate by the SSPI. As octreotide suppresses endogenous insulin secretion and insulin is infused, the ICR from the insulin suppression test represents the exogenous ICR (ICRex). During the GGIT, ICR was calculated by dividing the insulin secretion rate AUC by the insulin AUC and represented the endogenous ICR (ICRen). ICRen was also calculated at every glucose infusion rate change (every 40 minutes) as the ratio between insulin secretion rate and insulin concentration. All calculations of ICR were adjusted for BSA.
Definition of insulin resistance and obesity
A SSPG concentration ≥ 10 mmol/L was defined as being insulin resistant based on prospective studies predicting type 2 diabetes (15). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows: (fasting glucose × fasting insulin)/22.5 (16). Body mass index (BMI) was used to classify individuals as obese (BMI ≥ 30 kg/m2) or overweight (BMI 25–29.9 kg/m2). Participants were divided into three groups: insulin sensitive/overweight (IS-OW, n=27): insulin sensitive/obese (IS-OB, n=24); and insulin resistant/obese (IR-OB, n=41).
Statistical Analysis
All statistical analyses were conducted using SPSS (version 23 for Windows, Armonk, NY). Data are presented as mean ± SD unless otherwise specified. Independent t-test or chi-square test was used to compare the mean and proportion, respectively, between the groups. Peak ICRen and nadir ICRen during the GGIT were also compared. Adjustments were not made for multiple comparisons. Pearson’s correlation coefficients between ISR AUC and experimental variables were calculated. Linear regression models were also used to predict ISR AUC adjusted for age, sex, BMI, glucose AUC and SSPG concentration. We also conducted linear regression analysis to predict fasting insulin concentration adjusted for age, sex, BMI, fasting glucose, SSPG, ICRen and ISR AUC.
RESULTS
Table 1 summarizes the results of the IST and GGIT in the 3 experimental groups. The groups did not differ in terms of age or sex distribution. Focusing initially on the impact of obesity, per se, on the experimental variables, we compared IS-OW vs. IS-OB. By selection, SSPG concentrations were not different in these two groups, but BMI was significantly higher in IS-OB individuals. There was no difference in glucose concentrations including glucose tolerance categories. However, fasting plasma insulin concentrations were higher in the IS-OB group, associated with higher values for insulin AUC, C-peptide AUC, insulin secretion rate AUC, and β-cell sensitivity during the GGIT. The marginally higher HOMA-IR in the IS-OB group likely reflects the higher fasting insulin concentration in the IS-OB group versus the IS-OW group and not differences in insulin sensitivity. There were no other differences noted between the 2 groups in SSPI and insulin clearance rate, including comparisons of ICRex (during the IST) and ICRen (during the GGIT). In addition, as seen in Supplemental Table 1, adjusting for differences in body surface area did not change the nature of the differences between the IS-OW and IS-OB subgroups. Thus, after correcting for body surface area (BSA), plasma insulin concentrations during the GGIT were still higher in the IS-OB group than in the IS-OW group (Insulin AUC/BSA; 446 ± 195 vs. 292 ± 101, P=0.001) and ISR also were higher (ISR AUC/BSA; 960 ± 265 vs. 643 ±199, P= 0.001).
Table 1.
Anthropometric and biochemical characteristics of the study subjects
| Insulin Sensitive Overweight (ISOW) |
Insulin Sensitive Obese (ISOB) |
P value ISOW vs. ISOB |
Insulin Resistant Obese (IROB) |
P value ISOB vs. IROB |
||
|---|---|---|---|---|---|---|
| N | 27 | 24 | 41 | |||
| Age (yrs) | 56.1±8.4 | 54.4±9.8 | 0.516 | 55.8±8.4 | 0.561 | |
| Sex (men) | 12 (43%) | 9 (36%) | 0.610 | 18 (43%) | 0.580 | |
| BMI (kg/m2) | 27.9 ±1.3 | 33.5±2.4 | <0.001 | 33.7±2.3 | 0.798 | |
| Glucose Tolerance State | 0.771 | 0.030 | ||||
| NGT | 9 (33%) | 7 (29%) | 6 (15%) | |||
| Isolated IFG | 10 (37%) | 11 (46%) | 9 (22%) | |||
| Isolated IGT | 3 (11%) | 1 (4%) | 5 (12%) | |||
| Combined IFG/IGT | 5 (19%) | 5 (21%) | 21 (51%) | |||
| Insulin Suppression Test | ||||||
| Fasting Glucose (mmol/L) | 5.6 ±0.4 | 5.8±0.6 | 0.176 | 5.8±0.5 | 0.915 | |
| Fasting Insulin (pmol/L) | 85±33 | 103±32 | 0.039 | 153±84 | 0.002 | |
| HOMA-IR | 3.1±1.3 | 3.8±1.4 | 0.051 | 5.7±3.2 | 0.009 | |
| SSPG (mmol/L) | 6.5±2.1 | 6.9±1.9 | 0.478 | 13.1±2.0 | <0.001 | |
| SSPI (pmol/L) | 448±99 | 458±84 | 0.698 | 571±156 | <0.001 | |
| ICRex (L·min−1·m−2) | 0.522±0.123 | 0.501±0.09 | 0.514 | 0.416±0.106 | 0.002 | |
| Graded Glucose Infusion Test | ||||||
| Glucose AUC (mmol·L−1·4h) | 30.0±3.5 | 30.7±3.6 | 0.442 | 32.8±3.4 | 0.019 | |
| Insulin AUC (pmol·L−1·4h) | 564±226 | 923±424 | <0.001 | 1415±669 | <0.001 | |
| C-peptide AUC (nmol·L−1·4h) | 4.4±1.4 | 6.3±1.8 | 0.001 | 8.1±2.6 | 0.001 | |
| Insulin secretion rate AUC (pmol·min−1· 4h) |
1241±452 | 1990±603 | <0.001 | 2589±981 | 0.003 | |
| Beta cell sensitivity (slope, pmol·min−1 ·[mmol/L]−1) |
104±57 | 159±103 | 0.019 | 166±120 | 0.810 | |
| ICRen (L·min−1·m−2) | 1.20±0.29 | 1.14±0.27 | 0.449 | 0.95±0.26 | 0.006 | |
Data are presented as mean ± standard deviation or number (percentage).
IS, insulin sensitive; IR, insulin resistant; nonOB, non-obese; OB, obese; BMI, body mass index; NGT, normal glucose tolerance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; HOMA-IR, homeostasis model assessment of insulin resistance; SSPG, steady-state plasma glucose; SSPI, steady-state plasma insulin; ICRex, exogenous insulin clearance rate; AUC, area under the curve; ICRen, endogenous insulin clearance rate; BSA, body surface area
Turning to the effects of differences in insulin resistance, a different story emerges. To begin with, although by selection the BMI values were not different, SSPG concentration was approximately twice as high in the IR-OB group as compared to IS-OB persons who were equally obese. In addition, IR-OB group had higher prevalence of individuals with IGT. Fasting plasma insulin concentrations were also higher in the IR-OB group, and HOMA-IR, insulin AUC, C-peptide AUC and ISR AUC were also significantly higher (P≤0.003). In contrast to comparison between IS-OW and IS-OB, higher fasting insulin concentrations in IR-OB were associated with a significant decrease in average insulin clearance during both the IST and GGIT (IR-OB vs. IS-OB). There were no IR-related differences in β-cell sensitivity (See Supplemental Figure 1 for comparison in the dose-response relationship between plasma glucose and ISR).
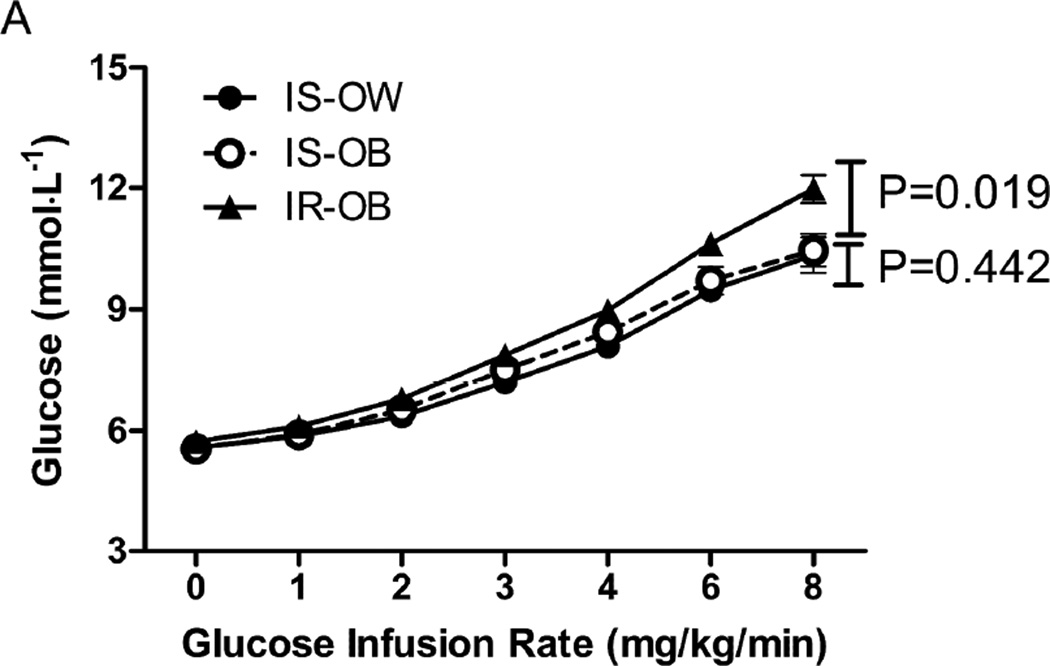
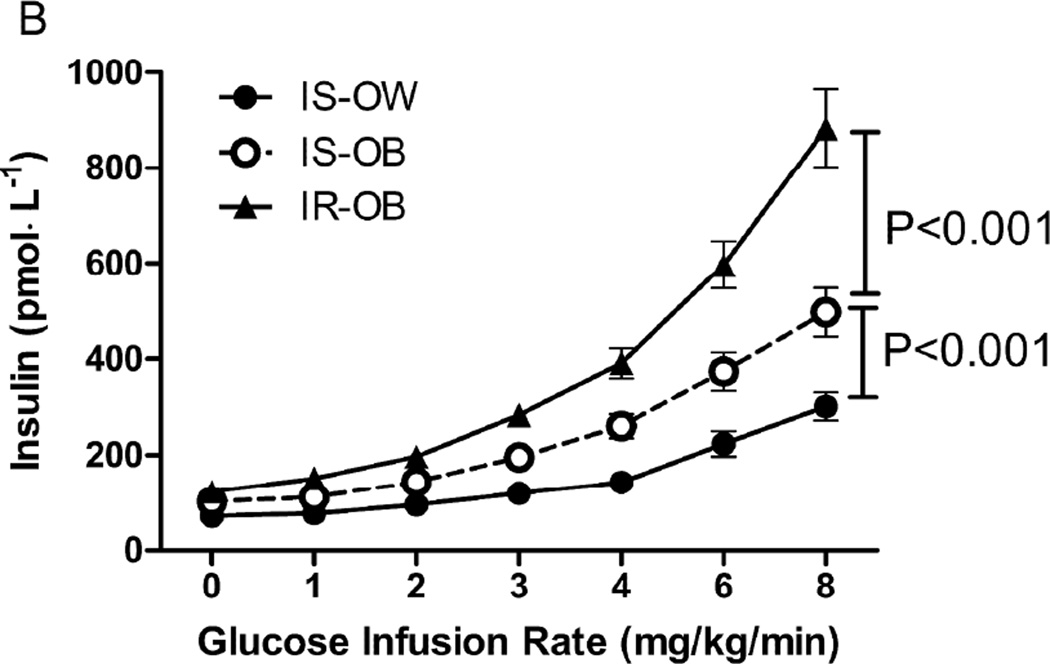
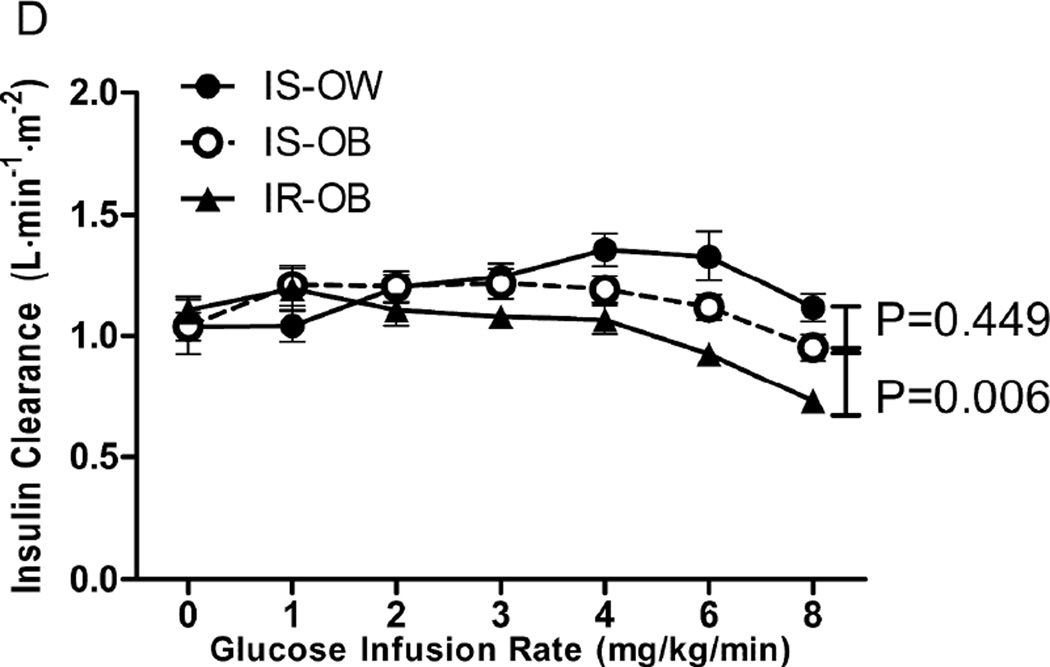
A more direct comparison in the 3 groups of the changes observed during the GGIT is shown in Figure 1. It can be seen that the IR-OB group was different from the other 2 groups in every category. Specifically, they had significantly higher plasma glucose (1.A) and insulin (1.B) concentrations in response to the glucose infusion during the GGIT, associated with the highest insulin secretion rate (1C) and the lowest insulin clearance rate (1.D). The IS-OB group had higher insulin concentrations than the IS-OW group (1.B), associated with significantly higher insulin secretion rates (1.C), but the two IS groups did not differ in terms of plasma glucose concentrations (1.A) or ICRen (1.D).
Figure 1.
Glucose (A), Insulin (B), insulin secretion rate (ISR, C) and insulin clearance rate (ICR, D) during the GGIT among insulin-sensitive/overweight (IS-OW; n= 27), insulin-sensitive/obese (IS-OB; n= 24) and insulin resistant/obese (IR-OB; n= 41) groups. P-values represent comparison in area under the curve (AUC) between groups.
ICRen had the most interesting pattern. Whereas glucose (1.A), insulin (1.B) and ISR (1.C) increase steadily with increase in intravenous glucose infusion, ICR rises then falls, likely due to the saturation of insulin clearance as insulin concentration increases. When ICRen were compared at each glucose infusion rate, IR-OB had significantly lower ICRen compared with IS-OB during the last 2 glucose infusion rates. ICRen were not significantly different between IS-OW and IS-OB (See Supplemental Table 2). We also compared ICRen at each glucose infusion rate with basal (fasting) ICRen within each group (See Supplemental Table 2). In IS-OW and IS-OB groups, ICRen rises significantly from basal rate and never significantly declines below basal rate. In the IR-OB group, the rise in ICRen is never significantly above basal rate and significantly decreases from basal rate during the last 2 glucose infusion rates.
We also evaluated the relationship between plasma insulin concentration and ICRen to understand whether differences in ICRen were predominately driven by insulin concentration or insulin resistance. As seen in the Supplemental Figure 2, ICRen initially rises with increasing plasma insulin concentration and then declines in all 3 groups. However, as reported above, the decline in ICRen is only significant in the IR-OB group, when plasma insulin concentration is at or above 600 pmol/L. Although it appears that IS-OW has the highest ICRen, peak ICRen did not differ among the 3 groups (P=0.30). The nadir ICRen was lowest in the IR-OB group (0.68 ± 0.21, P=0.009). Nadir ICRen was not significantly different between IS-OW and IS-OB (0.83 ± 0.24 vs. 0.83 ± 0.21, P=0.98).
To evaluate the relationship among obesity (BMI), insulin resistance (SSPG concentration), and insulin secretion, multiple linear regression analysis was performed as shown in Table 2. The relationships between ISR AUC and both BMI and SSPG concentration remained statistically significant. Male sex and lower insulin clearance rate were also associated with higher ISR.
Table 2.
Univariate and multivariate linear regression analyses of insulin secretion rate (ISR) with anthropometric and biochemical parameters
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| β | P-value | β | P-value | |
| Age | 0.017 | 0.864 | −0.026 | 0.695 |
| Sex (women vs. men) | 0.354 | <0.001 | 0.334 | <0.001 |
| BMI | 0.475 | <0.001 | 0.359 | <0.001 |
| Glucose AUC | 0.169 | 0.088 | −0.106 | 0.150 |
| SSPG | 0.565 | <0.001 | 0.413 | <0.001 |
| ICRex | −0.552 | <0.001 | −0.216 | 0.006 |
The independent variable is ISR AUC during the GGIT. Beta, standardized beta coefficient; BMI, body mass index; AUC, area under the curve; SSPG, steady-state plasma glucose; ICRex, exogenous insulin clearance rate
Finally, we also evaluated predictors of fasting insulin concentration (Supplemental Table 3) when adjusted for age, sex, BMI, fasting glucose, ISR AUC, ICRen, and SSPG. We found independent effects of ISR and ICR on fasting insulin concentration.
DISCUSSION
In a recent publication (1), we provided evidence that fasting hyperinsulinemia was present in individuals with obesity, whether they were insulin sensitive or insulin resistant. However, since insulin clearance did not vary as a function of obesity in either the insulin-resistant or insulin-sensitive subgroups, we speculated that obesity, per se, increases plasma insulin concentration primarily by increasing insulin secretion, not decreasing insulin clearance. The current findings extend these observations, and support our hypothesis by showing that insulin sensitive individuals with obesity have significantly higher plasma insulin concentrations and insulin secretion rates than equally insulin sensitive persons who are overweight, without any differences in insulin clearance between the 2 groups.
The impact of insulin resistance on insulin secretion and clearance is qualitatively different than the effect of obesity. Perhaps this qualitative difference is best appreciated by inspection of Fig. 1, which shows that all 3 experimental groups had an increase in plasma insulin concentration (1.B) and insulin secretion rate in response to the glucose infusion (1.C), whereas insulin clearance rate only decreased in the IR-OB population (1.D). In addition to emphasizing that both insulin resistance and obesity increase insulin secretion and insulin concentration, the data in Fig.1 demonstrate the relatively dramatic degree of hyperinsulinemia (1.B) in persons who are both obese and insulin resistant, as they have the highest insulin secretion rate and lowest insulin clearance rate.
Although the effects of obesity and insulin resistance on insulin secretion and insulin clearance appear relatively straight-forward, understanding the pathophysiological mechanisms responsible for the changes noted are far from clear. Focusing initially on insulin secretion, the notion of an increase in insulin secretion in insulin resistant persons in an attempt to maintain glucose tolerance is a recognized phenomenon (17). However, it is not clear why obesity, in the absence of insulin resistance, should increase insulin secretory function and insulin concentrations as shown in this study. In response to similar findings of an increase in insulin secretion in individuals with obesity, Ferrannini, at al. (2), concluded that “insulin resistance is not the only mechanism through which obesity enhances insulin secretion.” They went on to suggest that “signals originating in the central nervous system” play an important role in the increased insulin secretion and hyperinsulinemia associated with obesity. Others have also speculated on the effects of nocturnal free fatty acids to increase fasting insulin concentrations in obesity (18); however, nocturnal free fatty acids were also associated with increase in insulin resistance and thereby cannot explain the hyperinsulinemia in insulin sensitive individuals with obesity.
Although the existence of an association between insulin resistance and decreased insulin clearance seems to be well-recognized (6–8), in the absence of relevant data the reason why this association exists can only be speculated upon. At the simplest level it could be viewed as a homeostatic response to insulin resistance; a decrease in hepatic insulin clearance enables a greater proportion of pancreatic insulin secretion to reach the peripheral circulation to help overcome the resistance to insulin-stimulated glucose disposal. This conjecture is consistent with liver perfusion studies showing that removal of insulin by the liver is via a saturable system (19), thereby increasing the likelihood of the enhanced insulin secretion rate exceeding the clearance threshold. Alternatively, the liver is the main regulator of insulin clearance, and it has been shown that increases in hepatic fat content characteristic of insulin resistant individuals is associated with decreased hepatic insulin clearance and increased plasma insulin concentrations (20). Obviously, identifying the mechanism responsible for the association between insulin resistance and decreased insulin clearance requires additional investigation.
In conclusion, the results presented provide relatively straight-forward evidence of the complex interrelationship that exists among obesity, insulin resistance, insulin secretion, and insulin clearance. Put most simply, plasma insulin concentration and insulin secretion are increased in individuals with obesity, irrespective of degree of insulin resistance, but a decrease in insulin clearance is confined to the subset of individuals who are also insulin resistant. Although ISR is likely the primary driver of plasma insulin concentration, our results also show that as plasma insulin concentration increases, ICR decreases. The decrease in ICR is likely due to the saturation of insulin clearance mechanisms and significantly contributes to the plasma insulin concentration in insulin resistant individuals. However, it should be pointed out that this conclusion is based on an analysis of data collected for prior studies, primarily involving individuals of Caucasian ancestry. In addition, we had a smaller number of insulin sensitive individuals and did not have an insulin-resistant group who were overweight, which could have further strengthened our findings. We also measured ICRen under non-steady-state condition. Nevertheless, the results between ICRen and ICRex, which was measured under steady state conditions, were concordant. Furthermore, the pathophysiological mechanism(s) that account for our findings remain to be defined. On the other hand, we are unaware of other studies focused on these issues that have: 1) directly measured insulin secretion and insulin clearance, rather than estimating these variables based on changes in plasma insulin in response to various stimuli; and 2) quantified the impact of obesity, per se, on insulin clearance, independent of the co-existence of insulin resistance. Hopefully, our findings will stimulate others to evaluate our findings, as well as to explore the mechanisms accounting for the changes in plasma insulin concentration, insulin secretion, and insulin clearance associated with obesity and/or insulin resistance.
Supplementary Material
What is already known about this subject?
Individuals with obesity have increased insulin concentrations compared to their lean counterparts.
Differences in insulin secretion and/or insulin clearance could explain the hyperinsulinemia in obesity.
The mechanism for hyperinsulinemia in individuals with obesity remains unclear.
What does your study add?
Insulin sensitive individuals with obesity secrete more insulin than individuals who are equally insulin-sensitive but nonobese.
Decrease in ICR is confined to the subset of individuals with obesity who are also insulin resistant.
Obesity, per se, increases plasma insulin concentration primarily by increasing insulin secretion, and not decreasing insulin clearance.
Acknowledgments
FUNDING: This project was supported in part by an NIH/NCRR CTSA award number U L1 RR025744.
The authors would like to thank study volunteers and the st aff and nurses in the Stanford Clinical and Translational Research Unit for their invaluab le assistance with our metabolic studies.
Footnotes
Disclosure: The authors declared no conflict of interest.
REFERENCES
- 1.Kim MK, Reaven GM, Chen YI, Kim E, Kim SH. Hyperinsulinemia in individuals with obesity: Role of insulin clearance. Obesity (Silver Spring) 2015;23:2430–2434. doi: 10.1002/oby.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones CN, Abbasi F, Carantoni M, Polonsky KS, Reaven GM. Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am J Physiol Endocrinol Metab. 2000;278:E501–E508. doi: 10.1152/ajpendo.2000.278.3.E501. [DOI] [PubMed] [Google Scholar]
- 4.Goodarzi MO, Cui J, Chen YD, Hsueh WA, Guo X, Rotter JI. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am J Physiol Endocrinol Metab. 2011;301:E402–E408. doi: 10.1152/ajpendo.00013.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olefsky JM, Reaven GM, Farquhar JW. Effects of weight reduction on obesity: studies of carbohydrate and lipid metabolism. J Clin Invest. 1974;53:64–76. doi: 10.1172/JCI107560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzo C, Hanley AJ, Wagenknecht LE, Rewers MJ, Stefanovski D, Goodarzi MO, et al. Relationship of insulin sensitivity, insulin secretion, and adiposity with insulin clearance in a multiethnic population: the insulin Resistance Atherosclerosis study. Diabetes care. 2013;36:101–103. doi: 10.2337/dc12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marini MA, Frontoni S, Succurro E, Arturi F, Fiorentino TV, Sciacqua A, et al. Differences in insulin clearance between metabolically healthy and unhealthy obese subjects. Acta diabetologica. 2014;51:257–261. doi: 10.1007/s00592-013-0511-9. [DOI] [PubMed] [Google Scholar]
- 8.Lee MK, Rhee EJ, Kim MC, Moon BS, Lee JI, Song YS, et al. Metabolic Health Is More Important than Obesity in the Development of Nonalcoholic Fatty Liver Disease: A 4-Year Retrospective Study. Endocrinol Metab (Seoul) 2015;30:522–530. doi: 10.3803/EnM.2015.30.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Abbasi F, Lamendola C, Liu A, Ariel D, Schaaf P, Grove K, Tomasso V, Ochoa H, Liu YV, Chen YD, Reaven G. Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes Care. 2013;36:3276–3282. doi: 10.2337/dc13-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Liu A, Ariel D, Abbasi F, Lamendola C, Grove K, Tomasso V, Ochoa H, Reaven G. Effect of salsalate on insulin action, secretion, and clearance in nondiabetic, insulin-resistant individuals: a randomized, placebo-controlled study. Diabetes Care. 2014;37:1944–1950. doi: 10.2337/dc13-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pei D, Jones CN, Bhargava R, Chen YD, Reaven GM. Evaluation of octreotide to assess insulin-mediated glucose disposal by the insulin suppression test. Diabetologia. 1994;37:843–845. doi: 10.1007/BF00404344. [DOI] [PubMed] [Google Scholar]
- 12.Knowles JW, Assimes TL, Tsao PS, Natali A, Mari A, Quertermous T, et al. Measurement of insulin-mediated glucose uptake: direct comparison of the modified insulin suppression test and the euglycemic, hyperinsulinemic clamp. Metabolism. 2013;62:548–553. doi: 10.1016/j.metabol.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30:387–392. doi: 10.2337/diab.30.5.387. [DOI] [PubMed] [Google Scholar]
- 14.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 15.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2007;292:E1590–E1598. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 19.Mondon CE, Olefsky JM, Dolkas CB, Reaven GM. Removal of insulin by perfused rat liver: effect of concentration. Metabolism. 1975;24:153–160. doi: 10.1016/0026-0495(75)90016-5. [DOI] [PubMed] [Google Scholar]
- 20.Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709–E1715. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.